1. Introduction
Raoellidae form a small group of ‘bunolophodont’ cetartiodactyls. They have so far been unambiguously reported only from Middle Eocene deposits of the Indian Subcontinent, including localities from India (Kalakot region: Kashmir, Subathu Formation; Sahni et al. Reference Sahni, Bhatia, Hartenberger, Jaeger, Kumar, Sudre and Vianey-Liaud1981; Kumar & Sahni, Reference Kumar and Sahni1985; Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007) and Pakistan (Ganda Kas area: Kuldana Formation; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001; Gali Jhagir area: Chorgali Formation; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001; Chorlakki: Kuldana Formation, ‘Mami Khel’ Formation; Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987). Additional to these undisputed reports, some alleged raoellids have also been reported from outside the Indian Subcontinent, in the Middle Eocene of Mongolia (Haqueina haichinensis, Khaichin Ula locality; Vislobokova, Reference Vislobokova2004a, Reference Vislobokovab), Myanmar (‘raoellid indet.’, Pondaung Formation; Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007) and China (?Indohyus yuanchuensis, Rencun locality; Coombs & Coombs, Reference Coombs and Coombs1977).
The Raoellidae initially encompassed three genera endemic to the Indian Subcontinent according to Sahni et al. (Reference Sahni, Bhatia, Hartenberger, Jaeger, Kumar, Sudre and Vianey-Liaud1981), Raoella, Kunmunella and Khirtharia. Subsequent authors have significantly changed the content of the family and included taxa from outside the Indian Subcontinent. For example, Thewissen, Gingerich & Russell (Reference Thewissen, Gingerich and Russell1987) referred to the Raoellidae Indohyus (including Raoella and Kunmunella), Khirtharia, Bunodentus, Metkatius, all known from the Indian Subcontinent only, and possibly Haqueina, a genus initially reported from Middle Eocene deposits of Pakistan (Dehm & Oettingen-Spielberg, Reference Dehm and Oettingen-Spielberg1958) and then described in Mongolia (Vislobokova, Reference Vislobokova2004a, Reference Vislobokovab). Later on, Thewissen, Williams & Hussain (Reference Thewissen, Williams and Hussain2001) identified Khirtharia (including Bunodentus as proposed by West, Reference West1980), Metkatius, Haqueina, Indohyus and Kunmunella as raoellids. More recently, Theodor, Erfurt & Métais (Reference Theodor, Erfurt, Métais, Prothero and Foss2007) included the genera Khirtharia, Indohyus, Kunmunella and Metkatius in the Raoellidae, Haqueina being considered as a representative of the Dichobunidae (Table 1). The apparent palaeogeographical extension of the Raoellidae directly depends of the unstable systematic content of the family. This instability is mainly owing to the lack of clear definition of the group. More recently, since they were proposed as sister taxon to Cetacea (Geisler & Uhen, Reference Geisler and Uhen2003, Reference Geisler and Uhen2005; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001), raoellids have become central to questions of cetartiodactyl evolution. However, despite their key position within the Cetartiodactyla, recent large-scale phylogenetic analyses included few raoellid taxa: none in O'Leary & Gatesy (Reference O'Leary and Gatesy2008); two in Thewissen et al. (Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007, Khirtharia + Indohyus); only one in Theodor & Foss (Reference Theodor and Foss2005, referred to as ‘raoellid’); only one in Geisler et al. (Reference Geisler, Theodor, Uhen, Foss, Prothero and Foss2007, referred to as ‘Raoellidae’); only one in Spaulding, O'Leary & Gatesy (Reference Spaulding, O'Leary and Gatesy2009, Indohyus), and none of these authors discussed the characters supporting the monophyly of the group.
Table 1. Generic systematics of the Raoellidae according to different authors

Non-Indo-Pakistani taxa are indicated by a star.
In this study we address the question of raoellid taxonomic content and definition, through a revision of the dental features of the family, substantiated by a phylogenetic analysis of dental characters. This work is primarily based on a re-examination of some artiodactyl specimens from Shanghuang (Middle Eocene, China) IVPP V12763.1 and IVPP V12764.2, an m3 and a p4, respectively, initially identified as the oldest suoid remains by Métais et al. (Reference Métais, Qi, Guo and Beard2008) and here referred to the Raoellidae. The systematic attribution of putative non-endemic raoellid specimens from Mongolia (Vislobokova, Reference Vislobokova2004a, Reference Vislobokovab), Myanmar (Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007) and China (Coombs & Coombs, Reference Coombs and Coombs1977) is also tested. Dental differences between raoellids and other cetartiodactyls, including dichobunids, suoids, anthracotheriids and basal cetaceans are here discussed and sent back to early cetartiodactylan history.
2. Abbreviations
GSI – Geological Survey of India, Kolkata, India; IVPP – Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China; NMMP−KU – National Museum−Myanmar−Paleontology−Kyoto University (stored in the National Museum, Yangon, Myanmar); UM2 – Université de Montpellier 2, Montpellier, France. M/m – upper/lower molars; P/p – upper/lower premolars.
3. Systematic palaeontology
Order CETARTIODACTYLA Montgelard, Catzeflis & Douzery, Reference Montgelard, Catzeflis and Douzery1997
Family raoellidae Sahni et al. Reference Sahni, Bhatia, Hartenberger, Jaeger, Kumar, Sudre and Vianey-Liaud1981
Genus ?Khirtharia Pilgrim, Reference Pilgrim1940
?Khirtharia cf. major (Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987) comb. nov.
Figure 1
2008 Suoidea indeterminate (1) Métais et al., fig. 2e
2008 Suoidea indeterminate (2) Métais et al., fig. 2f, g

Figure 1. ?Khirtharia cf. major from Shanghuang (IVPP V12763.1) in (a) occlusal; (b) lingual; (c) labial view. Scale bar = 1 cm.
Differential diagnosis. Differs from other raoellid species by a weaker and more mesially located ‘hypolophid’; differs from other Khirtharia species by more elongated lower m3 with a wider and more massive hypoconulid lobe, a more expressed groove pattern on the mesial lobe (endofossids and postfossids), more pronounced preprotocristid, postprotocristid and metacristids, and an entoconid lacking a postentocristid; differs from Kunmunella and from Indohyus by lower mesial cuspids deeply separated by a wide longitudinal groove, the presence of a lower prehypocristid (cristid obliqua) and no accessory central cristid.
Stratigraphical and geographical provenance. Middle Eocene Shanghuang fissure filling B (Early Sharamurunian; Métais et al. Reference Métais, Qi, Guo and Beard2008), Jiangsu Province, coastal China.
Description. This material has initially been described by Métais et al. (Reference Métais, Qi, Guo and Beard2008). However, some additional features can be added to that primary description. We follow here the nomenclature defined by Boisserie et al. (Reference Boisserie, Lihoreau, Orliac, Fisher, Weston and Ducrocq2009) based on dental homologies within ‘Suiformes’, because it best allows comparison between bunodont to sublophodont forms.
