INTRODUCTION
The genus Leishmania includes parasitic protozoa responsible for a species-dependent spectrum of diseases extending from localized, self-healing cutaneous lesions to more severe visceral infections. In endemic areas of the Americas, Leishmania (Viannia) braziliensis is the major causative agent of tegumentary leishmaniasis (Grimaldi, Tesh and McMahon-Pratt, 1989; Canto-Lara et al. 1998). This parasite is genetically heterogeneous (Cupolillo et al. 2003), exhibiting substantial enzyme polymorphism. This protozoa has been associated to a broad range of clinical manifestations – from simple cutaneous ulcers to disseminated lesions and mucosal involvement, a very destructive form of leishmaniasis (Saravia et al. 1985; Marsden, 1986; Weigle et al. 1986; Grimaldi and Tesh 1993; Cupolillo et al. 2003). The plasticity of L. (V.) braziliensis and its interaction with the human host, producing distinct clinical forms, represents a challenge to researchers looking for a better understanding of the infection's natural history.
The life-cycle of Leishmania parasites involves 2 principal developmental stages: the amastigote form, usually confined in mammalian macrophages, and the promastigote form, found within the sand fly insect vector. The digenetic parasite's mechanism of differentiation presented in diverse vertebrate and invertebrate hosts requires the parasite to possess the enzymatic tools necessary for quick and efficient response to dramatic environmental change. Proteases have been implicated to play crucial roles in parasitic cytoadherence, tissue invasion activities, survival and proliferation in host cells and virulence (Coombs, 1982; McGrath et al. 1995; Klemba and Goldberg, 2002; Sajid and McKerrow, 2002). Both metalloproteases and cysteine proteases have been implicated as putative determinants for infection and virulence factors of several species of Leishmania (Coombs, 1982; Chang and Chang, 1986; Russell and Wilhelm, 1986; Liu and Chang, 1992; Brittingham et al. 1995; Mottram et al. 1996, Mottram, Brooks and Coombs, 1998; Mahmoudzzadeh-Niknam and McKerrow, 2004). In addition, differential expression of protease profiles in virulent and avirulent promastigotes of Leishmania (L.) amazonensis has been described (de Araujo-Soares et al. 2003). In this manuscript, the protease activities of various Leishmania (V.) braziliensis strains, from Brazilian and Colombian patients presenting diverse clinical manifestations, were characterized and compared using whole-promastigote extracts and extracellular secretions.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma Chemical Company (USA). Stock solutions of 2,2-dipyridyl (20 mM), 1,10-phenantroline (200 mM) and pepstatin A (1 mg/ml) were solubilized in ethanol, whereas E-64 (6 mM), EDTA (500 mM) and EGTA (500 mM) were dissolved in water. PMSF (250 mM) was diluted in isopropanol, and TLCK (10 mM) was stocked in methanol. All these protease inhibitors were maintained at −20 °C.
L. (V.) braziliensis strains and culture
Five Leishmania (V.) braziliensis strains were used: (i) 2 were isolated from both cutaneous and mucosal lesions of 1 Colombian patient (International Center for Training and Medical Research, CIDEIM, Cali, Colombia, kindly provided by Dr Nancy Saravia) and (ii) 3 from cutaneous, disseminated, and mucosal lesions of different patients (University Hospital of Federal University of Bahia, Brazil, strains deposited in the Leishmania Type Culture Collection, Instituto Oswaldo Cruz by Dr Edgar Carvalho). Clinical presentation, geographical origin and strain identity are summarized in Table 1. The promastigote forms of the parasite were grown at 25 °C in Schneider's medium supplemented with 20% (v/v) heat-inactivated fetal bovine serum with passes every 4 days until reaching a density of 1×109 cells/ml. The cells were then harvested in late log phase of growth by centrifugation at 3000 g for 5 min and washed twice in 0·01 M phosphate-buffered 0·9% NaCl (PBS), pH 7·2. Parasite density was estimated by counts in a haemocytometer, and the viability was assessed using the Trypan blue dye exclusion test.

