INTRODUCTION
Microhabitat selection in parasites, like any other organisms, should ultimately be driven by fitness consequences, though at a proximate level it often results from simple mechanisms, such as fixed responses to specific external cues (Sukhdeo and Sukhdeo, Reference Sukhdeo and Sukhdeo1994). Ectoparasites of fish, i.e. monogeneans and copepods, have been the subject of numerous studies on microhabitat selection, partly because most of them show very restricted distributions in specific sites (Rohde, Reference Rohde1993, Reference Rohde1994). In part, this may be due to physical factors, such as water flow over the gills or body surface of fish, with the parasites found only in sites from which they do not get dislodged (e.g. Davey, Reference Davey1980; Etchegoin and Sardella, Reference Etchegoin and Sardella1990; Loot et al. Reference Loot, Poulet, Reyjol, Blanchet and Lek2004). However, given that many apparently suitable sites are typically left vacant on the complex physical landscape that is provided by the external surfaces of fishes, explanations are required for the limited use of the available space by monogeneans and copepods.
A prominent and widely accepted hypothesis is that these ectoparasites, which generally occur at low densities, have been selected to concentrate at very specific attachment sites to facilitate mate location and reproduction (Rohde, Reference Rohde1979, Reference Rohde1991, Reference Rohde1993). Although this hypothesis may well apply to monogeneans, some results on parasitic copepods do not support this hypothesis, with the existing support in fact consisting mostly of circumstantial evidence (Timi, Reference Timi2003; Poulin, Reference Poulin2007). One glaring weakness of the vast majority of earlier studies is that the fitness associated with particular microhabitats is actually never quantified and related to the narrow site selection observed in most cases. Demonstration that reproductive success is greater in some infection sites than others has been achieved for some types of parasites (Sukhdeo, Reference Sukhdeo1990; Chilton et al. Reference Chilton, Bull and Andrews1992), but is missing in the case of fish ectoparasites, despite the wealth of information available on their extreme site selectivity.
Ectoparasitic copepods are good models to address this issue. In some families, like the Lernaeopodidae, females are anchored in one fixed position on the fish for their entire adult life (Kabata, Reference Kabata1981); they are visited and fertilized by the much smaller males (Raibaut and Trilles, Reference Raibaut and Trilles1993). Female copepods produce eggs in clutches contained in paired egg sacs. The number of eggs carried by a given female in a pair of egg sacs may vary over time, from clutch to clutch (e.g. Tedla and Fernando, Reference Tedla and Fernando1970). However, this number represents the output a female managed to achieve, for a given body size, in a particular microhabitat. It should therefore be possible to relate microhabitat selection in copepods to some measures (copepod body size, reproductive output per clutch) of fundamental relevance to their lifetime fitness. In a species where individuals vary in their selection of microhabitats, this approach should therefore allow for comparisons that can provide insights into the ultimate reasons for narrow site specificity. There are, in fact, several species in which site selection, although strict, nevertheless varies sufficiently among individuals to allow this kind of analysis. In particular, in some species of copepods (Timi, Reference Timi2003), as in some monogeneans (Rohde, Reference Rohde1993, Reference Rohde1994; Whittington and Ernst, Reference Whittington and Ernst2002), the attachment sites of parasites change as a function of the size or age of the fish host; linking the fitness of parasites with these movements could allow adaptive explanations to be tested.
