Introduction
Autoimmune and thyroid conditions account for a substantial proportion of the morbidity and mortality in the US. Reference Wang, Wang and Gershwin1 Nearly 24 million Americans suffer from at least one autoimmune disease, such as lupus or rheumatoid arthritis (RA), and almost all autoimmune diseases are known to decrease life expectancy. Reference Dinse, Parks and Weinberg2 Additionally, an estimated 20 million Americans have some form of thyroid disease, the causes of which are often autoimmune in nature but not fully elucidated. Reference Feller, Snel and Moutzouri3,Reference McLeod and Cooper4 Co-occurrence of both an autoimmune and thyroid condition is also likely, particularly among women Reference Antonelli, Ferrari, Corrado, Di Domenicantonio and Fallahi5,Reference Conigliaro, DʼAntonio and Pinto6 who bear the bulk of the disease burden. Reference Ortona, Pierdominici, Maselli, Veroni, Aloisi and Shoenfeld7 Epidemiologic studies on both sets of conditions have examined many risk factors, including demographic, lifestyle, genetic, and environmental risk factors. Reference Bel Lassen, Kyrilli, Lytrivi and Corvilain8-Reference Rees, Doherty, Grainge, Davenport, Lanyon and Zhang10 However, one area that has been neglected is the association between early life exposures with risk of autoimmune and thyroid conditions.
The Developmental Origins of Health and Disease hypothesis, or “Barker hypothesis,” postulates that diseases arising in childhood and later in life result from exposure to environmental factors in utero and during early childhood. Reference Barker11 The hypothesis has been frequently supported by chronic conditions that affect individuals later in life, such as cardiovascular disease, cancer, disability, and type 2 diabetes. Reference Ryckman, Rillamas-Sun and Spracklen12-Reference Spracklen, Wallace and Sealy-Jefferson15 There is also evidence that prenatal factors may influence the development of the immune system and propensity to develop asthma and other allergic disorders in childhood. Reference Chandran, Demissie, Echeverria, Long, Mizan and Mino16,Reference Xu, Li, Sheng, Liu, Tang and Chen17 Although alterations of the immune and endocrine system can persist into adulthood, few studies have considered the relationship between markers of early life growth and development and either adult onset-autoimmune or thyroid conditions that develop later in life, Reference Brix, Kyvik and Hegedüs18-Reference Parks, DʼAloisio and Sandler21 especially given that endocrine transitions in women (i.e., puberty, pregnancy, and menopause) increase their susceptibility to autoimmune conditions. Reference Desai and Brinton22
Thus, in the current study, we sought to investigate the potential associations between an individual’s birthweight and risk for (1) thyroid conditions, including underactive thyroid, overactive thyroid, and goiter; and (2) autoimmune conditions, including RA, lupus, multiple sclerosis (MS), ulcerative colitis (UC), and Crohn’s disease (CD). To evaluate the associations, we used the Women’s Health Initiative (WHI) Observational Study (OS), a well-characterized cohort of postmenopausal women.
Methods
Study population
The WHI is an ongoing prospective cohort study designed to study major causes of chronic disease in postmenopausal women. Briefly, 161,608 postmenopausal women aged 49–81 at enrollment and had a life expectancy of at least 3 years were recruited from the general population at 40 US clinical recruitment sites between 1993 and 1998. Reference Anderson, Manson and Wallace23,Reference Manson, Chlebowski and Stefanick24 Participants could have enrolled into overlapping clinical trials (WHI-CT; n = 67,932) or the long-term OS (WHI-OS; n = 93,676). The analytic cohort for this study only included women in the WHI-OS. Detailed information about the WHI’s study design, recruitment, and implementation has been described elsewhere. Reference Anderson, Manson and Wallace23,Reference Prentice and Anderson25 All study protocols were approved by the Institutional Review Board of each participating clinical center, and all participants provided written informed consent at study initiation.