Zymographic assays
Protease activities present both in whole-parasites and extracellular secretions were investigated as previously described (Melo-Braga, da Rocha-Azevedo and Silva-Filho, 2003). Briefly, for proteolytic activities in whole-parasite extracts, 1×109 promastigotes were disrupted in 1% Triton X-100 containing 10 mM Tris-HCl at pH 6·8. For the investigation of the secreted protease analyses, 1×109 promastigotes were washed 3 times in cold PBS pH 7·2, resuspended in 1 ml of RPMI 1640 medium plus 25 mM HEPES and incubated for 30 min to 3 h at 25 °C. Afterwards, cell suspensions were centrifuged, and the resulting supernatants were filtered in a 0·22 μm pore membrane filter. Both whole-parasite extracts as well as filtered supernatants were fractioned on SDS-PAGE (10%) copolymerized with 0·1% fish gelatin (Lockwood et al. 1987). The gels were loaded with 30 μg of protein per slot. Following electrophoresis, the resulting gels were washed twice for 30 min at 4 °C in 0·1 M sodium acetate buffer pH 3·5, 5·5, 7·0 or 9·0 containing 2·5% Triton X-100. Protease activity was detected by incubating the gels in the reaction buffer (0·1 M sodium acetate, 1 mM DTT) at pH 3·5, 5·5, 7·0 or 9·0 at 37 °C during 4 or 24 h in the case of extracts or for 48 h in the case of supernatants. The resulting bands were visualized by staining the gels with 0·2% (w/v) Coomassie blue R-250, 50% (v/v) trichloroacetic acid and 10% (v/v) acetic acid. The approximate molecular weight for zymographic studies was calculated using a protein commercial molecular weight standard. All experiments were performed in triplicate, with similar results obtained in at least 3 separate cell suspensions.
Inhibition assays
The lysates of both promastigotes and supernantants were prepared as aforementioned. Each of the following protease inhibitors was added to the reaction buffer: 10 μM E-64; 10 mM EDTA; 10 mM EGTA; 500 μM PMSF; 10 μM TLCK; 5 μM pepstatin A and 10 mM 1,10-phenanthroline for 4 or 48 h for protease activities of extract and supernatant, respectively. Hydrolysis was detected as previously described.
RESULTS
Protease activities
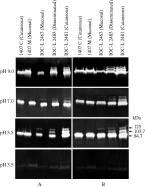
Both extracts and supernatants from promastigotes of L. (V.) braziliensis were assessed for protease activities using gelatin SDS-PAGE. The whole-cell extracts of Colombian strains, 1407 cutaneous and 1407 mucosal isolated from the same patient, presented the same protease activity profile, with a prominent band of 84·7 kDa (Fig. 1A). The Brazilian strain, IOC-L 2463 isolated from a patient with mucosal leishmaniasis, displayed a protease profile with 2 bands of 84·7 and 125 kDa. Two clones of this strain were assayed and the same proteolytic activity profile was observed (data not shown). The strains isolated from disseminated (IOC-L 2483) and cutaneous (IOC-L 2481) cases from the same locality in Brazil exhibited an extra band of 103·7 kDa (Fig. 1A). In addition, the reference strain (MHOM/BR/1973/M2903) was also analysed and an identical band profile to the disseminated (IOC-L 2483) and cutaneous (IOC-L 2481) strains was observed (data not shown). In order to verify the effect of pH on proteolytic profiles, a range of pH was evaluated (Fig. 1). Proteolytic activities of promastigote extracts were optimal at pH 5·5 for all the analysed strains. At pH 7·0 and pH 9·0 no significant increases or decreases in those enzymatic activities could be detected, and at pH 3·5, all activities were substantially reduced (Fig. 1A). All activities were verified after 4 h of proteolysis in reaction buffer. To check the possibility of protease secretion to extracellular medium, 109 living promastigotes were incubated in RPMI medium without fetal bovine serum for a period of 3 h. Subsequently, the suspension was centrifuged for cell removal, and the supernatant was submitted to zymographic assays. The profile of secreted proteases was identical to that observed for whole-cell extracts in both Brazilian and Colombian strains. Nevertheless, when the secretion products from the parasites were loaded onto gelatin gels, enzyme activities were only detected after 48 h of incubation in reaction buffer (Fig. 1B).

