Variations in thyroid hormone (TH) bioactivity allow animals to adapt their metabolic balance to different environmental conditions, changes in nutrient requirements and allowances, and to homeorhetic changes during different physiological stages (Todini, Reference Todini2007). TH synthesis depends on iodine availability and the thyroid gland mostly secretes 3-3′-5-5′-tetraiodothyronine or thyroxine (T4), which is monodeiodinated by selenium-containing enzymes to 3-3′-5-triiodothyronine (T3), the active form stimulating oxygen utilization and heat production in every cell of the body, mostly by the binding to nuclear receptor containing zinc (Utiger, Reference Utiger, Felig, Baxter and Frohman1995; Freake et al. Reference Freake, Govoni, Gud, Huang and Zinn2001; Köhrle et al. Reference Köhrle, Jakob, Contempre and Dumont2005). TH are known to stimulate lactation (Tucker, Reference Tucker2000) and play a permissive role for galactopoietic hormones, such as prolactin and somatotropin (Kahl et al. Reference Kahl, Capuco, Binelli, Vanderkooi, Tucker and Moseley1995). Different ratios of blood/milk TH in various mammals suggest species differences in permeability of the blood-mammary barrier and/or in the conversion of T4 to T3 in mammary gland cells (Akasha & Anderson, Reference Akasha and Anderson1984). Several studies have dealt with TH in milk of various mammalian species (Slebodzinski et al. Reference Slebodzinski, Brzezinska-Slebodzinska, Styczynska and Szejnoga1999) and in mares’ mammary gland there are two types of 5′-monodeiodinase enzyme (Slebodzinski et al. Reference Slebodzinski, Brzezinska-Slebodzinska, Nowak and Kowalska1998), allowing a significant intra-mammary T3 generation. Active T3 in colostrum and milk could play different physiological roles, such as a source of systemic T3 and a paracrine action supporting lactogenesis in the mother; on the other hand, physiological effects on the suckling offspring may be systemic and/or within the gastrointestinal tract, including intraluminal digestion and maturation of enzyme systems (Sheard & Walker, Reference Sheard and Walker1988), macromolecule absorption by the intestine (Slebodzinski et al. Reference Slebodzinski, Brzezinska-Slebodzinska, Nowak and Kowalska1998), responsiveness to gastrin and gastric secretion (Murray & Luba, Reference Murray and Luba1993).
The traditional utilization of donkeys (Equus asinus) as dairy animals has recently attracted substantial scientific interest with regard to human nutrition. Donkey milk composition is similar to human milk, except for the low and variable average total solid and fat content (Salimei, Reference Salimei, Fuquay, Fox and McSweeney2011). Clinical evidence shows that donkey milk is well tolerated by infants with cows’ milk allergy (Monti et al. Reference Monti, Bertino, Muratore, Coscia, Cresi, Silvestro, Fabris, Fortunato, Giuffrida and Conti2007; Vita et al. Reference Vita, Passalacqua, Di Pasquale, Caminiti, Crisafulli, Rulli and Pajno2007) and its use is reported to be useful in the treatment of human immune-related diseases and in the prevention of atherosclerosis (Tafaro et al. Reference Tafaro, Magrone, Jirillo, Martemucci, D'alessandro, Amati and Jirillo2007). Moreover, an anti-proliferative and anti-tumour effect of active components of asses’ milk has been observed in vitro (Mao et al. Reference Mao, Gu, Xu, Zhang, Yang and Ren2009).