The mesial-most part of the isolated m3 IVPP V12763.1 is broken and the bases of the mesial cuspids, including the labial half of the protoconid, is lacking. The full height of the crown can only be appreciated on the labial wall of the hypoconid (Fig. 1c). The crown of the tooth is low and it bears four blunt cuspids, the two mesial ones being clearly stronger than the distal ones. The thickness of the enamel is visible on the broken parts of the mesial cuspids (Fig. 1b, c); it is 1.3 mm thick at the protoconid tip. Wear is almost absent in this specimen, which leaves the most ephemeral features of the crown visible (Fig. 1a). The preserved part of the protoconid bears small and blunt endo- and postprotocristids. The metaconid bears sharp pre- and postectometacristids and a short postmetacristid. A very light endometacristid might also be present. The premetacristid is labially lined by a clear groove and the postmetacristid is well delimited by two deep grooves (Fig. 2b). The postectometacristid connects distally to the mesial end of the ectoentocristid to form a continuous mesio-distal structure that constitutes the lingual wall of the tooth. The transverse valley separating the mesial and distal cusps is widely open; it is almost straight and it joins the buccal and lingual walls of the tooth. The centre of the tooth is occupied by a flat and low surface with slightly wrinkled enamel. There is no trace of an accessory cusplet that could be recognized as a mesoconulid. The hypoconid is very wide and it bears a sharp prehypocristid (cristid obliqua) that might be connected to the very base of the postectoprotocristid (not observable on this broken part of the specimen). This structure forms the buccal wall of the tooth. A very low endohypocristid is identifiable; its lingual-most extremity is thin and sharp (Figs 1a, 2b). This small crest most probably would be obliterated in early wear. The endohypocristid is distally separated from the posthypocristid by a deep groove. The posthypocristid is robust and enlarged distally. The latter, lingually shifted at its distal end and labially lined by a posthypofossid, runs towards the prehypocristulid. Both crests are clearly separated by a deep groove. The entoconid is labio-lingually slender and separated from the hypoconid by a deep groove. It only displays a smooth crest oriented towards the internal part of the tooth, which could be interpreted as the preentocristid or as the endoentocristid. We name this crest endoentocristid because of its connection to the endohypocristid. The lingual wall is delimited by a sharp ectoentocristid. The hypoconulid is strong and as high as other distal cuspids. It is divided by an oblique groove, almost separating it into two equal parts.

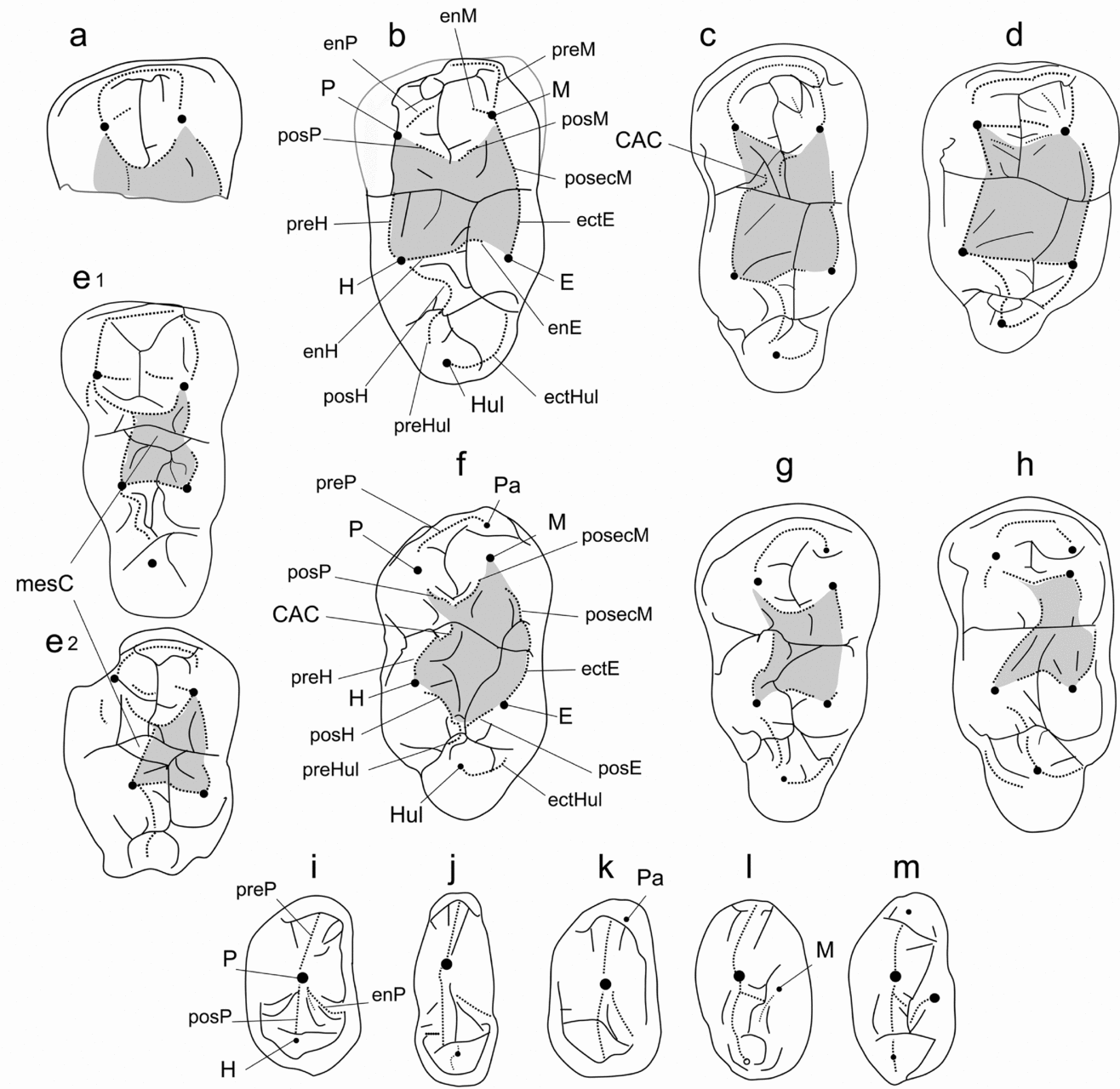
Figure 2. Comparison of lower cheek tooth structure of some Eocene cetartiodactyls. m2–3: (a) Indohyus major; (b) ?Khirtharia cf. major; (c) Kunmunella transversa; (d) Khirtharia dayi; (e1) Siamochoerus banmarkensis; (e2) Siamochoerus viriosus; (f) Gujaratia pakistanensis; (g) Dichobune leporina; (h) Gobiohyus orientalis. p4: (i) Khirtharia dayi; (j) Indohyus indirae; (k) IVPP V12764.2; (l) Siamochoerus; (m) Palaeochoerus quercyi. Abbreviations: CAC – central accessory cristid; E – entoconid; ectHul – ectohypocristulid; ectE – ectoentocristid; enE – endoentocristid; enH – endohypocristid; enP – endoprotocristid; enM – endometacristid; H – hypoconid; Hul – hypoconulid; M – metaconid; mesC – mesoconulid; P – protoconid; Pa – paraconid; posH – posthypocristid; preHul – prehypocristulid; posecM – postectometacristid; posM – postmetacristid; posP – postprotocristid; posE – postentocristid; preH – prehypocristid; preM – premetacristid; preP – preprotocristid. Grey areas indicate the surfaces corresponding to the crushing basin.
