Ketosis, the most common metabolic disorder of dairy cows, always occurs during the first 3 weeks after parturition (LeBlanc, Reference LeBlanc2010). It is characterised by increased levels of ketone bodies and decreased glucose in blood. The dairy cows with plasma ketone bodies [acetoaectate (ACAC)] over 1 mm and blood glucose below 2·5 mm are considered to be clinically ketotic (Kremer et al. Reference Kremer, Noordhuizen-Stassen, Grommers, Schukken, Heeringa, Brand and Burvenich1993; Xu et al. Reference Xu, Liu, Li, Xia, Zhang and Wang2010). Growing evidence indicates that hyperketonemia is associated with elevated levels of blood interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) in rat hepatocytes, human brain microvascular endothelial cells and U937 monocytes (Hoffman et al. Reference Hoffman, Cheng, Passmore, Carroll and Hess2002).
The proinflammatory cytokines IL-6, interleukin-1β (IL-1β) and TNF-α (Stentz et al. Reference Stentz, Umpierrez, Cuervo and Kitabchi2004) are secreted by macrophages lymphocytes and other cell types in response to various stresses (Bruun et al. Reference Bruun, Helge, Richelsen and Stallknecht2006). Studies demonstrated that lipopolysaccharide (LPS) induced the production of IL-6, IL-1β and TNF-α in the Kupffer cells of cows (Gerszten et al. Reference Gerszten, Garcia-Zepeda, Lim, Yoshida, Ding, Gimbrone, Luster, Luscinskas and Rosenzweig1999). Enhanced proinflammatory status is associated with reduced antioxidant capacity, which is associated with the development of mastitis and endometritis in dairy cows during the transition period.
In dairy cows, propionic acid is the main precursor for glucose production via hepatic gluconeogenesis (Li et al. Reference Li, Li, Bai, Chen, Deng, Liu, Zhang, Liu and Wang2012). Furthermore, dairy cows experience negative energy balance (NEB) caused by a low dry matter intake and an increased demand of glucose (Wathes et al. Reference Wathes, Cheng, Fenwick, Fitzpatrick and Patton2011). The NEB initiates fat mobilisation and a subsequent increase in non-esterified fatty acid (NEFA) concentration. At high concentration, NEFA are not completely oxidised but instead are metabolised into ketone bodies such as ACAC in hepatocytes. It is now evident that the liver can regulate systemic inflammatory responses and is the target of inflammatory stimuli, which develop inflammatory responses via releasing cytokines like IL-1β, TNF-α and IL-6 (Dasarathy, Reference Dasarathy2008). Elevated levels of proinflammatory cytokines (TNF-α) in serum have correlation with liver damage (Manco et al. Reference Manco, Marcellini, Giannone and Nobili2007). As the major cellular component of liver, hepatocytes are easily subject to locally released cytokines (Park et al. Reference Park, Chen, Kim, Brown, Kolls, D D'Agati and Lee2011).
We hypothesised that ACAC and glucose are associated with the increased blood proinflammatory cytokines levels in the dairy cows with ketosis. Therefore, the aim of this study was to determine the molecular mechanism of ACAC and glucose on the increased IL-1β, IL-6 and TNF-α in cow hepatocytes, which could be helpful to understand the mechanism of inflammatory liver damage induced by ketosis in dairy cows.