Baseline measures
Upon entry into the WHI-OS, all women completed structured, self-administered questionnaires to collect information on demographics; lifestyle factors; and medical, reproductive, and family histories. Participants were asked to report their birthweight as one of the following categories: <6 pounds (lbs.), 6–7 lbs. 15 oz, 8–9 lbs. 15 oz, and ≥10 lbs. The collection of birthweight by category has previously been validated (Spearman r = 0.75). Reference Troy, Michels and Hunter26 Women were also asked to report if they were born 4 or more weeks premature or were part of a multiple pregnancies (specifically, a twin or triplet). Further, a physical assessment was performed by trained staff to gather accurate anthropometric and other clinical measures at baseline. Participants were also asked to bring all current medications with them to the physical assessment.
Outcome definitions and measurement
Data on prevalent thyroid and autoimmune conditions were obtained at baseline through self-administered questionnaires. Women were first asked to report if a doctor had ever told them that they had a thyroid problem (yes/no/donʼt know). If they answered yes, they were then asked a series of subquestions (yes/no/donʼt know) about specific thyroid conditions. We included the following thyroid conditions in our analyses: any thyroid gland problem, overactive thyroid, underactive thyroid, and goiter.
We included the following prevalent autoimmune conditions in our analyses: any autoimmune disease, MS, RA, lupus, and CD/UC. We defined prevalent MS, lupus, and CD/UC as a self-report of a physician diagnosis of “MS,” “systemic erythematosus (‘lupus’ or SLE),” or “UC or Crohn’s disease,” respectively. As the self-reported variable for RA has been poorly validated within WHI and often includes cases of osteoarthritis, as shown in a previous study, Reference Walitt, Constantinescu and Katz27 we classified women as having prevalent RA if they simultaneously met the following criteria: (1) self-reported type of arthritis as “rheumatoid arthritis” ; and (2) use of disease-modifying antirheumatic drugs (DMARDs).
Clinical outcomes were reported by participants annually through in-person, mailed, and/or telephone questionnaires. Beginning in the third year of follow-up, women were asked to self-report any new lupus, RA, underactive thyroid, and/or overactive thyroid diagnoses that had occurred since the last completed survey. Women were considered as incident cases for each outcome if they responded “yes” to a recent diagnosis of lupus, underactive, and/or overactive thyroid. Similar to prevalent RA, women were required to indicate a recent diagnosis of RA and use DMARDs to be classified as an incident case of RA. Because medication coding only occurred in the third year of follow-up, our incident RA analyses were censored at the third year of follow-up.
Exclusion criteria
For all of our analyses, women were excluded if they reported being born premature (n = 7282), reported being a twin or a triplet (n = 1616), or were missing information on birthweight category (n = 11,751). The final sample size for the case-control analysis was n ≤ 80,806. Additionally, women who had reported at baseline that they had previously been diagnosed with lupus, RA, underactive thyroid, or overactive thyroid prior to enrollment were excluded from the Cox proportional hazards model for that same condition, resulting in a final sample size of n ≤ 75,624.
Statistical Analyses
Baseline characteristics of the study participants by birthweight category (<6 lbs., 6–7 lbs. 15 oz, 8–9 lbs. 15 oz, and ≥10 lbs.) were examined using t-tests for continuous variables and chi-square tests for categorical variables. Logistic regression models were used to estimate odds ratios (OR) and their associated 95% confidence intervals (95% CI) between a woman’s birthweight and prevalent autoimmune (any, RA, MS, lupus, and UC/CD) or thyroid (any, underactive, overactive, goiter) conditions. Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% CI between a woman’s birthweight and incident cases of lupus, RA, underactive thyroid, and overactive thyroid. For birthweight, we used “6–7 lbs. 15 oz” as the referent category as infants born full-term within this weight range are considered to be of normal birthweight for female births between 1930-1950 in the US. Reference Johnson, Choh, Soloway, Czerwinski, Towne and Demerath28 Covariates selected for inclusion in our models were well-known risk factors for most autoimmune and/or thyroid conditions including age (continuous), race/ethnicity (categorical), region (categorical), BMI (continuous), smoking status (categorical), Reference Costenbader and Karlson29 education (categorical), neighborhood socioeconomic status (NSES; continuous), and alcohol use (categorical). Because there is controversy in the field of life course epidemiology as to whether or not adult lifestyle factors, such as BMI, should be adjusted for in statistical models, we present results unadjusted, partially adjusted, and fully adjusted for demographic and lifestyle factors. Reference Farland, Correia and Dodge30,Reference Tu, West, Ellison and Gilthorpe31 Because of prior associations between female hormones and both thyroid and autoimmune conditions, Reference Desai and Brinton22,Reference Santin and Furlanetto32 we additionally examined the use of female hormones (ever; yes/no) and pregnancy (ever been pregnant; yes/no) as covariates; however, inclusion of neither variable altered our results. All statistical tests were two-sided, and P-values < 0.05 were considered statistically significant. Each outcome was considered independently with birthweight; therefore, we did not correct for multiple testing. For conservative interpretation, a Bonferroni-adjusted significance threshold of P < 0.0038 (0.05/13) could be considered. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Results from the comparison of baseline characteristics are outlined in Table 1. Women born weighing <6 lbs. were more likely to be younger, have a lower NSES, identify as non-white, be a current smoker, and have an autoimmune condition than women born weighing ≥6 lbs. Women born weighing ≥10 lbs. were more likely to be older and have a higher BMI at baseline than women born weighing <10 lbs. Characteristics of participants included in the survival analyses are provided in Supplemental Table 1.