Among bioactive molecules, leptin, ghrelin and IGF-1 were quantified in donkey milk (Salimei et al. Reference Salimei, Rosi, Maglieri, Polidori, Fantuz and Varisco2005, Reference Salimei, Rosi, Maglieri, Magistrelli and Fantuz2007; Magistrelli et al. Reference Magistrelli, Rosi, Amicone, Maglieri and Salimei2008). The presence of T3 (near 4·0 ng/ml) in donkey milk was observed by Todini et al. (Reference Todini, Malfatti, Salimei and Fantuz2010) but there is a lack of knowledge about potential factors (environmental, physiological, dietary) able to affect TH concentrations in milk and blood of donkeys. Several trace elements are directly involved with TH synthesis (I), metabolism (Se) and action (Zn), as well as thyroid gland function, protection and health (Lauwerys & Lison, Reference Lauwerys and Lison1994; Uriu-Adams & Keen, Reference Uriu-Adams and Keen2005; Gärtner, Reference Gärtner2007; Soldin & Aschner, Reference Soldin and Aschner2007; Kandhro et al. Reference Kandhro, Kazi, Afridi, Kazi, Baig, Arain, Sirajuddin Shah, Sarfraz, Jamali and Syed2009; Giray et al. Reference Giray, Arnaud, Sayek, Favier and Hıncal2010). Therefore the aim of the present on-field study was to evaluate whether TH concentrations in milk and blood of lactating donkey (i) change with the advancing lactation and (ii) can be affected by dietary trace element supplements.
Material and Methods
Animals and diet
During a 3-month period 16 clinically healthy lactating donkeys (3–7 parities) were used to provide milk and blood samples. At the first sampling time, jennies were 32–58 d from foaling, averaging 205·4 kg of live weight and 3·43 Body Condition Score (on a 1–5 scale; Martin Rosset, Reference Martin Rosset1990). Animals were randomly divided into two homogeneous groups: control (CTL) and trace element-supplemented (TE). All the jennies were fed 8 kg of meadow hay and 2·5 kg of mixed feed divided into two meals daily. Hay, common to both groups, contained (per kg) 22·1 mg Zn, 6·9 mg Cu, 113·2 mg Fe, 17·3 mg Mn, 0·13 mg I, 0·02 mg Se and 0·14 mg Co. Mixed feed fed to control group was not supplemented with any trace element and contained (per kg) 58·1 mg Zn, 8·6 mg Cu, 538·3 mg Fe, 91·9 mg Mn, 0·82 mg I, 0·1 mg Se and 0·25 mg Co. Mixed feed for TE group had the same ingredients as CTL but was supplemented with a commercial trace element premix based on dietary recommendations for lactating mares (Martin Rosset, Reference Martin Rosset1990). The trace element supplementation provided 163 mg Zn (zinc oxide), 36 mg Cu (copper sulphate), 185 mg Fe (ferrous carbonate), 216 mg Mn (manganese oxide), 3·2 mg I (calcium iodate), 0·67 mg Se (sodium selenite) and 2·78 mg Co (cobalt sulphate) per kg mixed feed. The mixed feed was gradually administered starting at the first sampling time, immediately following milking. Jennies were housed with the foals that were separated from the dam 3 h before mechanical milking.
Samplings and hormone assays
Individual milk and blood samples were collected every 2 weeks (7 sampling times) at 11·00 during the mechanical milking session (Salimei, Reference Salimei, Fuquay, Fox and McSweeney2011). Aliquots of whole milk samples were utilized for iodothyronines extraction with alkaline ethanol at low temperature, as described by Slebodzinski et al. (Reference Slebodzinski, Brzezinska-Slebodzinska, Nowak and Kowalska1998) and by Todini et al. (Reference Todini, Malfatti, Salimei and Fantuz2010). Briefly, 2 ml of cold (−20°C) alkaline ethanol (pH 9·0 with NH4OH) were added to 1 ml of whole donkey milk, mixed thoroughly with a glass rod, vortexed and left in the freezer (−20°C) for 24 h. Then the milk-ethanol mixture was vortexed, left in the freezer for a further 24 h and then centrifuged at 3500 g at 0°C for 30 min. Supernatant was collected and stored at −20°C. Twenty-four hours before the assay, these supernatant samples were diluted 1:1 with the assay buffer. Blood samples were collected by jugular venipuncture in evacuated tubes (Venoject, Terumo Europe NV, Leuven, Belgium) containing K3-EDTA as anticoagulant. Tubes were immediately centrifuged (2500 g for 15 min) and the plasma aliquots stored at −20°C until assayed. Total concentrations of T3 in extracted milk samples (T3M) and T3 and T4 in blood plasma (T3P and T4P) were assayed using commercially available enzyme immunoassay (EIA) kits for clinical use in humans (T3KT1EW and T4KT2EW; Radim, Rome, Italy), which are competitive-type ELISA. Assays were performed by the automated processor Brio 2 reader (Seac, Firenze, Italy). The assay kits were expressly validated for donkey species, as previously described (Todini et al. Reference Todini, Malfatti, Salimei and Fantuz2010). Intra- and inter-assay coefficients of variations were respectively: 5·2 and 7·9% for T3M, 3·9 and 6·3% for T3P, 4·0 and 3·1% for T4P. All samples of each animal were analysed for each hormone within the same assay session, and each microplate was used for 2 animals for each of both experimental groups. Hormone concentrations were determined as the mean of duplicate determinations.