Here, we used this approach on the copepod Neobrachiella spinicephala (Ringuelet, 1945) (Lernaeopodidae), parasitic on the Brazilian sandperch, Pinguipes brasilianus Cuvier, 1829, along the Atlantic coast of Argentina (Etchegoin et al. Reference Etchegoin, Timi and Lanfranchi2006). These parasites attach to either of 4 locations on their hosts: the lips, the margins of the operculum, the bases of the pectoral fins, or the bases of the pelvic fins. Preliminary observations have suggested that the female copepods display a different distribution on large hosts than on small hosts. Given that females are attached for life in one place on the body of the host (via insertion of a bulla deep into host skin), this apparent difference cannot be explained by movement on the fish as the latter grows, but may instead reflect different site selection by new recruits based on host size. In an attempt to determine which attachment site yields the greatest benefits, and why copepods appear to shift their preferred location based on the size of their host, we analysed individual data on a large collection of female N. spinicephala. While taking a range of other variables into account, we focused specifically on attachment site, and addressed the following questions. (i) Is there a significant shift from certain attachment sites toward others as a function of host size? (ii) How does attachment site influence the body size attained by adult females? (iii) After correcting for the parasite's body size, how is reproductive output affected by the site of attachment? Our analysis is one of the first to directly link fitness correlates with site selectivity in fish ectoparasites, and it provides insights into the selective forces that have restricted attachment to very specific sites on the host.
MATERIALS AND METHODS
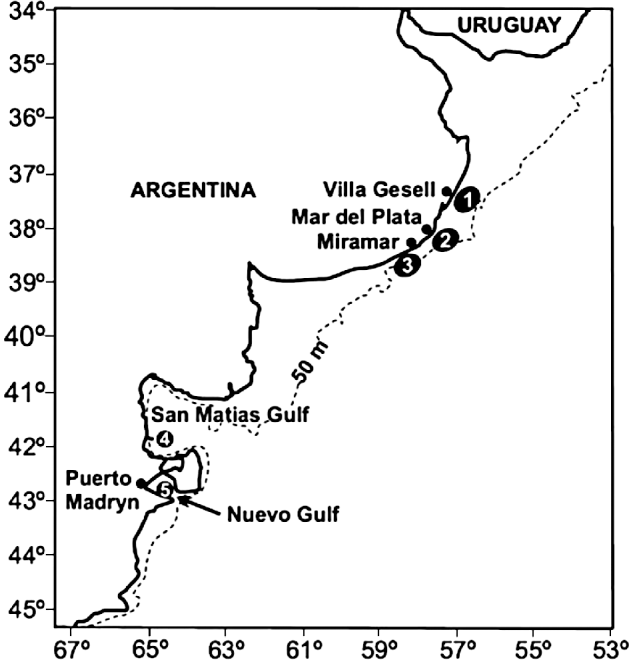
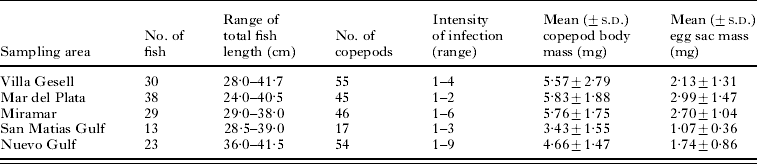
In total, 480 fish were obtained from commercial fishermen from 5 areas along the Atlantic coast of Argentina (Fig. 1), in all seasons. Previous investigations showed little difference in prevalence of the copepod among localities or seasons (Timi et al. Reference Timi, Lanfranchi and Etchegoin2009), but these factors were considered in all analyses here. All fish were used to estimate prevalence of N. spinicephala as a function of host size, and then only fish harbouring N. spinicephala were retained for subsequent analyses. In the laboratory, each fish was measured (total length) and then examined for copepods. Neobrachiella spinicephala was the only copepod species found on these fish. One monogenean, Microcotyle pseudopercis Amato and Cezar, 1994, and the praniza larvae of gnathiid isopods, occurred at low abundance on the gills (Timi et al. Reference Timi, Lanfranchi and Etchegoin2009), and thus not in the same microhabitats as the copepods; they were therefore not considered here. Male copepods are occasionally seen attached to females, but were only rarely found in our samples; for this reason, and because they are very small (<1 mm), their presence is unlikely to affect resource competition, and thus they were not considered further.

Fig. 1. Map of the Atlantic coast of Argentina, showing the location of the 5 areas where the fish host Pinguipes brasilianus was sampled: (1) Villa Gesell; (2) Mar del Plata; (3) Miramar; (4) San Matias Gulf; (5) Nuevo Gulf.