4. Systematic reattribution of the ‘suoid’ material from Shanghuang
4.a. Rejection of IVPP V12763.1 as a suoid
The specimen IVPP V12763.1 was initially considered a suoid by Métais et al. (Reference Métais, Qi, Guo and Beard2008) based on the overall bunodonty, the cusped hypoconulid and the system of grooves and bulbous crests interpreted as an incipient ‘furchenplan’ (Métais et al. Reference Métais, Qi, Guo and Beard2008, p. 1127). According to the same authors, IVPP V12763.1 compares most closely with the earliest suoids known from the Late Eocene of Asia, especially Siamochoerus, in the general arrangement of its cuspids and crests, the trigonid transversely wider than the talonid and by the duplicated hypoconulid on the m3. Another important character supporting the suoid affinities of IVPP V12763.1 would be the swelling of enamel linking the hypoconid and the entoconid, which might be interpreted as incipient massive crests.
The systematic attribution of Eocene artiodactyls is often problematic because of the uncertain phylogenetic relationships within the Cetartiodactyla, generating obvious problems of character polarization. The Diacodexeinae are represented in the Holarctic Province and might represent a paraphyletic assemblage. Although they share the same basic structure, regarded as the primitive condition among the Cetartiodactyla, Early Eocene Diacodexeinae already present a complicated molar pattern, with several accessory structures (Fig. 2f). A rudimentary ‘furchenplan’ is present, with endo- and postfossids on mesial cuspids. The crest pattern is also well developed with postcristids and postectocristids on mesial cuspids. The distal cuspids bear two cristids that could be interpreted as the prehypocristid (cristid obliqua) and posthypocristid on the hypoconid and ectoentocristid and postentocristid on the entoconid, respectively (Fig. 2f), according to the dental nomenclature proposed by Boisserie et al. (Reference Boisserie, Lihoreau, Orliac, Fisher, Weston and Ducrocq2009). On the m3, the posthypocristid joins the hypoconulid via a small crest located on the mesio-labial corner of the conule, here recognized as the prehypocristulid. This cristid pattern appears as symplesiomorphic among cetartiodactyls (Fig. 2f).
Bunodont cusps and a trigonid wider than the talonid are observed in various ungulate groups and, as such, cannot be advocated as diagnostic characters for suoids. The same remark can be expressed for the double hypoconulid, which corresponds to the presence of a lingual groove cutting the hypoconulid lobe, observed in several other cetartiodactyl groups such as the Raoellidae, Diacodexeidae and Dichobunidae. Figure 2 exemplifies the latter lower molar pattern. The swelling of enamel linking the hypoconid to the entoconid corresponds to the ‘hypolophid’ described in basal artiodactyls, and is also present in most ‘non-diacodexeine’ Eocene families (Fig. 2b–d, g–h). These characters common to suoids and specimen IVPP V12763.1 are also shared by other cetartiodactyls and might be symplesiomorphies (for discussion of these characters see the phylogenetic analysis in Section 5).
Suoids differ from the primitive artiodactyl lower molar pattern by: (i) the absence of a paraconulid; (ii) the presence of a precristid on the mesial cuspids; (iii) the presence of internal cristids on the distal cuspids (hypolophid), recognized as the endohypocristid and endo- or preentocristid; (iv) a larger entoconid; and (v) a more reduced central part of the molars, occupied by a central accessory cusp (Fig. 2e v. 2f; Orliac, Antoine & Ducrocq, Reference Orliac, Antoine and Ducrocq2010; Orliac et al. in press). The specimen IVPP V12763.1 from Shanghuang only shares with suoids the absence of paraconulid, internal cristids on the distal cuspids and rudimentary endocristids on the mesial cuspids. The proportion, height and disposition of the cuspids greatly differ from those of suoids (Fig. 2b v. 2e). The crest and groove pattern of IVPP V12763.1 is not consistent with that of suoids, including Eocene forms like Siamochoerus (Fig. 2b v. 2e). Compared to suoids, the groove pattern of IVPP V12763.1 is reduced (no groove on the entoconid) and shallower; there is neither trace of a central accessory cristid (termed CAC in Fig. 2) nor a mesoconulid. The longitudinal groove separates the mesial cuspids, interrupting the postcristids, whereas in suoids the groove network is interrupted by postcristids of the mesial lobe and by the central accessory cusps. The lingual wall of IVPP V12763.1 is bounded at the level of the occlusal surface by the postectometacristid and ectoentocristid, connected to each other. This morphology is never observed in suoids. In suoids, these two lingual cristids are separated by the median groove and the lingual flank of the cuspids does not form the lingual wall of the tooth (Fig. 2b v. 2e). All these differences support exclusion of IVPP V12763.1 from the Suoidea.
4.b. Referral of IVPP V12763.1 from Shanghuang to the Raoellidae
Métais et al. (Reference Métais, Qi, Guo and Beard2008, p. 1127) noticed the morphological similarity between IVPP V12763.1 and Khirtharia but rejected the attribution of the former to the Raoellidae because a longitudinally wider trigonid, more reduced hypoconulid lobe on m3, more developed transverse crests and absence of accessory grooves on lower molars characterize raoellids. However, a narrow trigonid can be observed in raoellids such as Kunmunella and Indohyus, which also exhibit a large hypoconulid lobe on m3 (Fig. 2c). The groove pattern of the lower molars of raoellids is shallow but can be seen in unworn specimens of Khirtharia and Kunmunella (cf. Fig. 2c–d). The general structure of IVPP V12763.1 is similar to that of some raoellid species in terms of crests and grooves (Fig. 2b v. 2c–d). The most distinctive feature of IVPP V12763.1 is the wide and flat area occupying the centre of the tooth (coloured in grey in Fig. 2). This morphology is observable in raoellids and recognized as the ‘crushing basin’ (Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007). The crushing basin is a concave surface receiving a cusp of the opposing cheek tooth during occlusion. In raoellids, this basin is very wide and the four cuspids of the lower molars are far apart. The basin is delimited by labial, lingual, mesial and distal ‘lophids’ composed of the postcristids of the mesial cuspids, the postectoprotocristid and the prehypocristid on the labial side, the two endofossids of the distal cuspids and the ectoentocristid and postectometacristids on the lingual side. The internal part of the hypoconid is flattened and stretched and it lodges against most of the protocone during occlusion. A deep crushing basin is also observed in the Diacodexeinae. It is, however, smaller than that of raoellids as the prehypocristid (cristid obliqua) points towards the centre of the tooth. There are no endocristids (hypolophid) bordering the crushing basin distally in the Diacodexeinae. All these characters substantiate the referral of IVPP V12763.1 from Shanghuang to the Raoellidae.