Materials and methods
Isolation and culture of primary bovine hepatocytes
Neonatal calf was provided by the Experimental Animal Centre of Jilin University (Changchun, China) and the procedures were approved by the Institutional Animal Care and Use Committee of Jilin University (Changchun, China). Hepatocytes were collected and cultured as described in previous studies (Zhang et al. Reference Zhang, Li, Gao, Liu, Kong, Li, Wang, Zhang, Wang and Zhang2012; Li et al. Reference Li, Li, Song, Shi, Ding, Yang, Liu, Chen, Li and Wang2013, Reference Li, Huang, Gu, Du, Lei, Yuan, Sun, Wang, Li and Liu2015). The caudate lobe of the liver was obtained under sterile conditions from a female Holstein calf anesthetised with thiamylal sodium and was quickly placed on a sterile bench. The caudate lobe was perfused with perfusion solution A (140 mm NaCl, 6·7 mm KCl, 10 mm HEPES, 2·5 mm glucose and 0·5 mm EDTA in the final volume of 1 l, pH was adjusted to 7·4, 37 °C) to wash the bloodstains on the surface in order to reveal the blood vessel on the cross-section of the caudate process. Then the caudate lobe was perfused with perfusion solution B (140 mm NaCl, 6·7 mm KCl, 30 mm HEPES, 2·5 mm glucose and 5 mm CaCl2, pH was adjusted to 7·4, 37 °C) at the rate of 50 ml/min for 3 min. When the liquid became clear, it was time to start the second step of perfusion digestion with perfusion solution C (500 mL perfusion solution B plus 100 mg collagenase IV, 37 °C) at the rate of 20 mL/min. After digestion, 100 mL of basic medium with 0·2% calf serum was added and the liver capsule, blood vessels, fat and connective tissue were removed. Any parts of the liver caudate lobe that were incompletely digested were cut away and the liver amicula was torn by forceps. The hepatocyte suspension obtained was washed twice with basic medium (4 °C) and centrifuged for 10 min at 500 rpm before re-suspension in adherent medium. Viability counts were obtained by trypan blue staining and cell enumeration using a haemocytetometer. Cell density was adjusted to 2 × 106 cells/ml with adherent culture medium and 12 ml hepatocytes suspension were seeded into a 6-well tissue culture plate (2 ml/well) and incubated at 37 °C in 5% CO2. After a 4-h attachment period, the medium and unattached cells were separated and replaced with growth medium containing 5% fetal cows serum. Every 24 h, the medium was replaced with fresh medium. Culturing hepatocytes were observed every day under a phase contrast microscope and the status of adherence and the morphological changes were compared. Before treatment with glucose or ACAC, cells were starved of serum overnight. There was a period of 12 h glucose-free medium culture before bovine hepatocytes were treated with glucose.
ACAC and glucose treatment
After bovine hepatocytes were cultured for 72 h, ACAC (Sigma Aldrich, St Louis, Mo, USA, 0–4·8 mm) and glucose (Sigma Aldrich, St Louis, Mo, USA, 0–5·55 mm) with or without PDTC (Sigma Aldrich, St Louis, Mo, USA, 10 µm) were added to the culture medium (RPMI-1640 medium plus 10% foetal calf serum) respectively, with three replicates in one group. After pretreatment with PDTC for 1 h, bovine hepatocytes were cultured for 24 h, supernatant was centrifuged at 1000 g for 10 min and stored at −80 °C for further analysis.
Real-time fluorescence quantitative PCR (qRT-PCR)
Total RNA was extracted from hepatocytes using RNAiso Plus (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) according to the instructions and was transcribed into cDNA using reverse transcription kit (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China). The expression levels of mRNA were quantified using SYBR Premix Ex Taq II(TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) on an ABI Prism 7500 (Applied Biosystems, USA). Relative expression levels of IL-1β, IL-6 and TNF-α mRNA were analysed by 2−ΔΔct, using beta actin as the reference. The primers for IL-1β, IL-6, TNF-α and β-actin are shown in Table 1 (Shi et al. Reference Shi, Li, Deng, Li, Sun, Yuan, Song, Wang, Li and Li2015).
Table 1. The primers sequences of the genes

Cytokine secretion in cell culture supernatant
The IL-6, IL-1β and TNF-α concentrations in cell culture supernatant were determined using commercially available kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China), according to the manufacturer's instructions.
Western blotting
Western blotting was performed as described (Li et al. Reference Li, Li, Yang, Xiao, Fu, Deng, Ding, Wang, Liu and Li2014; Shi et al. Reference Shi, Li, Deng, Li, Sun, Yuan, Song, Wang, Li and Li2015) with primary antibodies against NF-κB p65 (Abcam, Cambridge, MA), phospho-IκBα (Cell Signalling Technology, Danvers, MA), IκBα (Cell Signalling Technology, Danvers, MA), histone (Santa Cruz, CA, USA) and β-actin (Santa Cruz, CA, USA). The appropriate peroxidase-conjugated secondary antibodies were used. An immunodetection analysis was performed using an enhanced chemiluminescence solution (ECL, Pierce Biotechnology Inc., Chicago, IL, USA).
Statistical analysis
Dates are expressed as means ± sd. Dates analysis was performed using SPSS17.0 (SPSS Inc, Chicago, IL, USA). Groups were compared by one-way analysis of variance followed by the Duncan's LSD test. P < 0·05 was considered to be significant and P < 0·01 was considered to be highly significant.