For each female copepod, the following variables were recorded: (i) site of attachment, which can be either the lip, the outer margins of the operculum, the base of the pectoral fins, or the base of the pelvic fin; (ii) the number of immediate neighbours, i.e. the number of other females attached to the same site, with left and right sides of the host body considered separately for the opercula and fins; (iii) the body mass of the female, excluding the egg sacs if any, to the nearest 0·1 mg and (iv) the mass of both egg sacs combined, for females that had them, also to the nearest 0·1 mg. Egg sac mass was used here as a proxy of the number of eggs per clutch. To justify this, we obtained the length and diameter of both egg sacs, and the maximum length and width of 10 randomly chosen eggs for each of a subset of female copepods. From these, we calculated the combined egg sac volume and the average volume of a single egg for each of these females. By dividing egg sac volume by average egg volume, we obtained the estimated number of eggs per combined egg sacs per female. We found that egg sac mass correlated strongly and positively with the estimated number of eggs (r 2=0·58, N=39, P<0·0001), and that average egg volume did not change as a function of increases in female body weight (P=0·265), making egg sac mass a reliable proxy of the number of eggs per clutch regardless of female body size.
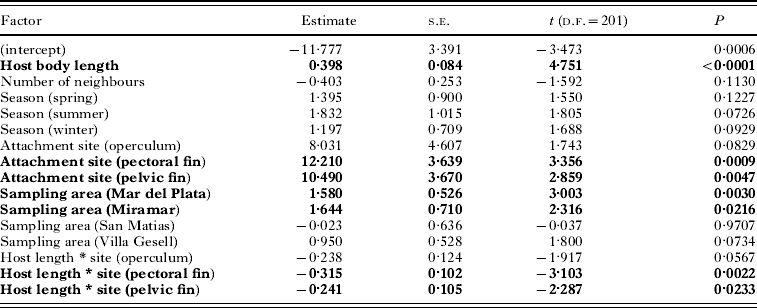
All statistical analyses were conducted in the R environment (version 2.9.1; R Development Core Team, 2009). We first determined whether copepods attach to different sites on their host's body as a function of the latter's size. To do this, we divided copepods into 2 groups: those attached to the host's lip or the margins of the operculum, and those attached at the base of either the pectoral or pelvic fins. This created a binary response variable that was used in a logistic regression, with fish body length and sampling area, and their interaction, entered as predictor variables. Other potential predictors were not included because there was no a priori reason to expect them to matter. For instance, season of capture was not included because copepods do not move after attachment, and the season of capture does not indicate when the parasites attached to their host. Also, the number of neighbours at the time of sampling does not tell us how many there were when the copepod attached in a particular site.
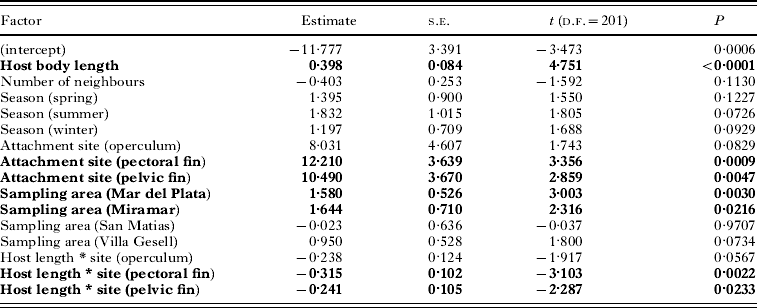
Second, we investigated the determinants of copepod body size using a generalized linear model (GLM), with copepod body mass as the response variable, and choosing a Gaussian error structure and identity link function. The predictors in the GLM were host body length, the number of neighbours at a particular attachment site (treated as a factor: 0, 1, or ⩾2 neighbours), sampling area (5 areas), season of capture (4 seasons), and attachment site on the host (4 sites: lip, operculum, pectoral fin and pelvic fin). Only the interaction between host body length and site of attachment was included, after exploratory analyses revealed that other second-order interactions were all non-significant.
