Introduction
Major depressive disorder (MDD) has been classified by the World Health Organization as the leading cause of disability and illness worldwide.1 With a global prevalence of over 300 million people, MDD poses a significant economic burden through its associated medical costs, mortality costs, and workplace costs.Reference Greenberg, Kessler and Birnbaum2 The core symptoms of depressed mood and anhedonia do not explicate the extent of disability observed with the disorder. Indeed, there are additional comorbid functional and cognitive dysfunctions that contribute to the debilitating nature of MDD.Reference Knight and Baune3
Given the complexity of MDD, it has proven difficult to treat, as evident by the rate of treatment-resistant depression (TRD). TRD is characterized as non-response to one or more treatments, with the likelihood of achieving remission decreasing with each subsequent treatment step.Reference Rush, Trivedi and Wisniewski4 TRD is experienced by approximately 12–20% of those with MDD.Reference Mrazek, Hornberger and Altar5 Of note, those with TRD have reported lower workplace productivity and social functioning, as well as depression rating scores indicative of more severe depression compared to those with non-TRD.Reference DiBernardo, Lin and Zhang6 Furthermore, the costs associated with TRD are higher compared to those with non-TRD, and it has been suggested that TRD is driving the capital costs associated with depression.Reference Fostick, Silberman and Beckman7 Given the prevalence of TRD and its associated functional debilitations, there is interest in developing both effective and fast-acting treatment options. Notably, fast-acting therapeutics may aid in reducing the capital cost associated with depression by decreasing absenteeism and presenteeism, wherein absenteeism and presenteeism have been shown to be major contributors to costs associated with MDD.Reference Evans-Lacko and Knapp8
First-line treatment for MDD typically includes a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI).Reference McIntyre, Suppes and Tandon9 The use of SSRIs and SNRIs in MDD accords with the monoamine hypothesis of depression. The monoamine hypothesis posits that MDD is caused by a deficit in monoamine systems (i.e., serotonin, noradrenaline, or dopamine). While SSRIs and SNRIs have demonstrated efficacy in alleviating mood symptomatology, there remains a high percentage of non-responders. Therapeutic strategies for non-responders include switching or combining antidepressants and augmenting antidepressant therapy with a non-antidepressant agent.Reference Al-Harbi10 Given the number of US Food and Drug Administration (FDA)–approved SSRIs for MDD (e.g., citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, vilazodone), the high prevalence of those with TRD and with non-response to monoamine antidepressants suggests that monoamine dysregulation does not fully account for depressive symptomatology and that other targets should be explored.11
During the past one to two decades, several alternative therapeutic targets have been identified, such as the glutamatergic pathway, the gamma-aminobutyric acid (GABA) pathway, the opioidergic pathway, and the inflammatory pathway. Not only do these novel pathways have the potential to offer effective alternative treatment for those with TRD, but benefits have also been observed in non-affective domains such as cognition.Reference Rosenblat, McIntyre and Alves12 In addition, alternative mechanisms of action may change the traditional treatment course by providing rapid symptomatic relief (i.e. hours), when compared to traditional antidepressants (e.g., 4–6 weeks).Reference Katz, Tekell and Bowden13 For example, suicidal ideation is a potential area of improvement with rapid antidepressant response. As it has been suggested that rapid time to response can mediate increased suicidal behavior occasionally observed after starting antidepressant treatment, such that suicidal risks decrease in those with early antidepressant response.Reference Jick, Kaye and Jick14 , Reference Teicher, Glod and Cole15 Improving the time to response could fundamentally change approaches to treatment and offer considerable improvements for patients. The current review covers the antidepressant action of the glutamatergic, GABAergic, opioidergic, inflammatory systems, and those associated novel therapeutics that have demonstrated considerable efficacy in recent clinical trials (Table 1 provides a list of all pharmaceutical agents discussed, sponsors, current trial phases, and regulatory affairs).