Fig. 1. Zymographic profiles and influence of pH on the protease activities of Leishmania (V.) braziliensis strains. Enzymatic activities detectable in each (A) whole-promastigote extracts after 4 h of reaction and (B) extracellular medium after 48 h of reaction.
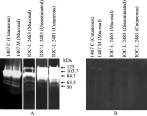
To evaluate the possible presence of other proteases expressed in low concentration or with little activity, whole promastigote extracts were incubated for 24 h in reaction buffer pH 5·5. Post-incubation, at least 2 additional weak bands between 50 and 65 kDa were observed (Fig. 2A).

Fig. 2. Zymographic analysis of proteases obtained from extracts of whole-promastigotes after 24 h of reaction at pH 5·5 and 37 °C in the absence (A) of protease inhibitor and in the presence (B) of 10 mM 1,10-phenanthroline. Two additional bands between 50 and 65 kDa can be observed in all lanes in the absence of protease inhibitor.
Time-course of secreted proteases
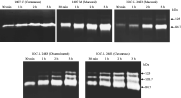
In order to check whether or not the release of proteases into the extracellular medium might possibly vary throughout the incubation times of the parasite in RPMI medium, 4 culture supernatants, each one corresponding to 109 parasites/ml, were collected after 30 min, 1 h, 2 h and 3 h of parasite incubation in serum-depleted RPMI medium. After 48 h of incubation in reaction buffer, proteolytic activities increased progressively from 30 min to 3 h of secretion (Fig. 3). After 30 min, the first band to appear in all strains was the 84·7 kDa. In the Brazilian IOC-L 2481 cutaneous strain, the 2 additional bands of 103·7 kDa and 125 kDa also appeared at this stage. These 2 bands were visualized clearly in the IOC-L 2483 disseminated strain just after 2 h of secretion (Fig. 3). In the mucosal IOC-L 2463 strain, the second band of 125 kDa was visualized only after 3 h of secretion (Fig. 3). No change in the molecular weight of the protease activities was observed during the time-course of secretion. In these assays, the viability and motility were tested before, during and after 3 h of parasite incubation in RPMI medium as previously described.

Fig. 3. Extracellular proteases secreted by Leishmania (V.) braziliensis. Supernatants of living parasites were obtained and assayed as detailed in the Materials and Methods section. Secreted proteases were detected after 30 min and 1, 2 and 3 h of reaction with fish gelatin substrate. A progressive increase of protease activities was observed for all analysed strains. Parasite viability [ges ]95% was assessed before, during and after the time-course.
Inhibition assays
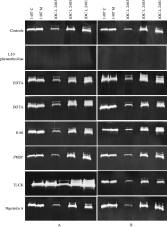
To characterize the classes of the observed proteases in L. (V.) braziliensis, several compounds known to inhibit different proteases were tested for their effects on the activity of both extract and supernatant proteases. It was found that 10 μM E-64, 10 mM EDTA, 10 mM EGTA; 500 μM PMSF; 10 μM TLCK and 5 μM pepstatin A had no inhibitory effect on the activity of promastigote extract and secreted proteases (Fig. 4). In contrast, 10 mM 1,10-phenanthroline, a strong and specific metalloprotease inhibitor, completely abrogated protease activities in all L. (V.) braziliensis strains (Fig. 4). This inhibitor also completely abrogated the 2 additional activities between 50 and 65 kDa (Fig. 2B). The fact that the parasite extract and secreted protease activities were blocked by 1,10-phenantholine indicates that these enzymes are metalloproteases or metal-dependent proteases.

Fig. 4. Protease activities detected in (A) whole-promastigotes extracts and (B) the supernatants of Leishmania (V.) braziliensis strains in the absence (controls) or presence of 10 mM 1,10-phenanthroline, 10 mM EDTA, 10 mM EGTA, 10 μM E-64, 500 μM PMSF, 10 μM TLCK, 5 μM pepstatin A, for 4 or 48 h for protease activities of extract and supernatant, respectively.