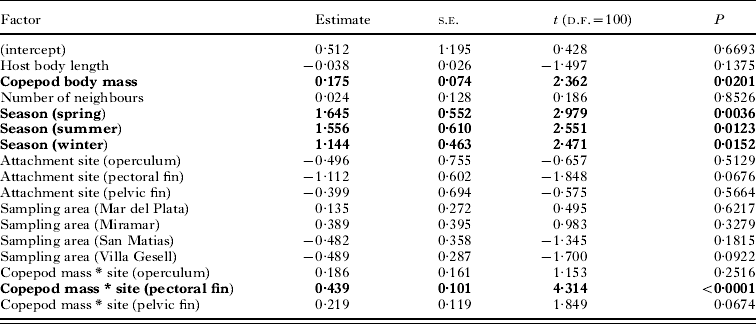
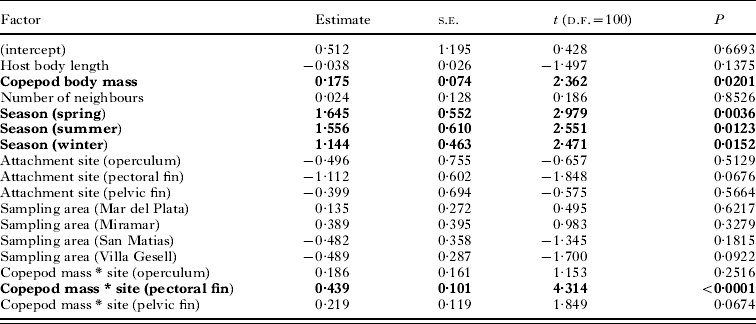
Third, we determined what affects copepod fitness, using a GLM with egg sac mass as the response variable, again choosing a Gaussian error structure and identity link function. The predictors in this GLM were copepod body mass, host body length, the number of neighbours at a particular attachment site, sampling area, season of host capture, and attachment site on the host (all factors as above). Once again, after exploratory analyses of all second-order interactions, we only included that between copepod body mass and site of attachment.
RESULTS
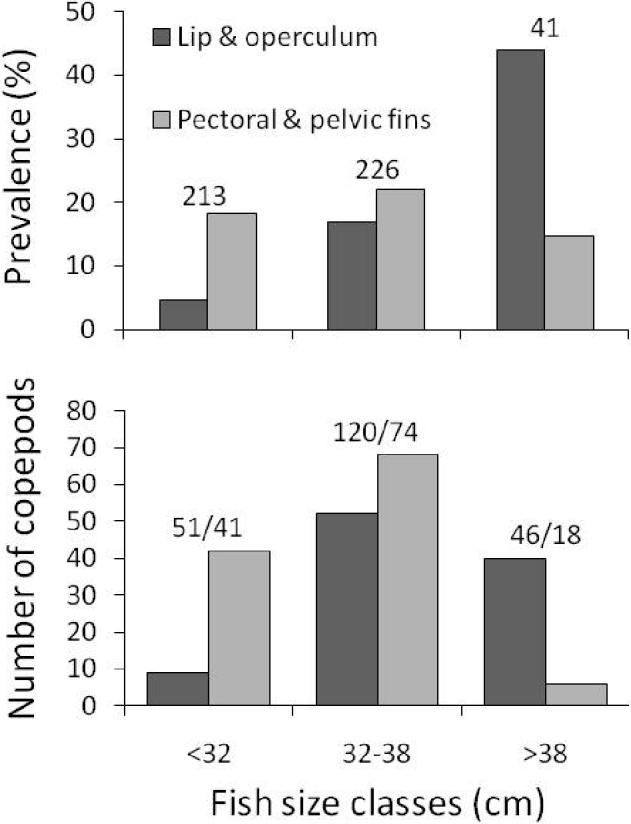
Prevalence of Neobrachiella spinicephala at the base of the pectoral or pelvic fins, i.e. the proportion of fish infected at those sites, was roughly the same independently of fish body length; however, prevalence on the lips or operculum was higher in fish of greater body length (Fig. 2). In total, 217 female N. spinicephala were recovered from 133 individual fish (Table 1). Of these females, 123 (56·7%) had egg sacs; because 6 females had either a missing sac, broken sacs, or sacs too small to be weighed, egg sac mass data were only available for 117 females. Of the fish included in the study, most (114 of 133, i.e. 85·7%) harboured only 1 or 2 copepods, and the maximum intensity of infection was 9 (Table 1). When there were more than 1 copepod per fish, they were often attached in different sites, such that 71% of copepods overall had no immediate neighbours.