Table 1. Baseline characteristics of 80,806 WHI study participants, by birthweight category
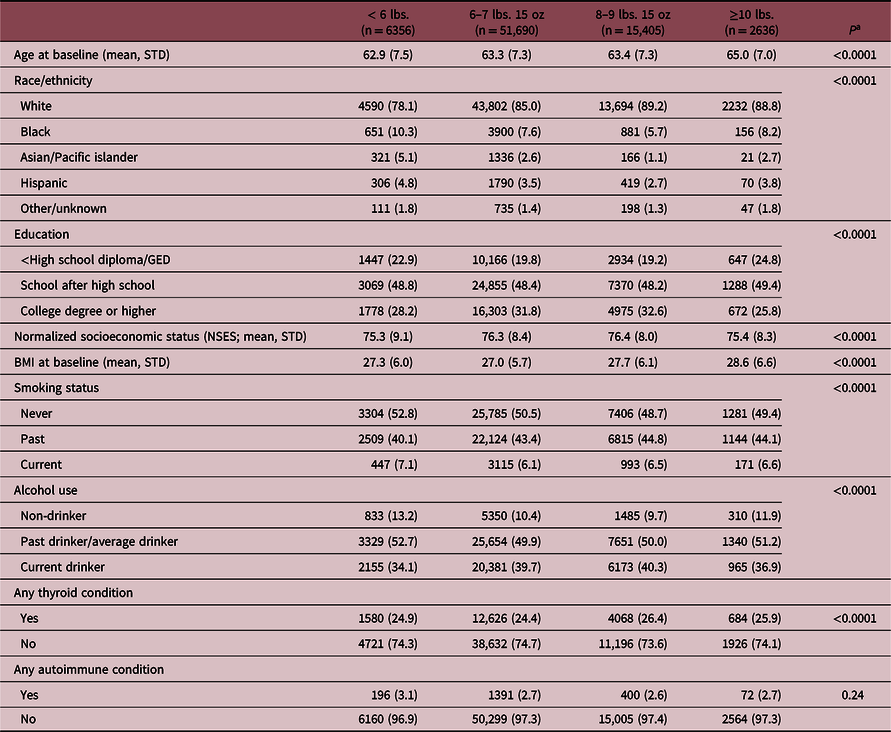
Numbers are N (%) for categorical variables or mean (standard deviation) for continuous variables.
a P-values are from t-tests and chi-square statistics.