Among raoellids, the m3 from Shanghuang is strongly reminiscent of Indohyus major from Chorlakki (Fig 2a v. 2b), although it is known by an m2 trigonid only (Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987). Both specimens are of comparable size. The labio-lingual width of IVPP V12763.1 can only be estimated because the base of the crown is broken but it might not have been much wider than 10 mm, which is the width of the specimen of I. major (Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987, table 3). Both specimens further present the same mesial cuspid structure. The premetacristid of both taxa presents a sharp angle at the metaconid level. The mesial cuspids bear clear postcristids. Mesial to their contact, the protoconid and the metaconid are separated by a wide valley, which is much wider than in Kunmunella and Indohyus, and deeper than in Khirtharia inflata and K. dayi. Both specimens show an important enamel crenulation. The specimen from Shanghuang is here referred questionably to Khirtharia as the specimen shares the following features: low molar crown, large groove separating the mesial cuspids, presence of a low prehypocristid and no accessory central cristid (based on the results of the phylogenetic analysis in Section 5.a). It is referred to ?K. cf. major because of the scarcity of available material from Chorlakki and Shanghuang, and because the specimens differ slightly in the trigonid grooves, which seem to be deeper in the specimen from Shanghuang.
Thewissen, Gingerich & Russell (Reference Thewissen, Gingerich and Russell1987) differentiate I. major from I. indirae by its larger size only. However, the mesial cuspids of I. major are more inflated and less acute than those of I. indirae (Thewissen et al. Reference Thewissen, Gingerich and Russell1987, fig. 6e v. 6f). By these characters, I. major is of intermediate morphology between the type species I. indirae and Khirtharia. The lower molar from Shanghuang differs from those of K. inflata and K. dayi by a deeper groove pattern on the mesial lobe, endofossids and postfossids. The preprotocristid, postprotocristid and metacristids are also sharper. The prehypocristid is lingually lined by a deep groove that is absent in other Khirtharia species. The ‘hypolophid’, particularly the endoentocristid, is reduced and more mesially located compared to that of other raoellids. The lower molar of ?K. cf. major from Shanghuang is also more elongated than that of other Khirtharia species and it bears a wider and more massive hypoconulid lobe, which recalls that of both Indohyus and Kunmunella. The postentocristid present in Khirtharia species is not observed in IVPP V12763.1. The latter differs from the m3 of Kunmunella by its lower mesial cuspids, whereas Kunmunella presents high mesial cuspids with almost vertical distal walls. Its mesial ‘lophid’ is constituted by the endocristids, the postcristids lying in a distal position. In ?K. cf. major, like in Khirtharia, the protoconid and the metaconid are more deeply separated by the wide longitudinal groove. The prehypocristid (cristid obliqua) is sharper in Kunmunella. The hypoconulid lobe of ?K. cf. major consists of two distinct cusps, whereas only one cusp occurs on this part of the third molar in Kunmunella. However, a labial groove is present in the latter and a small accessory cusplet is observed on the hypoconulid of Indohyus indirae (Kumar & Sahni, Reference Kumar and Sahni1985, fig. 7a).
A p4 from Shanghuang (IVPP V12764.2), found in the fissure filling D (Irdinmanhan, Middle Eocene), has also been referred to the Suoidea by Métais et al. (Reference Métais, Qi, Guo and Beard2008). While no character clearly indicates that this specimen is a raoellid, its simple structure would be compatible with the raoellid family. As stated by Métais et al. (Reference Métais, Qi, Guo and Beard2008), the material is too scarce to allow association of the m3 from fissure B with the p4 from fissure D (no connection, no comparison, no occlusion). The size difference between the two specimens is noticeable and might prevent referring the teeth to a single taxon. However, the species K. dayi from Lammidhan (Pakistan, M15796 and M15797; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001) presents a similar p4 v. m3 size difference (M15796 and M15797: p4 length 7.0 mm; m3 length 11.3 mm). The p4 IVPP V12764.2 bears a small paraconid (= mesiostylid). This stylid is not observed in Siamochoerus (Fig. 2l) but is present in suoids such as Palaeochoerus (Fig. 2m). The structure of the tooth deeply differs from that of basal suoids by its cingulid, crest and groove pattern (Fig. 2k v. 2l–m). However, the differences observed in terms of crests and groove location may be mainly owing to the lack of a metaconid on the specimen IVPP V12764.2. The p4 IVPP V12764.2 is close in shape to what is observed in the raoellid Khirtharia, but with a simpler structure (Fig. 2k v. 2l). Khirtharia is devoid of a parastyle. However, this structure is present but small in Kunmunella (H-GSP 97187; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001, fig. 5e–f).
5. Raoellidae definition and systematic content
Raoellid species were successively considered as choeropotamids (Ranga Rao, Reference Ranga Rao1971), dichobunids (Ranga Rao, Reference Ranga Rao1972), anthracotheriids (Sahni & Kumar, Reference Sahni and Kumar1971; West, Reference West1980) or heloyids (Pilgrim, Reference Pilgrim1940; Coombs & Coombs, Reference Coombs and Coombs1977) before they were given a familial status by Sahni et al. (Reference Sahni, Bhatia, Hartenberger, Jaeger, Kumar, Sudre and Vianey-Liaud1981). In order to provide a clear diagnosis of the family based upon synapomorphies, and to formally test the systematic content of the Raoellidae, we performed a phylogenetic analysis based on the available dental characters. Testing the phylogenetic relationships of the Cetartiodactyla is well beyond the scope here, and the taxa included have been chosen regarding their implication for raoellid systematics. Four taxa have been included for character polarization: the arctocyonid ‘condylarths’ Protungulatum and Hyopsodus, and the diacodexeid cetartiodactyls Diacodexis and Gujaratia. The dichobunid Dichobune, the heloyid Gobiohyus, and the hippopotamoids Siamotherium and Choeropotamus (sensu Orliac et al. Reference Orliac, Boisserie, MacLatchy and Lihoreau2010), have been included to test previous attribution of raoellid species proposed in the literature (Pilgrim, Reference Pilgrim1940; Ranga Rao, Reference Ranga Rao1971, Reference Ranga Rao1972; Coombs & Coombs, Reference Coombs and Coombs1977; Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987). As raoellids were more recently proposed as the sister taxon of cetaceans within the Cetartiodactyla (Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007), the basal archaeocete Pakicetus was also included.