Results
Increased cytokines secretion induced by ACAC and glucose in bovine hepatocytes
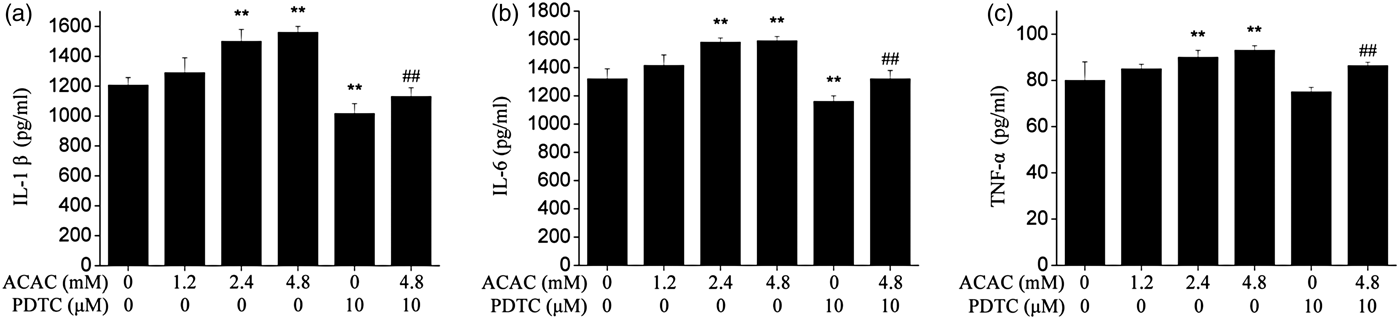
As shown in Fig. 1, the secretion of IL-1β, IL-6 and TNF-α increased in an ACAC dose-dependent manner and was significantly higher in 2·4 and 4·8 mm (P < 0·01) ACAC-treated groups than in the control group. Meanwhile, pretreatment with NF-κB inhibitor PDTC could alleviate this effect.

Fig. 1. Effect of different concentration of ACAC on secretion of IL-1β (a), IL-6 (b), and TNF-α (c) in cows hepatocytes. Hepatocytes (approximately 2 × 106 cells/well) were incubated in RPMI-1640 medium in the absence or presence of ACAC for 24 h. Cells which are not treated were considered as controls. The data shown were the Means ± sd of 3 replications in each treatment with 3 samples. Difference in values marked * were statistically significant (P < 0·05). Note that secretion of cytokines increased with increasing concentrations of ACAC.
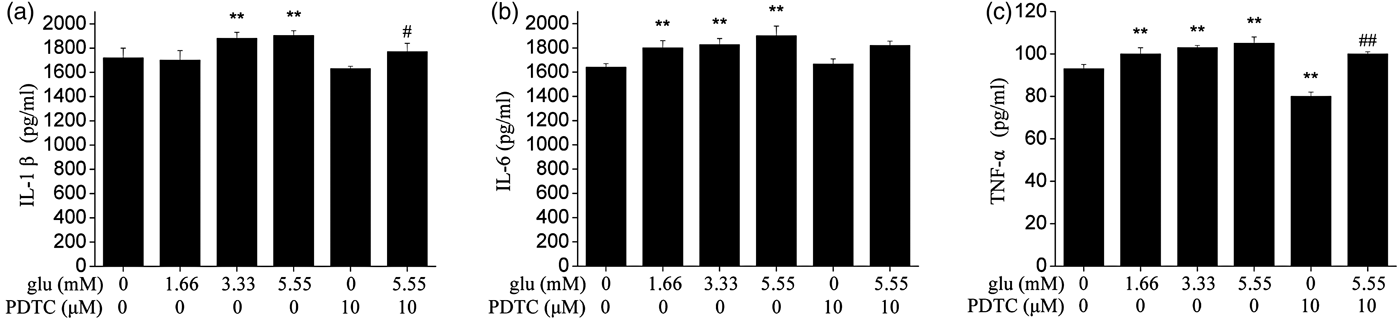
As shown in Fig. 2, compared with control group, the secretion of IL-1β increased significantly in 3·33 and 5·55 mm (P < 0·01) glucose-treated groups. The secretion of IL-6 and TNF-α increased in a glucose dose-dependent manner and was significantly higher in 1·66, 3·33 and 5·55 mm (P < 0·01) glucose-treated groups than in the control group. Meanwhile, the effect induced by glucose was alleviated after pretreatment with NF-κB inhibitor PDTC.

Fig. 2. Effect of different glucose concentrations on secretion of IL-1β (A), IL-6 (B), and TNF-α (C) in cows hepatocytes. Hepatocytes (approximately 2 × 106 cells/well) were incubated in RPMI-1640 medium in the absence or presence of glucose for 24 h. Cells which were not treated were considered as controls. The data shown were the Means ± sd of 3 replications in each treatment with 3 samples. Difference in values marked * were statistically significant (P < 0·05).