SDS-PAGE analysis
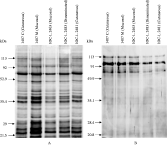
Analysis of both whole-promastigote extract and supernatant extractions was performed by SDS-PAGE and silver staining. Neither in the extract nor supernatant protein profile was any difference observed (Fig. 5).

Fig. 5. SDS-PAGE of whole-promastigote extracts (A) and supernatants (B) of Leishmania (V.) braziliensis.
DISCUSSION
Proteases of protozoan parasites play major roles in infection, survival and pathogenicity (Rosenthal, 1999; Sajid and McKerrow, 2002). These enzymes have been found in several Leishmania species (Fong and Chang, 1981; North and Coombs, 1981; Coombs, 1982; Pupkis and Coombs, 1984) and are involved in the establishment of intracellular parasitism as well as in the inhibition of host immune responses (Chang and Chang, 1986; Russell and Wilhelm, 1986; Liu and Chang, 1992; Mottram et al. 1996; Alexander, Combs and Mottram, 1998; Buxbaum et al. 2003). The cysteine, serine and metalloproteases activities of Leishmania (Leishmania) species have been well explored and described. In contrast, description of protease profiles of Leishmania (Viannia) is scarce, demanding further investigation (Leon et al. 1994).
Gp63 is the most abundant surface metalloprotease in Leishmania spp. (Etges, Bouvier and Bordier, 1986) and is expressed in both amastigote and promastigote stages. It is more abundant in the promastigotes, being released into the extracellular environment (Gardiner, Jaffe and Dwyer, 1984; Bouvier, Etges and Bordier, 1985; Colomer-Gould et al. 1985; Etges et al. 1985; Medina-Acosta et al. 1989; Medina-Acosta, Karess and Russell, 1993; McGwire et al. 2002; Jaffe and Dwyer, 2003). Gp63 is specifically involved in evasion of humoral lytic factors by rendering promastigotes resistant to complement-mediated cytolysis (Brittingham et al. 1995); attachment of promastigotes to macrophages via receptor-mediated endocytosis (Chang and Chang, 1986; Russell and Wilhelm, 1986); protection against degradation within macrophage phagolysosome (Chaudhuri et al. 1989) and migration through the extracellular matrix (McGwire, Chang and Engman, 2003).
Our results demonstrate that all analysed strains of L. (V.) braziliensis express abundant proteolytic activities between 84·7 and 125 kDa, probably corresponding to metal-dependent proteases as hydrolysis bands were abrogated by 1,10-phenanthroline – a potent inhibitor of metalloproteases. However, none of the herein enzymatic activities were affected either by EGTA or EDTA, both of which have been considered to be metalloprotease inhibitors of eukaryotic cells (Beynon and Bond, 2001), including parasitic protozoa. We, as yet, do not have a clear explanation to this fact. Nevertheless, we cannot exclude the possibility that the enzymes might be in such a configuration that during our reported assays the active sites of the enzymes may have been inaccessible to the chelators. Such an issue is under investigation.
The occurrence of metalloprotease activities in promastigotes of L. (V.) braziliensis as well as other Leishmania species (de Araujo-Soares et al. 2003), may be clearly observed among parasites collected from the late log phase of growth. It must be pointed out that in zymographic studies, the proteins are not completely denatured and reduced, and therefore, the molecular weight of proteolytic activities is estimated, thus perhaps not representing the true protease weight.
Considering that the supernatant after centrifugation and filtration displayed the same zymographic profile as the extract and that there was a progressive increase of protease activity during the secretion time-course, the presence of 2 different forms of this metalloprotease was demonstrated, an intracellular form and a soluble form which was secreted into the extracellular environment. The release of other enzymes, including metalloprotease gp63, has been reported in Leishmania promastigotes (Bates, Hermes and Dwyer, 1989; Jacobson and Schlein, 1993; Blum and Opperdoes, 1994; McGwire et al. 2002; Yao et al. 2002; Jaffe and Dwyer, 2003).