5.a. Phylogenetic analysis of dental characters
The data matrix composed of 37 characters (Table 2) controlled for 18 taxa (the data matrix and a detailed list of the material controlled are given in online Appendix 1 at http://journals.cambridge.org/geo) was treated under the assumption of the minimal model of unweighted parsimony, using PAUP 3.1 (Swofford, Reference Swofford1993), with a branch and bound search. Eighteen parsimonious trees (L = 67; CI = 0.55; RI = 0.77) were obtained. In the consensus tree (Fig. 3), two major clades of the Cetartiodactyla are supported: (a) a clade uniting the dichobunid Dichobune, the helohyid Gobiohyus, Haqueina of problematic affinities, the choeropotamid Choeropotamus, the anthracotheriid Siamotherium and the suoids Palaeochoerus and Siamochoerus; and (b) a clade gathering Pakicetus and the Raoellidae with Diacodexis and Gujaratia as early successive offshoots. The raoellid/cetacean relationship retrieved by Thewissen et al. (Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007) from multiple sources of morphological characters (dental, cranial and postcranial) is supported by our results based on dental characters only. The raoellid/cetacean clade is supported by nine unambiguous synapomorphies, among which two are not homoplastic: prehypocristid (cristid obliqua) labially oriented (81) and biradiculated P3 (371; this character was proposed as a cetacean synapomorphy by Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007). Basal cetaceans and raoellids further share wrinkled enamel (11; RI = 0.66), the presence of an endohypocristid (71; RI = 0.60), the absence of a mesiostylid on p4 (160; RI = 0.25), the absence of a parastyle on upper molars (200; RI = 0.66), labial walls of upper molar cusps rounded (251; RI = 0.80), the paracone of P4 higher than that of the molars (331; RI = 0.66) and the absence of a protocone on P3 (360; RI = 0.50). Raoellidae are unambiguously defined by the absence of a paraconid (20; RI = 0.85), a metaconule of the upper molars located distally to the paracone (271; RI = 0.66, convergently present at node I) and the endocristae of the P4 forming an anterior loph (301; RI = 0.50). The presence of an endoentocristid (51; RI = 0.66) and the loss of the hypoconid on m1–2 (130; RI = 1.00) are potential synapomorphies of the Raoellidae (DELTRAN optimization), but the distribution of these characters is ambiguous because of nonapplicable character states in Pakicetus. The Raoellidae form a pectinate clade with Kunmunella and Indohyus as first offshoots. Metkatius, Khirtharia and ?K. cf. major share a low molar crown (150; RI = 1.00). ?Khirtharia cf. major is closely related to Khirtharia; both taxa share an entoconid almost as high as the hypoconid (31; RI = 0.83, convergently observed at node F) and the loss of the central accessory cristid (90; RI = 1.00). IVPP V12763.1 from Shanghuang is the sister taxon to Khirtharia.
Table 2. Character list


Figure 3. Phylogenetic relationships of raoellid genera: strict consensus of the 18 parsimonious trees (L = 67; CI = 0.55; RI = 0.77). Below the branches are the number of unambiguous synapomorphies on the left and the Bremer support on the right (Bremer, Reference Bremer1994). The numbers in parentheses indicate the Bremer support when the specimen from Pondaung, which introduces a large amount of missing data, is excluded. V 12763.1 – specimen from Shanghuang described in this paper; KU 1765 – ‘Artiodactyla indeterminate’ from Pondaung; I. indirae – Indohyus indirae.
5.b. What is a raoellid?
Raoellids were recently defined as ‘extremely bunodont forms showing various degrees of lophodonty; the premolars are generally simple and trenchant; molars with reduced or absent paraconules on uppers, loss of the paraconid on lowers’ (Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007, p. 56). Based on our phylogenetic analysis, the Raoellidae present a reduction of accessory structures in lower and upper molars (paraconid (20), hypoconulid (130), parastyle (200)) and construction of a wide crushing basin by labial displacement of the prehypocristid of the lower molars (81, Fig. 2c–e, observed in both ‘sub-lophodont’ and ‘hyperbunodont’ species). Raoellids also present a specialization of their upper and lower premolars to form a complementary shearing device. The lower premolars are very trenchant and the strictly mesio-distal P3 is simplified by the loss of the paracone (360) and of the lingual root (371). The P4 is highly derived: the endocristae that are primitively present in the Cetartiodactyla (Fig. 4f, i–l) are mesial and high to form a transverse loph (301, Fig. 4i), and its paracone with sharp mesial and distal edges is high compared to the protocone (321).

Figure 4. Comparison of upper cheek tooth structure of some Eocene cetartiodactyls. Upper molar: (a) Kunmunella transversa; (b) Khirtharia inflata; (c) Haqueina haichinensis; (d) ‘artiodactyla indeterminate’ (NMMP−KU 1765, Tsubamoto et al. Reference Tsubamoto, Egi, Takai, Sein and Maung2005, fig. 2B); (e) Gujaratia pakistanensis; (g) Gobiohyus orientalis; (h) Palaeochoerus quercyi. P4: (f) Diacodexis secans; (i) Kunmunella transversa; (j) ?Indohyus yuanchuensis (after Young, Reference Young1937, fig 13); (k) Gobiohyus orientalis; (l) Palaeochoerus quercyi. Abbreviations: ectMul – ectometacristule; enM – endometacrista; enMul – endometacristule; enPa – endoparacrista; enPr – endoprotocrista; H – hypocone; M – metacone; Mul – metaconule; Pa – paracone; Pasl – parastyle; prePa – preparacrista; posM – postmetacrista; posMul – postmetacristule; posPa – postparacrista; pPul – preparacristule; prePaul – preparacristule; posPr – postprotocrista; Pr – protocone; preM – premetacrista; preMul – premetacristule; prePr – preprotocrista; Pul – paraconule.
Raoellids and cetaceans share high crowns in the posterior premolars (Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007). Indeed, our results indicate that raoellids and early cetaceans share derived characters corresponding to the development of prominent longitudinal cutting edges: sharp, biradiculated P3 lacking the protocone, a paracone of the P4 higher than that of the molars and a labial orientation of the prehypocristid (cristid obliqua) on the lower molars (corresponding to the opening of the crushing basin, completely open in pakicetids). Raoellids exhibit transverse structures on molars and premolars: a metaconule of the upper molars distal to the paracone (271) and the endocristae of the P4 forming an anterior loph (301), whereas basal cetaceans developed mesio-distal shearing structures, with an extreme modification of the molars with all transversal structures reduced. The Raoellidae and Pakicetus share several dental characters that can be correlated to a functional adaptation to shearing. Cetaceans can be defined by major changes in dental functions related to dietary changes (O'Leary & Uhen, Reference O'Leary and Uhen1999). According to our results, the ‘hyperbunodont’ molar morphology is derived among raoellids, indicating that, from a common shearing cheek tooth pattern, raoellids and cetaceans specialized in two different ways, raoellids shifting to a derived ‘omnivorous’ diet (shearing premolars associated with crushing molars). Isotopes indeed indicate different diet for raoellids and pakicetids (Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007).
The results of our phylogenetic analysis highlight that raoellids and suoids share numerous dental features. These similarities are here interpreted as convergences: the loss of the parastyle in the upper molars (200) and the rounded labial wall of the upper molars (251, both convergently present at the nodes B and J), and the loss of the paraconid (20, convergently occurring at the nodes B and H). These characters, together with symplesiomorphies explain the overall similarity between suoids and raoellids. This could as well explain why raoellids have been often referred to the Suiformes (e.g. Ranga Rao, Reference Ranga Rao1971, Reference Ranga Rao1972; Sahni & Kumar, Reference Sahni and Kumar1971; Vislobokova, Reference Vislobokova2004a).