Table 1. Summary of novel pharmaceuticals for treatment of MDD
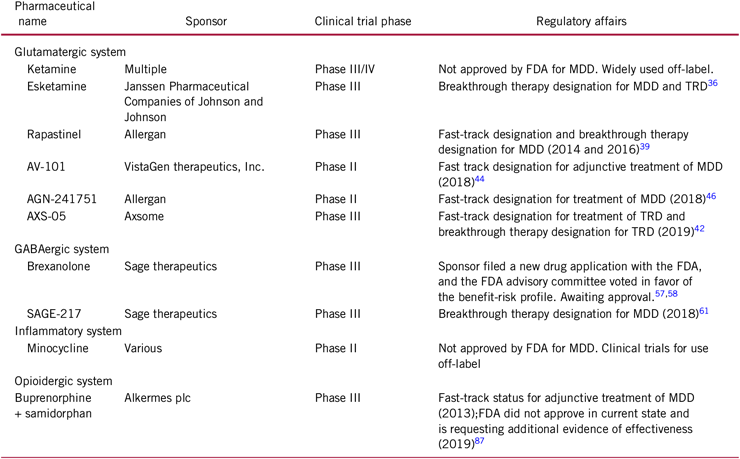
Note: FDA: US Food and Drug Administration; MDD: major depressive disorder.
Glutamate
Glutamate is the principal and most abundant excitatory neurotransmitter in the brain. A wealth of preclinical and clinical data posit that the glutamatergic system has a role in mood regulation.Reference Black16 , Reference Moriguchi, Takamiya and Noda17 Furthermore, neurological differences in glutamate, glutamine, and their metabolites have been observed in those with MDD. Those with MDD have lower neural concentrations of the aforementioned than healthy controls and their concentrations can be increased following successful treatment.Reference Moriguchi, Takamiya and Noda17 –Reference Pfleiderer, Michael and Erfurth19 Conversely, high plasma blood glutamate levels have been reported in those with MDD, and following antidepressant treatment with monoamine-based antidepressants, decreases in blood glutamate levels have been reported.Reference Inoshita, Umehara and Watanabe20 , Reference Kucukibrahimoglu, Saygin and Caliskan21 It follows that monoamine-based antidepressants impact the glutamatergic system and that the glutamatergic system may be involved in MDD.
Regulation of the glutamatergic system to treat mood disorders is typically achieved through interaction with glutamate release or glutamate receptors. Receptors are classified as ionotropic (i.e. forming a ligand gated ion channels) or metabotropic (i.e. activating coupled G-proteins). Various ionotropic receptors have been documented, including N-methyl-d-aspartate (NMDA), α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA), and kainic acid. Three groups of metabotropic glutamate receptors (mGluRs) have also been identified. Mechanistically, it has been suggested that NMDA antagonists increase the AMPA to NMDA neurotransmission ratio, which has shown to have antidepressant-like effects in preclinical models.Reference Andreasen, Gynther and Rygaard22 Furthermore, AMPA and the brain-derived neurotrophic factor (BDNF) have a bidirectional interaction, wherein increases in AMPA facilitate increases in BDNF expression, which can result in enhanced neuronal plasticity.Reference Gulyaeva23 Notably, dysregulation of this system can produce negative changes in neuronal plasticity associated with MDD.Reference Brunoni, Lopes and Fregni24
Novel therapeutics
Ketamine and ketamine-like agents
Ketamine
Ketamine is a high-affinity NMDA receptor antagonist. While not approved by the FDA for the treatment of MDD, ketamine has been increasingly used off-label for psychiatric disorders wherein the most common use was MDD.Reference Wilkinson, Toprak and Turner25 Ketamine is most often administered via an intravenous infusion over 40 minutes using sub-anesthetic doses in the range of 0.10–0.75 mg/kg.Reference Andrade26 Metabolism of ketamine occurs in the liver and is achieved primarily by CYP3A4 and in lesser amounts by CYP2B6 and CYP2C9.Reference Hijazi and Boulieu27 A meta-analysis exploring the efficacy of ketamine for use with MDD in randomized control trials found that those receiving ketamine had improved response rates versus placebo at 24 hours, 72 hours, and 7 days post-infusion, where response was defined as a minimum of 50% reduction in absolute Hamilton Depression Rating Scale scores (HAM-D), Montgomery–Åsberg Depression Rating Scale (MADRS), or significant improvements in the Clinical Global Impression Scale (CGI). Furthermore, results at all time points were highly significant (p < 0.00001).Reference Han, Chen and Zou28 Of clinical relevance is the time to response to ketamine wherein, following a single infusion, antidepressant effects can be observed within 4 hours.Reference Coyle and Laws29 Notably, the use of ketamine for MDD has been associated with psychotomimetic effects post-infusion and may have the potential for abuse.Reference Sanacora and Schatzberg30 Methods by which the aforementioned potential adverse events could be mitigated include dosing modification, co-administration with other agents, alternative routes of administration, and isometric formulations.Reference Cooper, Rosenblat and Cha31 Esketamine and, in particular, rapastinel have been suggested as alternative formulations that have demonstrated rapid response with fewer (particularly in the case of rapastinel) psychotomimetic