Table 2 shows the crude and demographic- and lifestyle-adjusted odds of thyroid and autoimmune conditions by category of birthweight. Birthweight was significantly and positively associated with odds for any thyroid condition (all types combined) at baseline (unadjusted, P-value, test for linear trend < 0.0001). The association remained statistically significant after adjustment for demographic (P-value, test for linear trend = 0.005) and lifestyle (P-value, test for linear trend = 0.02) factors, although the strength of the association was attenuated. Birthweight was associated with underactive thyroid in a U-shaped trend such that women weighing <6 lbs. [OR 1.13, (95% CI 1.04, 1.22)], 8–9 lbs. 15 oz [OR 1.09, (95% CI 1.03, 1.15)], or ≥10 lbs. [OR 1.14, (95% CI 1.02, 1.28)] at birth were all at increased odds for underactive thyroid. Further, a significant inverse association was observed between birthweight and the odds for overactive thyroid (adjusted, P-value test for linear trend = 0.009) such that women born weighing <6 lbs. had a 16% increase in odds [OR 1.16, (95% CI 0.99, 1.26)] and women born weighing ≥10 lbs. had a 33% decrease in odds [OR 0.67 (95% CI 0.50, 0.92)] compared to women born between 6–7 lbs. 15 oz. No significant associations were observed between birthweight and the odds for goiter, RA, MS, lupus, CD/UC, or any autoimmune disease (all types combined).
Table 2. Association of birthweight to thyroid and autoimmune conditions among women at baseline in the Women’s Health Initiative

a Demographic factors include age, race, region, and BMI.
b Lifestyle factors include smoking status, education, normalized socioeconomic status, and alcohol use.
c Policy from the Women’s Health Initiative will not allow researchers to report number of participants in cells with fewer than 20 individuals. As such, cells that contain fewer than 20 participants read “<20.”
Crude and demographic- and lifestyle-adjusted hazards ratios of incident thyroid and autoimmune conditions are presented in Table 3. Women born in the highest birthweight category, >10 lbs., had a greater risk for lupus in both crude [HR: 1.47 (95%CI 1.14, 1.94] and adjusted models [demographic-adjusted, HR = 1.43 (95% CI 1.08, 1.90); demographic- and lifestyle-adjusted, HR = 1.51 (95% CI 1.12, 2.03)] compared to women born weighing between 6 and 7 lbs. 15 oz. Women born weighing >10 lbs. also had a greater risk for underactive thyroid compared to women weighing 6–7 lbs. 15 oz at birth [unadjusted, HR = 1.18 (95% CI 1.05, 1.31]; however, this association was attenuated after adjustments for covariates. No associations were detected within the incident models for overactive thyroid or RA.
Table 3. Association of birthweight to incident thyroid and autoimmune conditions among women in the Women’s Health Initiative

a Demographic factors include age, race, region, and BMI.
b Lifestyle factors include smoking status, education, normalized socioeconomic status, and alcohol use.
c Policy from the Women’s Health Initiative will not allow researchers to report number of participants in cells with fewer than 20 individuals. As such, cells that contain fewer than 20 participants read “<20.”
Discussion
Within this large, well-characterized, nationwide cohort of postmenopausal women, we found that higher birthweight was associated with a higher risk for prevalent underactive thyroid and a lower risk for prevalent overactive thyroid. Further, women born in the highest birthweight category had a significantly higher risk for developing incident lupus compared to women born at a normal birthweight.
Previous studies examining the relationship between an individual’s birthweight and risk for thyroid function and/or conditions are sparse. An infant’s birthweight is related to their thyroid stimulating hormone levels at birth, Reference Korada, Pearce, Avis, Turner and Cheetham33,Reference Korevaar, Chaker, Jaddoe, Visser, Medici and Peeters34 but this association does not appear to persist into childhood or adulthood. Reference Korevaar, Chaker, Jaddoe, Visser, Medici and Peeters34,Reference Frost, Petersen, Hegedüs, Christiansen, Brix and Christensen35 Birthweight has also demonstrated inverse associations with free thyroxine (FT4) and triiodothyronine (T3) in older adult twin pairs, Reference Frost, Petersen, Hegedüs, Christiansen, Brix and Christensen35 as well as subclinical thyroid dysfunction and both thyroglobulin (TgAb) and thyroid peroxidase (TPOAb) autoantibodies, Reference Phillips, Barker and Osmond36,Reference Phillips, Cooper and Fall37 although a separate study found no association between birthweight and TgAB or TPOAb. Reference Brix, Hansen and Rudbeck38 No associations were observed between birthweight and autoimmune (i.e., Grave’s disease or Hashimoto’s thyroiditis) or nonautoimmune (i.e., simple and toxic nodular goiter) thyroid diseases, Reference Brix, Kyvik and Hegedüs18 however lower birthweights were shown to increase the risk for spontaneous hypothyroidism in older adults. Reference Kajantie, Phillips, Osmond, Barker, Forsén and Eriksson19 Moreover, low birthweight has consistently been associated with risk for congenital hypothyroidism, Reference Hashemipour, Hovsepian, Ansari, Keikha, Khalighinejad and Niknam39,Reference Zhou, Luo and Lin40 particularly transient congenital hypothyroidism. Reference Zhou, Luo and Lin40 Further, gestational diabetes, one of the leading causes of macrosomia (i.e., high birthweight), is an established risk factor for congenital hypothyroidism. Reference Zhou, Luo and Lin40,Reference Franco, Laura, Sara and Salvatore41 Consistent with the existing body of literature, results from our cross-sectional analysis indicate both low and high birthweights are associated with higher risk for underactive thyroid.