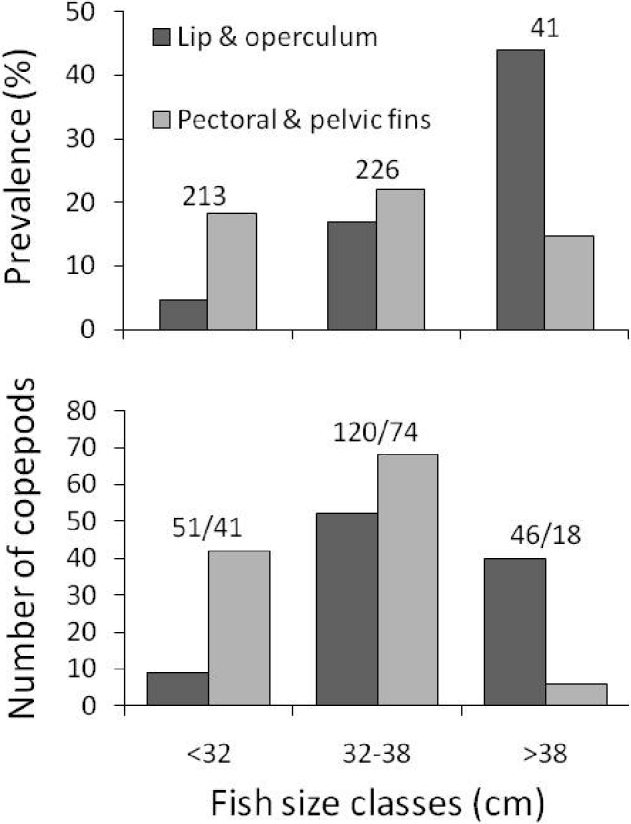
Fig. 2. Prevalence of infection (top) and number of female copepods, Neobrachiella spinicephala (bottom), at different attachment sites on fish of 3 different size classes. Numbers above the bars: numbers of fish examined (top) and numbers of copepods/numbers of infected fish (bottom).
Table 1. Summary statistics for the fish Pinguipes brasilianus infected by the parasitic copepod, Neobrachiella spinicephala, sampled from 5 localities along the Atlantic coast of Argentina

The logistic regression indicated that the site of attachment chosen by a copepod was influenced by both host body length (χ2=26·17, d.f.=1, P<0·0001) and area of sampling (χ2=22·01, d.f.=4, P=0·0002); the interaction of these two factors was not significant (χ2=1·80, d.f.=4, P=0·772). Copepods showed different patterns of attachment on fish of different sizes: they were much more likely to attach at the base of fins on small fish, and much more likely to attach on either the lip or the margins of the operculum on large fish (Fig. 2). The effect of sampling area is unlikely to be a real biological influence because it may be confounded by fish sizes. There was a significant difference in fish lengths among the 5 samples (F 4,212=43·31, P<0·0001). Fish from the Nuevo Gulf were generally longer (Table 1), so that this sample consisted mostly of medium-to-large fish, whereas the other samples comprised mostly small-to-medium fish.