5.c. Systematic content of the Raoellidae
According to our results, the Raoellidae is comprised of the genera Kunmunella, Indohyus, Metkatius and Khirtharia. This generic content of the family is congruent with that proposed by Theodor, Erfurt & Métais (Reference Theodor, Erfurt, Métais, Prothero and Foss2007). The close affinities of the specimen from Shanghuang (morphologically similar to the only specimen known of ‘I. major’) with Khirtharia, triggers the question of its generic attribution. Based on the results of our phylogenetic analysis, we chose to refer this material to ?Khirtharia, as it consists of very poor material.
In agreement with Theodor, Erfurt & Métais (Reference Theodor, Erfurt, Métais, Prothero and Foss2007), the genus Haqueina is not part of the raoellid clade contra Vislobokova (Reference Vislobokova2004a, Reference Vislobokovab). This result is congruent with the deep morphological differences observed between Haqueina and raoellid species for lower and upper molars (Fig. 2h v. 2c–e; Fig. 4c v. 4a–b), Haqueina being morphologically very close to Gobiohyus (Fig. 4c v. 4g). Theodor, Erfurt & Métais (Reference Theodor, Erfurt, Métais, Prothero and Foss2007) suggest that the isolated right M2 and M3 from Pondaung referred to as ‘Artiodactyla indeterminate 2’ (NMMP−KU 1765, 1742; Tsubamoto et al. Reference Tsubamoto, Egi, Takai, Sein and Maung2005, fig. 2b–c) could be raoellids. The teeth are bunodont and quadricuspidate as in raoellids, but their structure and crest pattern differ deeply from the latter (Fig. 4d v. 4a–b). On the M2, the paraconule is small and mesially located; it is strongly connected to the mesial cingulum by two small crests. In all raoellids, the crushing basin is mesially delimited by a transversal crest formed by a small endoparacrista connected to the paraconule and to the preprotocrista, and distally, it is delimited by the ‘hypoloph’ that corresponds to the junction of the endocristae of the distal cusps. The specimens from Pondaung do not present such transversal structures. Upper molars of raoellids exhibit a clear endometacrista, a structure that is not present in the specimens from Pondaung. According to our results, ‘Artiodactyla indeterminate 2’ from Pondaung is not part of the raoellid clade (Fig. 3).
Coombs & Coombs (Reference Coombs and Coombs1977) assigned Gobiohyus yuanchuensis Young, Reference Young1937 from Rencun (late Middle Eocene, China; Tsubamoto, Takai & Egi, Reference Tsubamoto, Takai and Egi2004) to ?Indohyus on the basis of the P4 morphology, the lack of a paraconule and a strong parastyle on M1–2, as well as lack of a paraconid on m3. Thewissen, Gingerich & Russell (Reference Thewissen, Gingerich and Russell1987) rejected the attribution of the specimen from Rencun to the Raoellidae on the basis of the bunodont cusps and the lack of transverse crests. The specimens of ?Indohyus yuanchuensis have been figured only in the original description of the species (Young, Reference Young1937). The drawings are unfortunately not very clear and wear has removed detailed structures of the cheek teeth. As stated by Coombs & Coombs (Reference Coombs and Coombs1977), the P4 is morphologically similar to that of raoellids (Fig. 4j), with a paracone bigger than the protocone (unfortunately the relative height of these cusps cannot be controlled), no postprotocrista (contrary to Gobiohyus) and no prominent parastyle. The presence of an endoparacrista cannot be verified from the drawing (Young, Reference Young1937, fig.13). The M2 has no paracone and the labial cusps have rounded labial flanks, as in raoellids (Fig. 4a–b). However, the ‘selenodont’ wear pattern of the M1 is clearly different from that of raoellids, and there is no trace of the crushing basin, or of the endometacrista, forming the distal loph (hypoloph) in Raoellidae. The M2 seems to exhibit a crushing basin, but the distal part of the tooth is broken and the presence of the hypoloph cannot be ascertained. On the left lower jaw fragment of ?I. yuanchuensis much of the structure of the m3 (only preserved tooth) is eroded or broken and no diagnostic character can be observed. Because of the difficulty in interpreting the drawings provided by Young (Reference Young1937, figs 13–14), we chose not to code ?I. yuanchuensis in our analysis. To conclude on the familial status of the material from Rencun, the material illustrated by Young (Reference Young1937) does not allow unambiguous referral to the Raoellidae. However, the morphology of the P4 could be compatible with an attribution to the raoellids, in agreement with Coombs & Coombs (Reference Coombs and Coombs1977). It is also noteworthy that the size of ?I. yuanchüensis is similar to that of ?K. major.
5.d. The sister taxon of the Raoellidae
In the original description of the family Raoellidae, Sahni et al. (Reference Sahni, Bhatia, Hartenberger, Jaeger, Kumar, Sudre and Vianey-Liaud1981) rejected their affinities with the coeval Heloyidae from Mongolia, or with the later Eocene Anthracotheriidae of Myanmar. Theodor, Erfurt & Métais (Reference Theodor, Erfurt, Métais, Prothero and Foss2007) rather suggested a local radiation of raoellids in the Indian Subcontinent, from an ancestry close to Gujaratia. Based on additional material, including cranial and postcranial remains, Thewissen et al. (Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007) advocated the Raoellidae as the sister taxon to the cetaceans. Our results, though based on a reduced number of characters and taxa, clearly discard close affinities with heloyids or with anthracotheriids and support Thewissen et al.'s (Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007) hypothesis of a sister taxon relationship between raoellids and cetaceans, both being nested within ‘diacodexeids’. Gujaratia is the sister taxon to the raoellid/Pakicetus clade. This relationship, previously proposed partly on geographical grounds (Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007), is here unambiguously supported by the presence of a paracone much higher than the protocone on P4 (321; RI = 1.00). Like other recent morphological analyses (e.g. Spaulding, O'Leary & Gatesy, Reference Spaulding, O'Leary and Gatesy2009; Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007), our results support a ‘Suiformes’ clade, i.e. grouping hippopotamoids (Choeropotamus and Siamotherium) and suoids (Siamochoerus, Palaeochoerus), instead of a Whippomorpha clade (i.e. grouping hippopotamoids and cetaceans; = Cetancodontamorpha sensu Spaulding, O'Leary & Gatesy, Reference Spaulding, O'Leary and Gatesy2009) so far chiefly supported by molecular data (e.g. Montgelard, Catzeflis & Douzery, Reference Montgelard, Catzeflis and Douzery1997; Waddell, Okada, & Hasegawa, Reference Waddell, Okada and Hasegawa1999; Marcot, Reference Marcot, Prothero and Foss2007). However, our taxonomic sampling cannot assess hippopotamid/cetacean relationship.