A small number of prior studies have considered the potential association between birthweight and each of the autoimmune conditions examined in this study. Among four studies considering birthweight as a risk factor for RA, one cohort study found no association Reference Mandl, Costenbader, Simard and Karlson20 while three additional case-control and cross-sectional studies observed positive associations with birthweight. Reference Carlens, Jacobsson, Brandt, Cnattingius, Stephansson and Askling42-Reference Svendsen, Kyvik and Houen44 Similarly for MS, one case-control and one cohort study found no association with birthweight Reference Gardener, Munger, Chitnis, Michels, Spiegelman and Ascherio45,Reference Ramagopalan, Valdar and Dyment46 while a second case-control study found individuals born weighing ≥4 kg were at increased odds for MS. Reference Luetic, Menichini and Deri47 Results among studies examining risk factors for lupus are inconsistent with two case-control studies observing no association with birthweight, Reference Arkema and Simard48,Reference Coleman, Naleway, Davis, Greenlee, Wilson and McCarty49 while a cohort study found a positive association Reference Simard, Karlson and Costenbader50 and a cross-sectional study found an inverse association with birthweight. Reference Parks, DʼAloisio and Sandler21 Lastly, two cohort studies found no associations between birthweight and either UC or CD. Reference Khalili, Ananthakrishnan, Higuchi, Richter, Fuchs and Chan51,Reference Mendall, Jensen, Ängquist, Baker and Jess52 Compared to our analyses, many of the aforementioned studies were conducted in populations of similarly aged or slightly younger women, Reference Mandl, Costenbader, Simard and Karlson20,Reference Parks, DʼAloisio and DeRoo43,Reference Gardener, Munger, Chitnis, Michels, Spiegelman and Ascherio45,Reference Simard, Karlson and Costenbader50,Reference Khalili, Ananthakrishnan, Higuchi, Richter, Fuchs and Chan51 including several from the Nurse’s Health Study I and/or II. Reference Mandl, Costenbader, Simard and Karlson20,Reference Gardener, Munger, Chitnis, Michels, Spiegelman and Ascherio45,Reference Simard, Karlson and Costenbader50,Reference Khalili, Ananthakrishnan, Higuchi, Richter, Fuchs and Chan51 While our cross-sectional results are consistent with those studies that found no association between birthweight and the various autoimmune conditions, our analyses are underpowered to find an association given the magnitude of association observed.