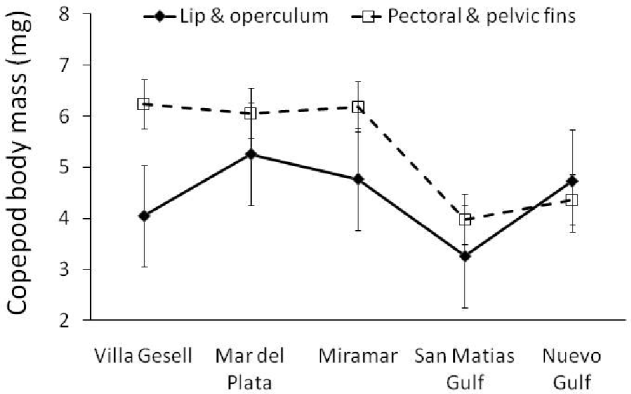
The body mass of the largest ovigerous female was almost 10 times that of the smallest, indicating substantial size variation among adult females. Our first GLM showed that copepod body mass was influenced by host body length, attachment site, area of sampling, and an interaction between host body length and attachment site (Table 2). Although it is significant, host body length explains very little of the variance in copepod body mass overall (<1%, based on the r 2 of a simple regression). The interaction between host body length and attachment site provides an explanation: the mass of a copepod attached to the lip of its host is explained to some extent by host body length (r 2=0·24), but that of copepods attached to other sites is not (all r 2 <0·015). The other 2 effects are clearer. First, copepods attached to the base of fins are almost always larger (by 32% on average) than those attached on the lips or the operculum (Fig. 3), except in the Nuevo Gulf area where very few copepods attached at the base of fins were collected because of the larger size of fish. Second, copepods from the more northern sampling areas tend to be larger-bodied than those from southern areas (Fig. 3).

Fig. 3. Mean (±s.e.) individual body mass of female copepods, Neobrachiella spinicephala, from different attachment sites on fish of 5 different sampling areas.
Table 2. Results of the GLM evaluating the effect of several factors (significant ones in bold) on body mass of individual female copepods, Neobrachiella spinicephala
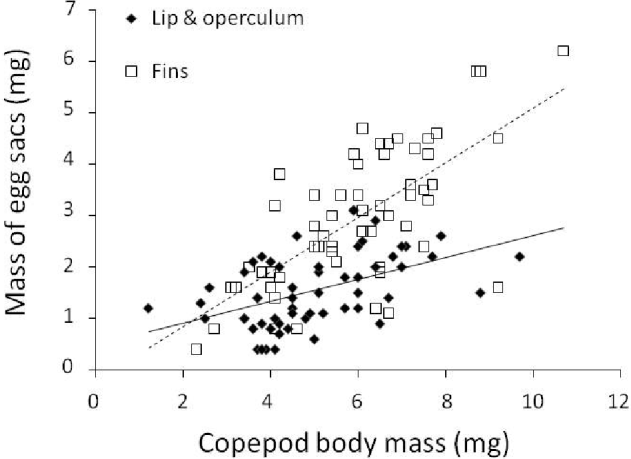
There was an approximately 20-fold variation among egg sac masses of all ovigerous females; although this may in part be due to eggs being at different developmental stages, it nonetheless indicates that different individuals achieve much larger reproductive output than others. Our second GLM demonstrated that the mass of egg sacs produced by female copepods was influenced by copepod body mass, the season of sampling, and an interaction between copepod body mass and attachment site (Table 3). Although egg sacs are generally smaller in southern sampling areas than in northern areas (see Table 1), this is likely to be a mere consequence of copepod body mass being lower in the southern areas, and the effect of sampling area was not significant in the GLM. The seasonal effect is manifested by slightly larger egg sacs for a given copepod body mass in summer, and to a lesser extent spring, than in winter or autumn (data not shown). The effect of copepod body mass, and its interaction with attachment site, is clear when considering different attachment sites separately (Fig. 4). For a given copepod body mass, the combined mass of egg sacs is almost always greater if the copepod is attached at the base of fins than to the lip or operculum; as an example, a female weighing 6 mg would produce 40% larger egg sacs if attached to the base of fins. Egg sac mass also rises more sharply as a function of copepod body mass for copepods attached at the base of fins than for those on the lips or operculum (Fig. 4).