Increased cytokines mRNA induced by ACAC and glucose in bovine hepatocytes
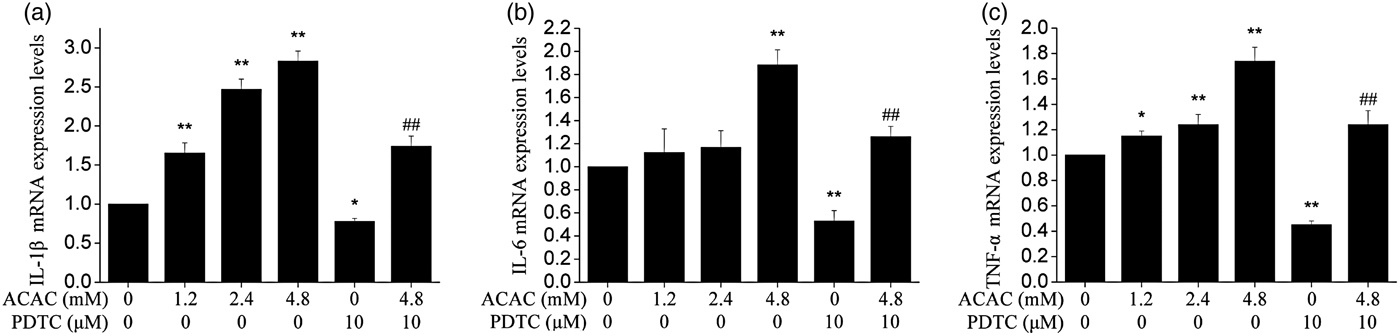
As shown in Fig. 3, compared with control group, the mRNA expression of IL-1β and TNF-α was significantly higher in the 1·2, 2·4 and 4·8 mm (Pp < 0·01) ACAC-treated groups than in the control group. The mRNA expression of IL-6 was increased in an ACAC dose-dependent manner and was significantly higher only in 4·8 mm ACAC-treated groups than in the control group. Meanwhile, pretreatment with NF-κB inhibitor PDTC could also alleviate this effect.

Fig. 3. Effect of different concentrations of ACAC on expression levels of IL-1β (a), IL-6 (b), and TNF-α (c) mRNA in cows hepatocytes. Cells with no treatment were considered as controls. The data shown were the Means ± sd of 3 replications in each treatment with 3 samples. Difference in values marked * were statistically significant (P < 0·05) vs. control. Note that expression levels of cytokines increased with increasing concentrations of ACAC.
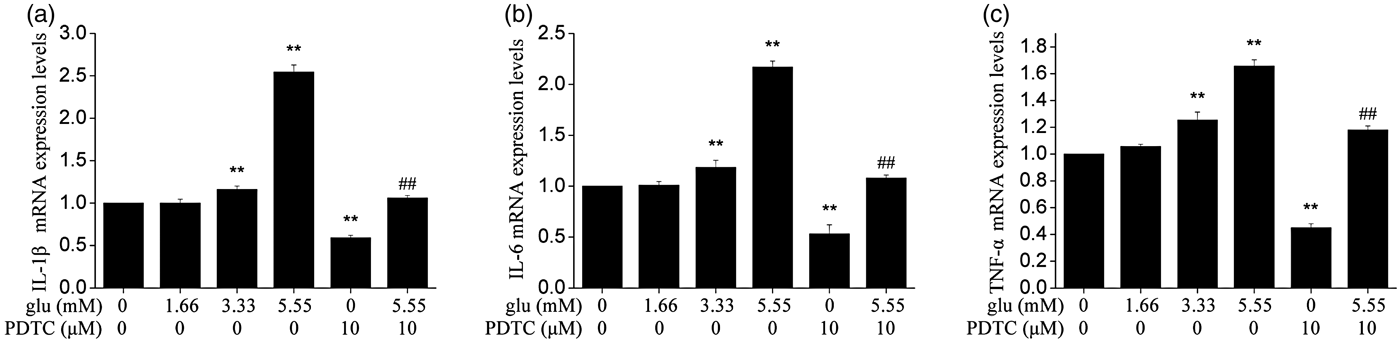
As shown in Fig. 4, compared with control group, the mRNA expression of IL-1β, IL-6 and TNF-α was significantly higher in both 3·33 and 5·55 mm (P < 0·01) glucose-treated group than in the control group. Meanwhile, the effect induced by glucose was alleviated after pretreatment with NF-κB inhibitor PDTC.