side effects.32 , Reference Preskorn, Macaluso and Mehra33 In addition to its rapid response, ketamine may have pro-suicidal effects such that ketamine has demonstrated efficacy in rapidly reducing suicidality in patients with TRD.Reference Serafini, Howland and Rovedi34 Notably, while the antidepressant action of ketamine is often attributed to its NMDA properties, one study has suggested that opioid receptors are involved in ketamine’s antidepressant effects. Indeed, when naltrexone (i.e., an agent that blocks neurological opioid receptors) is administered to ketamine responders’ pre-ketamine treatment, the antidepressant effects typically observed with ketamine are significantly attenuated relative to placebo. However, naltrexone pretreatment relative to placebo did not significantly impact ketamine-induced dissociation, suggesting that ketamine’s dissociative effects are largely independent of its action on the opioid receptors.Reference Williams, Heifets and Blasey35 Ultimately, further studies are needed to elucidate the mechanism of action of ketamine and explore the role of opioid receptors in relation to ketamine’s efficacy.
Esketamine
Esketamine is the S-enantiomer of ketamine (i.e. the R-enantiomer) and similarly, a NMDA receptor antagonist. Esketamine has been given breakthrough therapy designation from the FDA for both TRD and MDD with imminent risk for suicide.36 Esketamine is administered intranasally as a nasal spray and, to date, clinical trials have used self-administered doses from 14 to 84 mg over various time points (e.g., twice weekly, weekly, and biweekly).Reference Daly, Singh and Fedgchin37 Esketamine is metabolized similarly to ketamine by CYP3A4 and, in lesser amounts, by CYP2B6 and CYP2C9.Reference Hijazi and Boulieu27 The sponsor has released data from a phase 3 clinical trial with TRD wherein esketamine, in addition to a newly initiated oral antidepressant, had marginally significant (one-sided p = 0.010) improvements as measured by change from baseline in the MADRS total score. Furthermore, clinical response, wherein response was considered a ≥50% improvement in MADRS from baseline, was observed 24 hours post-dose and maintained through day 28 for participants receiving an esketamine and an oral antidepressant combination. The most common treatment emergent adverse events were metallic taste and headache, and other treatment emergent adverse events included nausea, vertigo, dizziness, and headache.32 In a clinical trial specifically evaluating the safety of esketamine, the most common treatment emergent adverse events were dizziness (75%) headache (36%), and dissociative symptoms (21%), wherein dizziness and nausea may be dose-related. Dissociative symptoms, as measured by the Clinician Administered Dissociative States Scale (CADSS), were apparent following intranasal dosing and reached their maximum at 30–40 minutes post-dose; however, they were resolved 2 hours post-dose. Notably, dissociative symptoms decrease with repeated dosing. Furthermore, hypertension was observed immediately following esketamine treatment. While symptoms typically resolved 2 hours post-treatment, they may persist post-treatment.Reference Daly, Singh and Fedgchin37 Recognizing the potential for adverse events, the FDA has approved a Risk Evaluation and Mitigation Strategy (REMS) to ensure proper usage of esketamine and mitigate the risks of esketamine-related adverse events. Strategies are in place at various levels including the healthcare setting, pharmacies, wholesalers, and patients. Overall, the REMS ensure that esketamine is dispensed only in a medically supervised, certified, healthcare setting. Further, patients are made aware of the possible serious adverse events, and there is continued monitoring of patients to both characterize the risks and support safe usage.38
Rapastinel