Fig. 4. Relationship between the combined mass of both egg sacs and the body mass of female copepods, Neobrachiella spinicephala, from different attachment sites on fish hosts.
Table 3. Results of the GLM evaluating the effect of several factors (significant ones in bold) on the combined mass of both egg sacs of individual female copepods, Neobrachiella spinicephala
DISCUSSION
Understanding site selection by parasites ultimately requires that parasite fitness be compared among different sites, in order to evaluate the consequences of attaching in one place on the host rather than in another. Here, we have observed 10-fold variation in adult female body sizes, and approximately 20-fold variation in reproductive output among these females, in the parasitic copepod Neobrachiella spinicephala. These are key fitness correlates, and the huge variation observed among females hints at the possibility that certain females might have greater success because of what attachment sites they occupy.
Some of the explanatory variables uncovered in our GLMs were expected to have an effect on either copepod body size or egg production. For instance, there was a latitudinal gradient in copepod body masses, with individuals from northern samples being generally larger than those from southern samples. This could be a direct consequence of temperatures experienced during growth. Experimental studies with other copepods of the family Lernaeopodidae show that copepods achieve smaller body sizes at cool temperatures than at warmer ones (Johnston and Dykeman, Reference Johnston and Dykeman1987). Along the Atlantic coast of Argentina, water temperatures are indeed warmer in the north, where the influence of the warm Brazil Current is felt, than in the south (Bakun and Parrish, Reference Bakun and Parrish1991). The parasite is absent from Pinguipes brasilianus populations off the coast of Brazil (Timi et al. Reference Timi, Lanfranchi and Luque2010), so further increases in body size in warmer waters cannot be confirmed. In addition, the seasonal effect detected with respect to the mass of egg sacs is similar to what has been seen in other species of parasitic copepods (Tedla and Fernando, Reference Tedla and Fernando1970). This seasonal pattern may also be explained by changes in water temperature throughout the year, since copepods in cold water generally produce smaller egg sacs, whether they are parasitic (Johnston and Dykeman, Reference Johnston and Dykeman1987) or free-living species (Abdullahi, Reference Abdullahi1990). These seasonal and geographical trends represent background influences that were corrected via the GLMs to allow other effects to be detected.
Another confounding effect that had to be taken into account was host body size. In some species of parasitic copepods, individuals attached to larger fish hosts attain larger sizes than conspecifics attached to small hosts (e.g. Van Damme et al. Reference Van Damme, Maertens, Arrumm, Hamerlynck and Ollevier1993). In our study, however, fish body size had only a minor influence on copepod body size. In fact, it is only among copepods attached to host lips that host body length correlates well with copepod body mass; for copepods attached to other sites, host body length does not relate to copepod body mass.
Our main focus was on attachment sites of N. spinicephala on its fish host, and their fitness consequences. First, we confirmed that preferred attachment sites change with host size: copepods are overwhelmingly found at the base of the pectoral and pelvic fins on small fish, and on the lips and margins of the operculum in larger fish. Such shifts in attachment locations as a function of host size have been reported in another copepod species (Timi, Reference Timi2003). They have also been documented in monogeneans, where individuals are not fixed for life but can shift their position as the host grows (Whittington and Ernst, Reference Whittington and Ernst2002). This is not an option for N. spinicephala, which is anchored permanently to one location. One explanation may be that the parasites attach to all the sites on small fish but mostly at the base of fins, and that only those on the lips and operculum survive without being dislodged as the fish ages and grows. This scenario could explain why host length and copepod body mass correlate well for copepods attached to the lips. However, it would require a lifespan for the parasite longer than what is likely for lernaeopodid copepods (1–6 months; Lester and Roubal, Reference Lester, Roubal and Woo1995). A more likely explanation is that infective copepodids arriving on a fish attach to different sites based on fish size. One reason for this may be that the skin at the base of fins may become too thick for attachment in larger fish, forcing arriving copepodids to seek the lips and operculum margins. Finally, differences between host size classes with respect to foraging strategies or habitat use may also affect patterns of contact between copepodids and fish, and influence where the former can attach to the latter.