6. Biostratigraphical and palaeogeographical implications
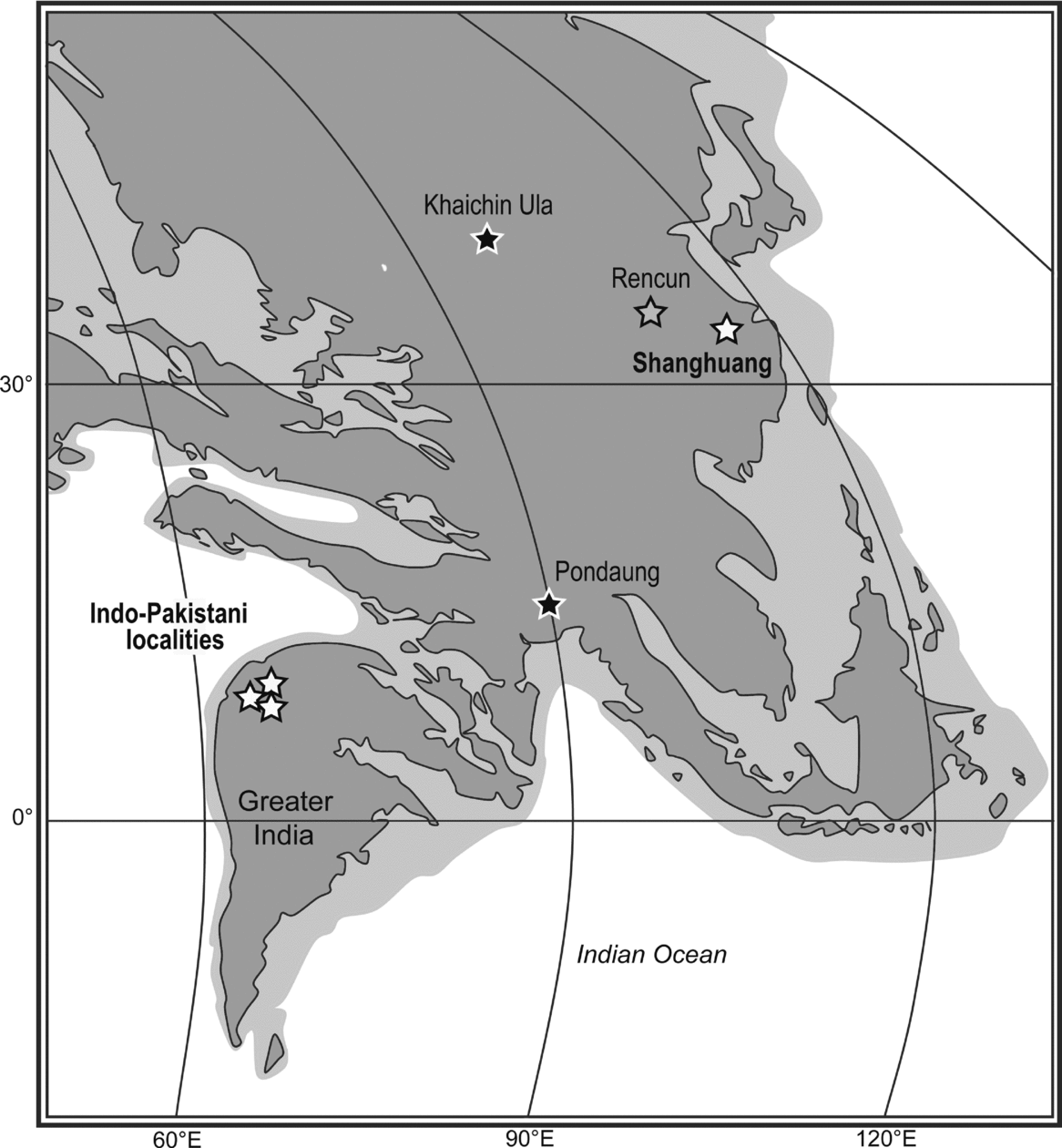
The endemism of the Raoellidae has been challenged by several recent works describing raoellids from outside the Indian Subcontinent: i.e. in Mongolia (Haqueina haichinensis, Khaichin Ula, early to middle Middle Eocene; Vislobokova Reference Vislobokova2004a, Reference Vislobokovab), in Myanmar (‘Artiodactyla indeterminate’, Pondaung, early Late Eocene; Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007) and in China (?Indohyus yuanchuensis, Rencun, late Middle Eocene; Coombs & Coombs, Reference Coombs and Coombs1977). Our results reject the attribution of the specimens from Mongolia and Myanmar to the Raoellidae, and the referral of ‘?Indohyus yuanchuensis’ from the late Middle Eocene site of China (Rencun, Sharamurunian ALMA; Tsubamoto, Takai & Egi, Reference Tsubamoto, Takai and Egi2004) is still dubious. On the other hand, the attribution of the m3 IVPP V12763.1 from Shanghuang to the Raoellidae documents the presence of the family outside the Indian Subcontinent and substantiates the easternmost occurrence of the Raoellidae. The remaining indisputable reports of the family are limited to the early Middle Eocene of Pakistan and India (Fig. 5).

Figure 5. Palaeogeographical reconstruction of South and South-East Asia at c. 50 Ma showing localities where raoellids were mentioned. Black stars – raoellid occurrences rejected in the present work; white stars – raoellid occurrences confirmed in the present work; grey star – dubious raoellid occurrence. The map is modified from R. Blakey (available at http://jan.ucc.nau.edu/~rcb7/RCB.html).
The fossil locality of Shanghuang (Jiangsu Province, coastal China) consists of five distinctive karstic infillings, Middle Eocene in age based on mammalian biochronology (Wang & Dawson, Reference Wang and Dawson1994; Dawson & Wang, Reference Dawson and Wang2001; Dawson, Huang & Wang, Reference Dawson, Huang, Li and Wang2003). The five fissures are considered to span a short interval of time (c. 1–2 Ma) of the Irdinmanhan ALMA (middle Middle Eocene, c. 45 Ma), with the fissure D being slightly older than the others (Beard et al. Reference Beard, Qi, Dawson, Wang and Li1994; Wang & Dawson, Reference Wang and Dawson1994; MacPhee, Beard & Qi, Reference MacPhee, Beard and Qi1995; Qi et al. Reference Qi, Beard, Wang, Dawson, Guo and Li1996; Métais, Guo & Beard, Reference Métais, Guo, Beard, Dawson and Lillegraven2004; Gebo et al. Reference Gebo, Dagosto, Beard and Ni2008). However, a late Middle Eocene age (Sharamurunian ALMA) has also been proposed for the fissures A, B and C (Dawson, Huang & Wang, Reference Dawson, Huang, Li and Wang2003; Métais et al. Reference Métais, Qi, Guo and Beard2008). The raoellid specimen from Shanghuang fissure filling B represents the youngest occurrence of the family, so far restricted to the early Middle Eocene in Indo-Pakistan. This species is also among the most derived raoellids according to the results of our analysis. The raoellid specimen from Shanghuang fissure filling B is morphologically very close to the fragmentary m2 described from Chorlakki, Pakistan (Figs 2a, b, 5; Thewissen, Gingerich & Russell, Reference Thewissen, Gingerich and Russell1987) and, as such, is designated ?Khirtharia cf. major. The Chorlakki fauna originates from a single locality, late Early or early Middle Eocene in age (Gingerich, Reference Gingerich2003), which would be slightly older than the Shanghuang karstic infillings if we consider them to span a short interval of the middle Middle Eocene (Irdinmanhan, Beard et al. Reference Beard, Qi, Dawson, Wang and Li1994; Gebo et al. Reference Gebo, Dagosto, Beard and Ni2008). The presence of ?K. cf. major in fissure B of Shanghuang (very close or conspecific to ?K. major from the early Middle Eocene locality of Chorlakki, Pakistan), would be more consistent with an Irdinmanhan age than with the Sharamurunian age proposed by Dawson, Huang & Wang (Reference Dawson, Huang, Li and Wang2003).