Because an individual’s birthweight may be a marker for a variety of in utero exposures, determining the biological mechanism(s) by which birthweight influences the risk for thyroid and autoimmune conditions is complex. Reference Class, Rickert, Lichtenstein and DʼOnofrio53 Infants born at lower birthweights are at a higher risk for intrauterine growth restriction, resulting in hypothalamic-pituitary-thyroid axis dysfunction. Reference Ward, Syddall, Wood, Chrousos and Phillips54 Additional studies have suggested that fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis may be one of the long-term changes that link low birthweight to adult disease, impacting cortisol responsiveness and, thus, chronic inflammation and autoimmunity. Reference Ward, Syddall, Wood, Chrousos and Phillips54 In fact, adults with RA, Reference Imrich55 lupus, Reference Gutiérrez, Garcia, Rodriguez, Rivero and Jacobelli56 and other autoimmune conditions Reference Gold, Mohr, Huitinga, Flachenecker, Sternberg and Heesen57,Reference Stasi and Orlandelli58 are known to have dysregulation of the HPA axis. At the other end of the growth spectrum, babies born weighing ≥10 lbs. (i.e., fetal macrosomia) are more likely to be delivered by women who are obese, gain too much weight during pregnancy, or diagnosed with type 2 or gestational diabetes. Reference Kc, Shakya and Zhang59 In conditions of fetal overnutrition, an imbalance of the appropriate set of nutrients needed for proper organ development results in the inability of the fetus to properly regulate its nutrient excess, Reference Regnault, Nijland, Budge and Morrison60 potentially resulting in altered endocrine programming that could influence thyroid function. Reference Phillips61
Strengths of our study include its large sample size with extensive phenotypic data collection at baseline. The prospective design of the WHI also allowed us to consider incident cases for four of our outcomes with up to 8 years of follow-up data available. We were also able to evaluate a broad spectrum of potential confounders that may account for the underlying association between birthweight and autoimmune or thyroid conditions.
Our study was limited to evaluating categories of birthweight based on self-report. While the most ideal birth data collection method would have been a quantitative measure obtained through medical records or birth certificates, self-reported birthweight by category has been shown to correlate with medical record data in validity studies (58%–87% correctly reported birthweight category). Reference Jaworowicz, Nie and Bonner62,Reference Wodskou, Hundrup, Obel and Jorgensen63 Further, we hypothesize that any exposure misclassification would be nondifferential. Additionally, it is possible that women born within the normal birthweight range also experienced intrauterine growth restriction, a key event of fetal programming, which we would expect would bias our results toward the null.
There are also potential limitations related to our outcomes data. All of the outcomes evaluated in this study were self-reported without confirmation or adjudication, which would result in at least some misclassification. For example, the American Thyroid Association suggests that up to 60% of individuals with thyroid disease are unaware of their condition, 64 which could result in a nontrivial number of women in our analyses being misclassified as controls. However, we would expect this to underestimate the association and bias our results toward the null. Despite the large sample size of the WHI, the number of participants diagnosed with most of the rare autoimmune conditions was comparatively small, resulting in a lack of power, particularly for the lowest and highest birthweight categories. We were also unable to distinguish between clinically overt and subclinical under- and overactive thyroid, or between autoimmune or nonautoimmune causes, so our results may not generalize to all thyroid conditions.
Another limitation of our study was our inability to adjust for all of the covariates that may be particularly important in the pathophysiology of our examined conditions. For example, the WHI did not collect information related to iodine levels or iodine consumption in the OS participants, and iodine deficiency is a well-known risk factor for thyroid dysfunction. Reference Leung and Braverman65 We also did not have data on other in utero pregnancy exposures, such as in utero tobacco smoking exposure, or other conditions relating to the pregnancy or mother’s reproductive health, such as polycystic ovarian syndrome, preeclampsia, or gestational diabetes. Further, data on the participant’s family history were only available for cardiovascular disease and related outcomes, cancer, and fracture history; no family history information was available for autoimmune or thyroid conditions.
In conclusion, we demonstrate that low birthweight (<6 lbs.) may be associated with higher risk for underactive thyroid and higher birthweights (≥10 lbs.) are associated with (1) higher risk for underactive thyroid and incident lupus and (2) lower risk for overactive thyroid. To our knowledge, this is the first study evaluating the association between birthweight and overactive thyroid, as well as the largest study conducted on the association between birthweight and underactive thyroid in adults. Our research provides additional evidence of the role of early developmental phenotypes in the development of later-life conditions, further illustrating the importance of targeted interventions during preconception and prenatal care aimed at reducing both high and low birthweights and, thus, the burden of thyroid and autoimmune conditions.
Acknowledgements
We thank the WHI investigators and staff for their dedication and the study participants for making the program possible.
Financial support
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN26801100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the Helsinki declaration of 1975, as revised in 2008. All study protocols were approved by the Institutional Review Board of each participating clinical center, and all participants provided written informed consent at study initiation.
Supplementary materials
For supplementary material for this article, please visit https://doi.org/10.1017/S204017442100057X