Second, to determine why copepodids might opt for different microhabitats on fish of different sizes, we looked at the consequences for copepod growth. We found that, independently of fish size or other confounding variables, copepods attached to the base of fins were larger than those on the lips or operculum; overall, copepods at the base of fins were on average 1·4 mg (or 32%) larger than those on the lips or operculum. Since copepods attached to the lips and operculum are more-or-less enclosed within skin folds, physical constraints may limit their eventual size. This would explain why the base of fins is a preferred location on small fishes, but it is unclear why copepods would attach elsewhere on larger fishes if this compromises their growth. Competition for space with other parasites is not an option in this system, as N. spinicephala occurs at very low abundance and there are no other parasites at the base of fins. As mentioned above, an increase in skin thickness at the base of fins might be the explanation for the shift in site selection. Alternatively, perhaps fin bases are more difficult to reach on a large fish if contact is first made around the head region via the ventilation currents passing through the mouth and gills (which is the ancestral, and still the main, point of contact with the host for parasitic copepods; Benz, Reference Benz1993).
Third, we examined the reproductive output per female associated with different sites of attachments. We measured reproductive output as the combined mass of both egg sacs. This may not always reflect the number of eggs produced, since some females may produce fewer, larger eggs whereas others might produce many small ones. This sort of trade-off between egg size and number is seen in comparisons across copepod species (Poulin, Reference Poulin1995). However, in intraspecific studies of parasitic copepods, this trade-off is not detectable (Timi et al. Reference Timi, Lanfranchi and Poulin2005). More importantly, in our system, the dimensions of individual eggs showed no detectable variation among females of different sizes (see Materials and Methods section). Therefore, the mass of egg sacs is a reliable correlate of egg numbers. We found that female copepods with greater body masses produced larger egg sacs, a general effect of body size on fecundity seen in many other species of parasitic copepods (Tedla and Fernando, Reference Tedla and Fernando1970; Emson et al. Reference Emson, Mladenov and Wilkie1985; Van Damme et al. Reference Van Damme, Maertens, Arrumm, Hamerlynck and Ollevier1993). More importantly, the interaction between copepod body mass and egg sac mass shows that for a given body mass, a female copepod will produce a larger pair of egg sacs if it is attached at the base of the host's fins than if it is attached to its lips or operculum. For instance, a typical female with a body mass of 6 mg would, on average and all else being equal, produce 40% larger egg sacs if attached to the base of fins than to the lips or operculum.
Overall, our results indicate that copepods attaching to the base of fins grow larger and produce more eggs than those attached to the lips and operculum. This conclusion is based on a ‘snapshot’ look at fecundity, i.e. a single clutch as opposed to lifetime fitness; a definitive assessment of the influence of attachment site on parasite fitness would require a full lifetime measurement of fitness, something that is not possible in wild species. Nevertheless, it suggests that site selection has major impacts on reproductive success. So why would the more advantageous sites be left vacant on larger hosts in favour of the lips and operculum, where growth and egg production will be lower? We suggest that changes in skin thickness as a function of host length may be the explanation. Other potential explanations include size-dependent differences between attachment sites with respect to (i) their accessibility by males seeking to fertilize females, (ii) the probability of dislodgement by water currents, and (iii) the probability of removal by cleaner organisms, with skin folds around the lips and the margins of the operculum providing protection compared to the exposed bases of fins. These, as well as other possibilities, remain to be explored. Our approach has nonetheless demonstrated that it is possible to obtain precise and quantitative estimates of fitness benefits associated with different sites of attachment, and that this should be used in more studies on microhabitat selection by parasites.
ACKNOWLEDGEMENTS
Financial support to J.T.T. was provided by grants from CONICET (PIP # 112-200801-00024) and ANPCYT (PICT # 02199).