Rapastinel is an NMDA modulator, behaving similarly to partial agonists of the glycine site. Rapastinel was granted fast track and breakthrough therapy designation by the FDA for adjunctive treatment of MDD.39 Rapastinel is administered with weekly intravenous doses of 1–10 mg/kg. There was a large placebo effect observed in a phase 2 clinical trial with rapastinel and placebo. Regardless, a rapid reduction in Hamilton Depression Rating Scale scores (HAMD-17) and Bech-6 (a six-item subscale from the HAMD-17) scores were also demonstrated; however, where rapastinel differed, the differences were marginally different from placebo (p < 0.05).Reference Ruhe, Dekker and Peen40 Seventy percent of participants showed a clinical response wherein a response was defined as 50% improvement from baseline in HAMD-17. Notably, higher doses such as 30 mg/kg demonstrated no antidepressant effects. Of clinical relevance, rapastinel demonstrated changes in Bech-6 scores within 24 hours of infusion, and no psychotomimetic effects were observed.Reference Preskorn, Macaluso and Mehra33 Preclinical trials suggest that rapastinel may have procognitive effects and there are ongoing clinical trials to evaluate this possibility in humans.Reference Rajagopal, Burgdorf and Moskal41
AXS-05
AXS-05 is a combinatory pharmaceutical that is a combination of bupropion and dextromethorphan. AXS-05 has been granted fast-track status by the FDA for TRD and breakthrough therapy designation.42 Dextromethorphan acts as a low-affinity NMDA receptor antagonist, a sigma-1 agonist, and inhibitor of the norepinephrine and serotonin systems with notably limited bioavailability.Reference Nguyen, Thomas and Lucke-Wold43 Bupropion is a norepinephrine and dopamine reuptake inhibitor and acts to increase the bioavailability of dextromethorphan. Available Phase II results from the sponsor indicated that AXS-05 met its primary endpoint in those with MDD. Specifically, there was a highly significant (p < 0.001) reduction in MADRS scores over 6 weeks compared to bupropion, and 47% of those who received AXS-05 achieved remission in comparison to 16% of those who received bupropion. Furthermore, the most commonly reported adverse events were nausea, dizziness, dry mouth, decreased appetite, and anxiety.44
Other potential future therapeutics
Throughout the year 2018, various glutamate-based therapeutics were granted fast-track status by the FDA. Notably, AV-101, a selective agonist of the NMDA receptor glycine binding site B, was given the fast-track designation for adjunctive treatment of MDD. AV-101 is expected to be administered orally and presents a novel mechanism of action. The sponsor suggested AV-101 has the potential to produce rapid therapeutic effects, similar to those of ketamine.45 Preclinical studies with AV-101 demonstrated rapid and persistent antidepressant effects in rodent models of depression and did not produce psychotomimetic effects.Reference Zanos, Piantadosi and Wu46
Another pharmaceutical agent in the pipeline is AGN-241751, an NMDA receptor modulator with oral administration. It was granted fast-track designation by the FDA for the treatment of MDD, and a phase 2 clinical trial is ongoing.47
Gamma-Aminobutyric Acid (GABA)
GABA is the primary inhibitory neurotransmitter in the brain. The GABAergic system interacts with various systems implicated in the pathophysiology of MDD such as the serotonergic system, noradrenergic system, and the hypothalamic-pituitary-adrenal (HPA) axis.Reference Slattery, Desrayaud and Cryan48 –Reference Verkuyl, Hemby and Joels50 The impact of the GABAergic system on MDD has been observed in preclinical and clinical models. For example, alterations of metabotropic type B GABA(GABAB) receptors in rodents result in anxiety and depression-like behavior.Reference Mombereau, Kaupmann and Gassmann51 GABA dysregulation is also observed in humans wherein decreased concentrations of GABA have been reported in those with MDD, and GABA concentrations have been shown to increase following treatment.Reference Sanacora, Mason and Rothman52 , Reference Bhagwagar, Wylezinska and Taylor53 These trends suggest that treatment for MDD normalizes GABA concentrations in the brain. Some treatment regimens employ GABA modulators such as benzodiazepines that modulate the ionotropic type A GABA (GABAA) receptor complex and are often administered alongside traditional antidepressants when patients exhibit anxiety or insomnia.54 The GABAA receptor is the most abundant inhibitory neurotransmitter receptor in the brain and, while altered function of this receptor has been associated with both anxiety and MDD, benzodiazepines have not shown sufficient therapeutic potential as monotherapy for MDD.Reference Sequeira, Mamdani and Ernst55 , Reference Benasi, Guidi and Offidani56 Regardless, targeting the GABAA receptor provides a potential novel mechanism of treatment for MDD in combination with traditional antidepressants.