Statistical analysis
Statistical analyses were performed by SPSS 12.0 program (SPSS, 2003). All the investigated variables were checked for normal distribution using the Kolmogoroff–Smirnoff test. In order to meet ANOVA assumptions (i.e. normal distribution and homogeneity of variance) data were logarithmic (T4P, T3P, T3M) or square root (T3P/T4P, T3M/T3P, T3M/T4P) transformed for statistical elaboration. Results presented in tables and figures are least square means±se from original data. Data were processed by analysis of variance for repeated measures in order to evaluate the effects of stage of lactation (time of sampling) (within subject factor) and dietary treatment (between subject factor) and their interaction. Data from the first sampling were used as covariate when significant. Differences between means were analysed by least significant difference. Associations between variables were examined by calculating simple linear correlation.
Results
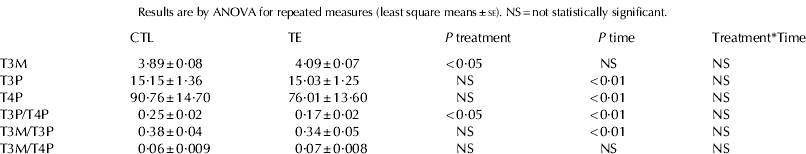
T3M, T3P and T4P values and their relative ratios are reported in Table 1. T3P and T4P concentrations were not affected by dietary treatment but T3M was significantly higher in milk from supplemented jennies. No stage of lactation×dietary treatment interaction was significant for any of the measured hormones.
Table 1. T3 concentration in milk (T3M, ng/ml) and blood plasma (T3P, ng/ml), T4 concentration in blood plasma (T4P, ng/ml) in lactating donkeys fed the control diet (CTL, n=8) and supplemented with trace elements (TE, n=8).
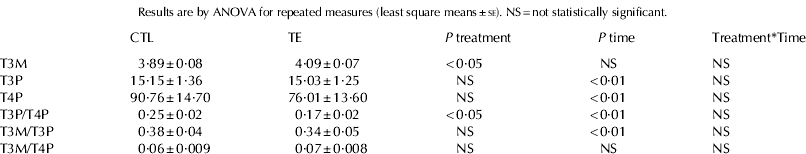
T3M (Table 1, Fig. 1) was not affected by stage of lactation and did not vary markedly among individuals (range of individual means±se: 2·9±0·08 to 5·99±0·21 ng/ml). T3M was not correlated with TH blood plasma concentrations.

Fig. 1. 3-3′-5-triiodothyronine (T3) concentration (ng/ml) in milk of donkeys fed the control diet (CTL, n=8) or supplemented with trace elements (TE, n=8).
Both T3P and T4P varied significantly during lactation (Table 1, Figs. 2 and 3). A widespread variability between individuals was observed (range of individual means±se: 4·5±0·4 to 37·1±4·7 ng/ml and 38·9±0·35 to 167·8±28·6 ng/ml, for T3P and T4P respectively). T3P and T4P were strongly correlated (r=0·65, P<0·01).