Clyde, Khan & Gingerich (Reference Clyde, Khan and Gingerich2003) proposed that modern orders of mammals dispersed into India during the initial collision with Asia, rather than from India (contra Krause & Maas, Reference Krause, Maas, Bown and Rose1990). They propose that the endemism of the early Middle Eocene faunas of the Indian Subcontinent (e.g. artiodactyls of the Kuldana and Kalakot formations) would indicate that early dispersal corridors were temporary, and that India was partially isolated by marine incursions during early Middle Eocene times, thus enhancing allopatric speciation (and endemic differentiation). Our results, although based on a reduced sample, would fit this scenario, with the differentiation of the cetacean/raoellid clade in India from a dichobunid-like ancestor of Holarctic origin close to Gujaratia pakistanensis. However, the earliest cetaceans (Pakicetidae) have been recovered from Lower Eocene sediments of India and Pakistan (West, Reference West1980; Gingerich & Russell, Reference Gingerich and Russell1990; Thewissen & Hussain, Reference Thewissen and Hussain1998; Thewissen, William & Hussain, Reference Thewissen, Williams and Hussain2001). This, in turn, implies, given this scenario, that the differentiation of endemic Indian Cetartiodactyla occurred well before early Middle Eocene time. The occurrence of the Raoellidae in South-East China, outside the Indian Subcontinent, during Middle Eocene time suggests that dispersal events occurred between the Indian Subcontinent and eastern Asia at the latest by Middle Eocene time. Two other small cetartiodactyls, Haqueina and Chorlakkia, are known both from the late Early Eocene or early Middle Eocene of Pakistan (H. haquei, Gandas Kas, Kuldana Formation; Dehm & Oettingen-Spielberg, Reference Dehm and Oettingen-Spielberg1958; C. hassani, Chorlakki, Kuldana Formation; Gingerich et al. Reference Gingerich, Russell, Sigogneau-Russell and Hartenberger1979) and from the Middle Eocene deposits of Mongolia (H. haichinensis and C. valerii, Khaichin Ula II; Vislobokova, Reference Vislobokova2004a). They further provide evidence of early Middle Eocene faunal dispersals between India and eastern Asia.
Among the small artiodactyls from Shanghuang, three diacodexeids have been described from Shanghuang, from fissures A (Jiangsudon shanghuangensis, Métais et al. Reference Métais, Qi, Guo and Beard2008) and D (‘cf. Diacodexis’ and ‘Diacodexeidae indet.’, Métais et al. Reference Métais, Qi, Guo and Beard2008). The occurrence of diacodexeids in Shanghuang is noteworthy because this family has an Early Eocene to early Middle Eocene stratigraphical range in Europe and North America (Theodor, Erfurt & Métais, Reference Theodor, Erfurt, Métais, Prothero and Foss2007), while the raoellid record is restricted to the early Middle Eocene of Indo-Pakistan (Thewissen et al. Reference Thewissen, Russell, Gingerich and Hussain1983; Thewissen, Williams & Hussain, Reference Thewissen, Williams and Hussain2001). Metais et al. (Reference Métais, Qi, Guo and Beard2008) suggested that the presence of diacodexeids in Shanghuang points to the persistence of very stable ecological conditions in coastal China during Early to Middle Eocene time, allowing notably the survival of forest-dwelling artiodactyls otherwise extinct elsewhere. During that period (c. 45 Ma ago), the palaeolatitude of India was ~ 20° farther south than today (10°N instead of 30°N), i.e. close to the equatorial humid belt (i.e. ~ 15°N; Mattauer, Reference Mattauer2002; Kent & Muttoni, Reference Kent and Muttoni2008), which implies that the latitudinal difference with Shanghuang (~ 35°N through Cenozoic times) was much more important than it is in present times (Fig. 5). However, global climate was much warmer during Middle Eocene time than today (Mid-Eocene climatic optimum; Zachos, Dickens & Zeebe, Reference Zachos, Dickens and Zeebe2008) and it is likely that warm and humid conditions (subtropical to tropical) occurred in coastal China, in contrast to the arid to semi-arid vegetation zone that extended at similar latitudes in China throughout Eocene time (Sun & Wang, Reference Sun and Wang2005, fig. 7b). There were neither significant climatic nor paleoenvironmental discrepancies between coastal Northern India and Shanghuang (coastal China) during Middle Eocene (Irdinmanhan) time, which allowed the dispersal/survival of ecologically exigent mammals such as the Raoellidae (aquatic waders; Thewissen et al. Reference Thewissen, Cooper, Clementz, Bajpai and Tiwari2007) in Eastern Asia.
7. Conclusions
Métais et al. (Reference Métais, Qi, Guo and Beard2008) tentatively identified an isolated m3 (specimen IVPP V12763.1) from the Middle Eocene of China as an indeterminate suoid, which implied that it was the earliest record of the superfamily. Our analysis and revision of this material led us to challenge this interpretation and we suggest instead that the Shanghuang specimen rather belongs to the Raoellidae. This is the only unquestionable record of raoellids outside the Indian Subcontinent so far, which significantly extends the geographical and chronological range of the family: this specimen represents the youngest and easternmost occurrence of the family. According to the results of our phylogenetic analysis, the Raoellidae include Kunmunella, Indohyus, Metkatius and Khirtharia. Haqueina from Mongolia and the ‘indeterminate artiodactyl’ from Pondaung previously tentatively referred to as raoellids (Theodor et al. Reference Theodor, Erfurt, Métais, Prothero and Foss2007) are excluded from the raoellid clade.
The occurrence of a raoellid species in Shanghuang implies that raoellids dispersed from the Indian Subcontinent to eastern Asia during late Early Eocene or Middle Eocene time. This somewhat tempers the hypothesis of Middle Eocene Indian endemism as well as that of eastern Asia provincialism.
Acknowledgements
We gratefully thank the heads and staff of the Muséum National d'Histoire Naturelle, Paris, France, who graciously provided access to their collections. Many thanks to B. Marandat, R. Tabuce, P.-O Antoine, L. J. Flynn and A. Ramdarshan for their help and for valuable comments on an early version of the manuscript. This work has been conducted with the support of the ANR-ERC programme Palasiafrica (ANR-08-JCJC-0017-01). This is ISE-M publication 2011-089. We also thank the Agence Nationale de la Recherche ANR-09-BLAN-0238.