Novel therapeutics
Brexanolone (SAGE-547)
Brexanolone is a positive allosteric modulator of the type GABAA receptor with parenteral administration. Following the filing of a new drug application wherein priority review status was granted, the FDA advisory committee voted in favor of the benefit-risk profile of brexanolone for treatment of postpartum depression.57 , 58 Of clinical relevance, if approved, brexanolone would be the first pharmaceutical indicated specifically for postpartum depression. Efficacy of brexanolone has been observed in clinical trials wherein women with postpartum depression received a single intravenous injection of 90, 60 µg/kg per hour, or placebo. Brexanolone, in comparison to placebo, produced a rapid antidepressant response that was marginally significant (p < 0.05) within 60 hours post infusion, where response was defined as a 50% reduction in HAM-D total score. Furthermore, response was sustained for up to 30 days. Brexanolone was generally well tolerated, with the most common treatment emergent adverse events being headaches, dizziness, and somnolence. Few treatment-related serious adverse events were observed, notably, the altered state of consciousness and syncope in one participant, and excessive sedation that was resolved following infusion cessation in five participants. No clinically significant changes in laboratory parameters were observed.Reference Meltzer-Brody, Colquhoun and Riesenberg59
SAGE-217
SAGE-217 is a positive allosteric modulator of the synaptic and extrasynaptic GABAA receptors.Reference Martinez Botella, Salituro and Harrison60 SAGE-217 was granted breakthrough therapy designation by the FDA for the treatment of MDD.61 Preclinical studies have demonstrated an improved drug metabolism and pharmacokinetics profile in comparison to brexanolone. Specifically, SAGE-217 offers low clearance, resulting in higher oral bioavailability and allowing for an oral route of administration.Reference Martinez Botella, Salituro and Harrison60 The sponsor has provided results from an open-label phase 2 clinical trial wherein participants with MDD received a daily oral dose of 30 mg. Improvements in depressive symptoms were observed 1 day post-treatment and persisted for 2 weeks following cessation of treatment wherein the differences observed between the treatment and placebo group were highly significant (p < 0.0001).Reference Gunduz-Bruce, Riesenberg and Sankoh62 Furthermore, a double-blind, randomized, and controlled phase 2 clinical trial wherein the participants also received a daily oral dose of 30 mg demonstrated that there was a mildly significant difference in HAM-D score observed on day 2 and maintained through day 28 (p = 0.0223).Reference Gunduz-Bruce, Silber and Rothschild63 SAGE-217 appeared to be well tolerated, though minor treatment-related adverse events were observed including sedation, headache, and dizziness.Reference Gunduz-Bruce, Riesenberg and Sankoh62
Immune-Inflammation
The macrophage theory of depression associates cytokines (e.g., macrophage monokines, interferon alpha, and tumor necrosis factor) with depressive symptomatology.Reference Smith64 The macrophage theory of depression is supported by the observation of increased concentrations of pro-inflammatory cytokines among those with MDD.Reference Dowlati, Herrmann and Swardfager65 The increase in cytokines may be indicative of particularly serious profiles of MDD, such as TRD or severe suicidal ideation. A recent study suggests that higher levels of inflammatory proteins are predictive of the severity of TRD.Reference Strawbridge, Hodsoll and Powell66 Furthermore, another study demonstrated that there are significantly higher levels of inflammatory markers in patients with MDD who have high suicidal ideation.Reference O’Donovan, Rush and Hoatam67 In addition to their involvement with mood symptomatology, cytokines are involved in cognitive functions such as attention, executive function, learning, and memory, which may contribute to the cognitive impairment observed in MDD.Reference McAfoose and Baune68 , Reference Pan, Park and Brietzke69 The proposed mechanism by which cytokines affect the nervous system is complex; in summary, they can evoke an excitotoxic effect by way of interactions between the monoamine, glutamate, and BDNF systems.Reference Felger and Lotrich70 Given their involvement in neurotransmitter systems and associated symptomatologic presentation, reduction of inflammatory cytokines presents a novel therapeutic pathway.