Fig. 2. 3-3′-5-triiodothyronine (T3) concentration (ng/ml) in blood plasma of donkeys fed the control diet (CTL, n=8) or supplemented with trace elements (TE, n=8).

Fig. 3. 3-3′-5-5′-tetraiodothyronine or thyroxine (T4) concentration (ng/ml) in blood plasma of donkeys fed the control diet (CTL, n=8) or supplemented with trace elements (TE, n=8).
T3M/T3P ratio (Table 1) significantly changed during lactation (P<0·01) but it was not affected by dietary treatment. T3M/T4P ratio (Table 1) did not vary during the study and between groups. T3P/T4P ratio (Table 1, Fig. 4) was significantly affected by stage of lactation and significantly lower in TE than in CTL group.

Fig. 4. T3/T4 concentrations ratio in blood plasma of donkeys fed the control diet (CTL, n=8) or supplemented with trace elements (TE, n=8).
Discussion
Results of the current study show ratio values of milk/blood T3 concentrations in agreement with that reported by Slebodzinski et al. (Reference Slebodzinski, Brzezinska-Slebodzinska, Styczynska and Szejnoga1999) in other species (milk T3 is approximately 1/3 of serum T3), and the lack of significant relationship between T3 in serum and milk confirms that reported earlier by those authors.
The presence of T3 in milk is originated both from serum transference, and from T4 deiodination within the mammary gland (Slebodzinski et al. Reference Slebodzinski, Nowak, Gawecka and Sechman1986), where deiodinase activity increases during early lactation as a function of increase in milk yield (Jack et al. Reference Jack, Kahl, St Germain and Capuco1994). Concentrations of T3 in milk found in the present study were rather stable among individuals, whilst TH blood levels were highly variable. Previously, a wide individual variation in blood T4 concentrations was reported in racehorses (Bayly et al. Reference Bayly, Andrea, Smith, Stenslie and Bergsma1996). Furthermore, it is noteworthy that T3 concentration in milk did not significantly change with the stage of lactation, contrary to the hormone concentrations in blood. In ruminants, blood TH levels are generally low at the beginning of lactation, afterwards gradually rising in does (Emre & Garmo, Reference Emre and Garmo1985; Riis & Madsen, Reference Riis and Madsen1985) and in ewes (Mitin et al. Reference Mitin, Mikulec and Karadjole1986). During late lactation, the increase of T4 concentration in blood seems to be related to the decrease of milk production (Bass, Reference Bass1989).
Trace element dietary supplementation of dairy jennies significantly increased the T3 content of milk while it did not affect TH plasma values. At present, we cannot match these statistical significances with any physiological significance, nor it is possible to explain whether this is due to an increased blood-milk transfer of T3, and/or to an increased deiodinase activity at the mammary gland level. To our knowledge, information on trace element allowances and hormone levels in dairy donkeys are lacking. Besides humans, studies have been carried out on other animal species, mostly ruminants and rodents, therefore any reference should be taken very cautiously for comparison. Many papers in the literature deal with trace element deficient/supplemented animals and pathological concerns, but all the experimental animals in the present study were healthy. Furthermore, we can only speculate about the combined effect of these trace elements together, rather than as a potential effect of any one of them. Opposite results (a decrease in the T4/T3 ratio in blood) were recently reported in buffalo calves supplemented with Se, Zn and Cu combined (Mudgal et al. Reference Mudgal, Garg, Dass and Varshney2011), probably as a consequence of an enhanced peripheral conversion of T4 to T3. It is well known that blood TH are low in iodine-deficiency, and in rats this was demonstrated to occur also regardless of Se or Zn status (Ruz et al. Reference Ruz, Codoceo, Galgani, Munoz, Gras, Muzzo, Leiva and Bosco1999). The iodine concentration of human milk varies widely with maternal iodine intake (Dorea, Reference Dorea2002) and iodine