Although the tested range of pH was suitable for serine, cysteine and metalloprotease detection (de Araujo-Soares et al. 2003; Silva-Lopez et al. 2004; Alves et al. 2005), only the third was observed, suggesting either metalloprotease predominance or that the other two were at extremely low levels, being undetectable and/or not expressed in promastigotes. In addition, it is important to emphasize that this metalloprotease activity as well as the inexistence of other proteases is limited to gelatin digestion. Therefore, we cannot exclude the possibility of L. (V.) braziliensis possessing cysteine proteases of which the activities might be detectable using other substrates. In this context, some proteases, such as cysteine and metalloproteases, have been characterized as developmentally regulated both in Leishmania spp. (Medina-Acosta et al. 1989; Robertson and Coombs, 1992; Medina-Acosta, Karess and Russell, 1993; Leon et al. 1994) and in other parasites (Kembla and Goldberg, 2002; Coppi et al. 2005).
Two additional hydrolysis bands between 50 and 65 kDa were observed in whole-cell extract assays when the reaction time was increased to 24 h, and these activities were also inhibited by 1,10-phenanthroline, similar to gp63. Finally, attention must be given to the fact that we cannot exclude the possibility of the bands located between 50–65 kDa, as well as any other of the bands observed, being the result of post-translational modification of the same protein.
The time of isolation and maintenance in culture did not influence the protease profiles observed in this study. The reference strain of L. (V.) braziliensis, which has been maintained in culture for several years, exhibits the same profile as the other Brazilian strains. These strains were passed in hamsters and were all cryopreserved while still infective (3rd passage). For the analysis conducted in this study, the stocks were maintained in vitro for less than 2 months while being processed. The Colombian strains exhibiting a different protease profile were maintained in vitro for at least 5 years, and they are no longer infective, considering that they produced no lesions whatsoever in hamsters under observation for up to 9 months (CIDEIM, unpublished data).
The nutritional requirement of the parasite in the insect vector has been suggested as the primary function for gp63 in Leishmania as well as other trypanosomatids (Etges, 1992; Schlein, 1993; Schneider and Glaser, 1993; Jaffe and Dwyer, 2003). It has recently been demonstrated that down-regulation of the metalloprotease gp63 affects the early development of L. (L.) amazonensis in experimental infections of Lutzomyia longipalpis (Hajmová et al. 2004). Considering these results, it is possible to hypothesize that differences observed among the metalloprotease profiles of the Colombian and Brazilian strains analysed herein might indicate geographical specialization of such enzymes to distinct vectors involved in the transmission of the parasite in these countries. The Brazilian and Colombian strains belonging to distinct zymodemes (IOC/Z27 and IOC/Z108 respectively) differ in 3 of the 18 loci employed in the MLEE characterization (unpublished results). These zymodemes probably correspond to clones adapted to different environmental conditions (Cupolillo et al. 2003). In Brazil, L. (V.) braziliensis is transmitted by L. intermedia and L. whitmani, whereas in Colombia L. spinacrasa is the incriminated vector for this Leishmania species (Young et al. 1987). Finally, we hypothesize that the invasive and immunomodulatory roles attributed to metalloproteases and other proteases in several protozoan infections (Bontempi and Cazzulo, 1990; Brittingham et al. 1999; Que and Reed, 2000; Buxbaum et al. 2003; McGwire et al. 2003) may be reflected in the profiles encountered among the Brazilian mucosal, cutaneous and disseminated strains isolated from different patients in the same geographical region and from the same zymodeme.
This work was partially supported by grants from the following Brazilian agencies: CNPq and FAPERJ (E.C.), and FIOCRUZ – FAPERJ (J.B.J.). P.C. is a Ph.D. student of the Cellular and Molecular Biology Program at Instituto Oswaldo Cruz and is a CNPq (PEC/PG Program, Brazil) Fellowship recipient. The experiments performed in this work comply with the current laws of Brazil.