Minocycline
Minocycline is a tetracycline antibiotic approved by the FDA for use with various bacterial infections.71 Minocycline has anti-inflammatory properties and has been used for treatment of rheumatoid arthritis, a chronic inflammatory disease.Reference Langevitz, Livneh and Bank72 Several clinical trials are underway exploring the efficacy of minocycline off-label for the treatment of MDD. Recently, results from published clinical trials have been summarized meta-analytically.Reference Rosenblat and McIntyre73 The dose used throughout various trials ranged from 100 to 300 mg/day. Pooled analyses revealed a significant large antidepressant effect (p = 0.005) with the use of minocycline in comparison to placebo. Furthermore, a favorable tolerability profile was observed such that there was no significant difference for study discontinuation between treatment and placebo groups. Notably, minocycline has demonstrated efficacy in treating the cognitive dysfunction observed with schizophrenia, particularly in the executive functioning domain.Reference Levkovitz, Mendlovich and Riwkes74 While this has yet to be evaluated in those with MDD, similar results could be expected as cognitive dysfunction has been associated with increased levels of C-reactive protein (i.e. a marker of inflammation) in MDD.Reference Krogh, Benros and Jorgensen75 At the time of this writing, there were several ongoing studies evaluating the efficacy of minocycline as augmentative therapy in MDD and in other mood disorders.
Opioidergic Systems
The opioid system is composed of three types of receptors (i.e. delta, kappa, and mu) which are expressed widely throughout the nervous system. While the opioid system has been commonly implicated in reward processing, pain, and addiction, alterations within the opioid system have also been associated with MDD.Reference Le Merrer, Becker and Befort76 –Reference Pecina, Karp and Mathew79 In addition, anhedonia, a core feature of MDD, has been observed with opioid use, suggesting that the opioid system may be involved in MDD pathophysiology.Reference Garfield, Cotton and Allen80 Various FDA-approved antidepressants, such as venlafaxine and mirtazapine, interact with the opioid system, though they are an SNRI and tetracyclic antidepressant, respectively.Reference Schreiber, Bleich and Pick81 Furthermore, preliminary evidence suggests that opioid agonist therapy has efficacy in treating mood disorders.Reference Tenore82 While a concern in the use of opioidergic treatment is the potential for abuse, agents such as naloxone or samidorphan can be used in conjunction with the opioid to mitigate such use.Reference Jordan and Morrisonponce83 , Reference Turncliff, DiPetrillo and Silverman84 The kappa-opioid receptor has demonstrated improvements in mood symptomatology and is of interest in novel therapeutic pathways for MDD.Reference Harrison85
Buprenorphine+samidorphan
Buprenorphine + samidorphan is a combinatory drug functioning primarily as a kappa-opioid antagonist and a paired mu-opioid agonist and antagonist to offer controlled opioid modulation. Buprenorphine + samidorphan was given fast-track status by the FDA for the adjunctive treatment of MDD; however, the FDA committee recently (November 2018) voted against the approval of buprenorphine + samidorphan. The official decision from the FDA (February 2019) states that it was unable to approve buprenorphine + samidorphan in its current state and requests additional substantial evidence of its effectiveness.86 , 87 Ongoing trials are, however, being conducted by the sponsor, and these results are pending. Clinical trials of buprenorphine + samidorphan have used doses from 0.5 mg/0.5 mg to 8 mg/8 mg, and a 1:1 dose has demonstrated the most efficacy and tolerability.Reference Ehrich, Turncliff and Du88 , Reference Ragguett, Rong and Rosenblat89 Buprenorphine undergoes hepatic metabolism wherein it is primarily processed by cytochrome P450 3A4 and, to a lesser extent, CYP 2C8.Reference Picard, Cresteil and Djebli90 Clinical trials have demonstrated mixed results for the efficacy of buprenorphine + samidorphan. A phase III trial did not meet its primary endpoint such that changes in MADRS from baseline to endpoint were not significant. However, there may have been a limitation regarding placebo responders, which was addressed in later trials.91 A phase III trial termed FORWARD-5 has achieved the primary endpoint such that buprenorphine + samidorphan 2 mg/2 mg produced a greater change in MADRS-6 and MADRS-10 in comparison to placebo with marginal significance (p = 0.026). Furthermore, a pooled analysis of safety results demonstrated that there were low incidences of serious adverse events and the majority of adverse events that occurred were mild or moderate, consisting of nausea, constipation, dizziness, and sedation. There were no clinically significant changes observed in vital signs, body weight, or echocardiogram parameters. Of particular relevance, there were no reported adverse events associated with abuse or dependence, and there were no reports of opioid withdrawal.Reference Fava, Thase and Trivedi92