supplementation was associated with increases of T4 concentrations in blood, without any effect or decreases on blood T3 in dairy cows (Franke et al. Reference Franke, Meyer, Wagner, Hoppen and Flachowsky2009), calves (Wichtel et al. Reference Wichtel, Craigie, Freeman, Varela-Alvarez and Williamson1996), buffaloes (Mudgal et al. Reference Mudgal, Garg, Dass and Varshney2011), Cashmere goats (Qin et al. Reference Qin, Zhu, Zhang, Zhou, Zhang and Jia2011), sheep (Bik, Reference Bik2003) and mouse (Yang et al. Reference Yang, Xu, Hou, Guo, Hao, Yao, Liu and Sun2006). In other words, milk iodine and blood TH seem directly dependent on the intake, whilst the active T3 in blood is more homeostatically controlled. The effects of Se supplementation on TH in blood may vary largely depending on the Se levels in the basal diet: with low basal Se intake, supplementation increases T3 and decreases T4 (Arthur et al. Reference Arthur, Morrice and Beckett1988; Wichtel et al. Reference Wichtel, Craigie, Freeman, Varela-Alvarez and Williamson1996; Awadeh et al. Reference Awadeh, Kincaid and Johnson1998; Abd El-Ghany & Tórtora-Pérez, Reference Abd El-Ghany and Tórtora-Pérez2010); otherwise no clear effect is observed (Chadio et al. Reference Chadio, Kotsampasi, Menegatos, Zervas and Kalogiannis2006; Mudgal et al. Reference Mudgal, Garg, Dass and Varshney2008; Qin et al. Reference Qin, Zhu, Zhang, Zhou, Zhang and Jia2011). Zinc is known to play a role also in thyroid hormone metabolism, as a co-factor of deiodinase enzymes, and may in part contribute to conversion of T4 to T3 in humans with low T3 syndrome (Nishiyama et al. Reference Nishiyama, Futagoishi-Suginohara, Matsukura, Nakamura, Higashi, Shinohara and Matsuda1994). In fact, Zn supplementation is effective in increasing circulating T3 in hypothyroidism (Kralik et al. Reference Kralik, Eder and Kirchgessner1996; Kandhro et al. Reference Kandhro, Kazi, Afridi, Kazi, Baig, Arain, Sirajuddin Shah, Sarfraz, Jamali and Syed2009) but at a lower dose (35 mg Zn/kg diet) than that used in the present study, did not affect blood TH levels in healthy calves (Mandal et al. Reference Mandal, Dass, Garg, Varshney and Mondal2008). In healthy animals, decreasing effects of Zn on serum T3 and T3/T4 ratio (as the results of the present study) are also reported, most likely due to increasing capillary permeability with the probable leakage of some serum proteins including the thyroid-binding proteins (Moustafa, Reference Moustafa2001). On this view, it could be hypothesized that also the passage of T3 from blood to milk can be facilitated by Zn.
The presence of bioactive molecules, similar to those found in humans, in milk supports the endogenous health-promoting properties of donkey milk and derivatives but it requires new strategies in the management of animals and milk in order to preserve the natural attributes of this product.
In conclusion, the content of T3 in donkey milk is not negligible, appears less variable among individuals than the hormone concentrations in blood, and is not significantly affected by the stage of lactation. Moreover, T3 content in donkey milk can be increased by nutritional strategies. Advancing knowledge about bioactive compounds in donkey milk therefore appears particularly opportune. Further interdisciplinary studies should clarify how intensive husbandry factors in an innovative farming enterprise might interact with the adaptive metabolic capacities of this docile species, its milk production and nutraceutical components. Dairy donkey breeding may have great potential as a tool for the sustainable development of marginalized areas.
This paper was partly funded by Università di Camerino (FAR L Todini, A Malfatti, F Fantuz). The authors gratefully acknowledge the valuable collaboration of Mr D Borghi, Mr G Borghi and Mrs T Faietti (Azienda Agricola Montebaducco, Salvarano di Quattro Castella, Reggio Emilia, Italy). The authors are also grateful to Mrs V Brunetti, Mr R Piloni, Dr P Mariani, Mr G Lebboroni (Università di Camerino) and Mr A El Jeddad (Az. Agr. Montebaducco) for technical assistance.