Conclusion
Although success has been found with traditional monoamine antidepressants, there remains a pronounced occurrence of TRD and a prolonged time to response. It is evident by the therapeutics in the pipeline for use with MDD that novel mechanisms of action outside the traditional serotonergic, noradrenergic, and dopaminergic pathways may be beneficial for treatment of MDD. Indeed, novel mechanisms of action allow for a wider selection of treatment options with various therapeutic targets, which may be of great benefit to those with MDD, considering the heterogeneity of the disorder. In particular, fast-acting therapeutics such as ketamine and rapastinel, and therapeutics that have an opportunity to uniquely target systems implicated in the pathophysiology of MDD such as buprenorphine + samidorphan, have the potential to produce cascading benefits throughout patient lives by both improving mood symptomatology and decreasing societal costs by decreased workplace absenteeism and presenteeism.Reference Adler, McLaughlin and Rogers93 While these novel therapeutics have shown some promising results, additional long-term clinical trials are warranted to determine both the long-term efficacy and monitor for treatment-related adverse events. Though many of the aforementioned therapeutics have rapid results and have shown efficacy in those with TRD, only with long-term clinical trials are we able to determine if these response rates will persist.
Disclosures
During the past 2 years, Dr. Roger S. McIntyre received consultation/speaker fees from the following pharmaceutical companies: Shire, Purdue, Otsuka, Janssen, Lundbeck, Pfizer, Neurocrine, Sunovion, Takeda, Allergan, and Minerva. Dr. Roger S. McIntyre also received research grants from Stanley Medical Research Institute and CIHR/GACD/Chinese National Natural Research Foundation. No other authors have disclosures to report.
Optional Posttest and CME Certificate
CME Credit Expires: July 31, 2022
Posttest Study Guide
The posttest can only be submitted online. The below posttest questions have been provided solely as a study tool to prepare for your online submission. Faxed/mailed copies of the posttest cannot be processed and will be returned to the sender. If you do not have access to a computer, contact NEI customer service at 888-535-5600.
Amelia is a 24-year-old patient with treatment resistant depression currently enrolled in a clinical trial involving esketamine. Esketamine is an antagonist at which of the following receptors?
A. AMPA
B. NMDA
C. mGluR
Marsha is a 31-year-old patient with postpartum depression who is intrested in enrolling in a clinical trial testing the novel antidepressant brexanolone. Brexanolone is hypothesized to work due to its actions with which of the following neurotransmitter systems?
A. GABA
B. Glutamate
C. Opioid
Clark is a 43-year-old patient with treatment resistant depression currently enrolled in a clinical trial testing the novel antidepressant SAGE-217. Compared to brexanolone, SAGE-217 differs in that it:
A. Is a positive allostric modulator of GABA-B receptors
B. Requires intravenous administration
C. Has higher oral availability
Patrick is a 34-year-old patient with MDD. Lab results indicate that this patient has increased levels of pro-inflammatory cytokines. Which of the following agents is hypothesized to ameliorate symptoms of depression primarily via its anti-inflammatory properties?
A. SAGE-217
B. Minocycline
C. Rapastinel
D. Buprenorphine/samidorphin combination
Optional Online Posttest and CME Certificate Instructions
Read the article
Complete the posttest and evaluation, available only online at www.neiglobal.com/CME (under “CNS Spectrums”)
Print your certificate (passing score = 70% or higher)
Questions? call 888-535-5600, or email CustomerService@neiglobal.com