Fig. 4. Effect of different glucose concentrations on expression levels of IL-1β (a), IL-6 (b), and TNF-α (c) mRNA in cows hepatocytes. Cells with no treatment were considered as controls. The data shown were the Means ± sd of 3 replications in each treatment with 3 samples. Difference in values marked * were statistically significant (P < 0·05) vs. control. Note that expression levels of cytokines increased with increasing concentrations of glucose.
NF-κB pathway activation induced by ACAC and glucose in bovine hepatocytes
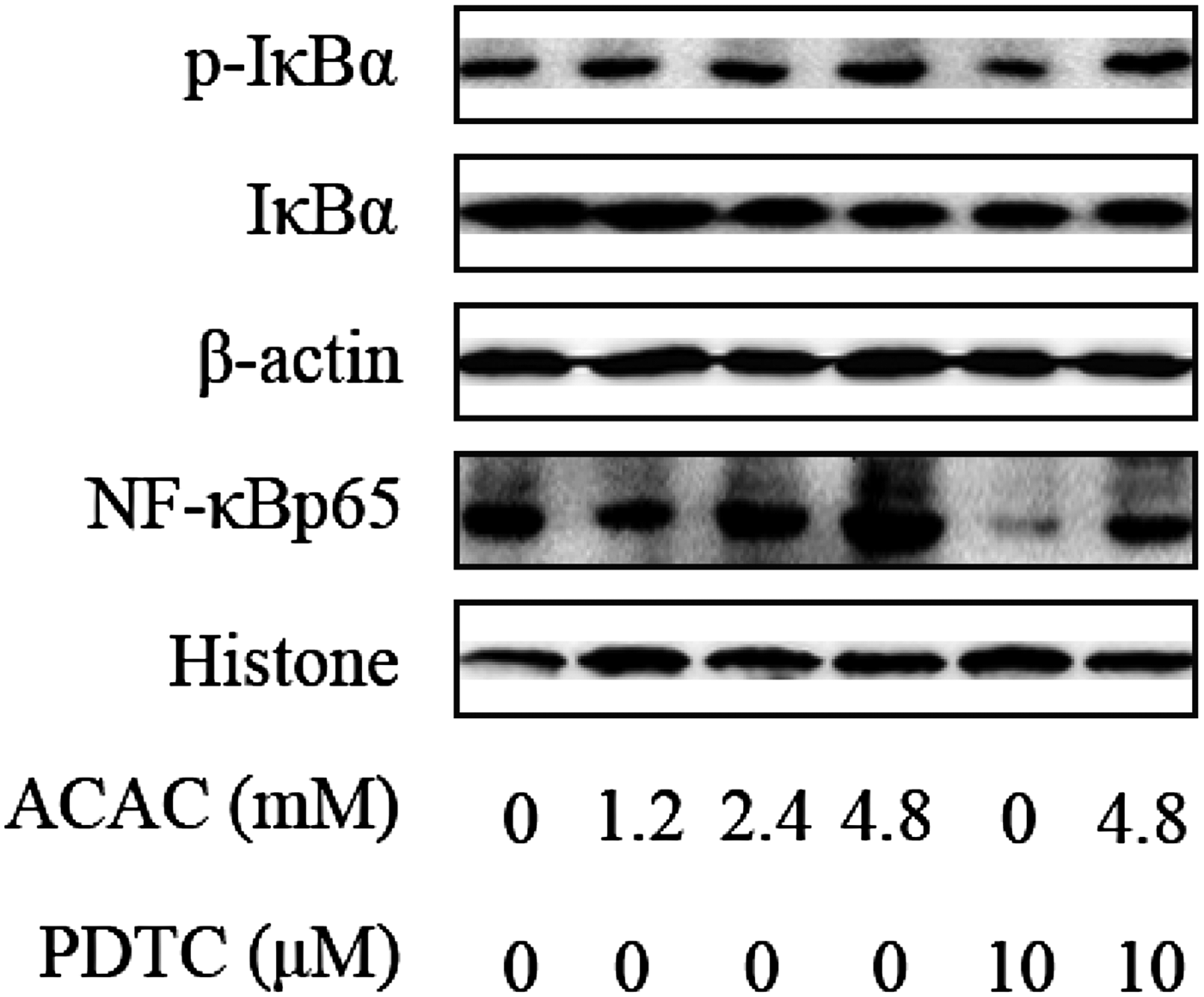
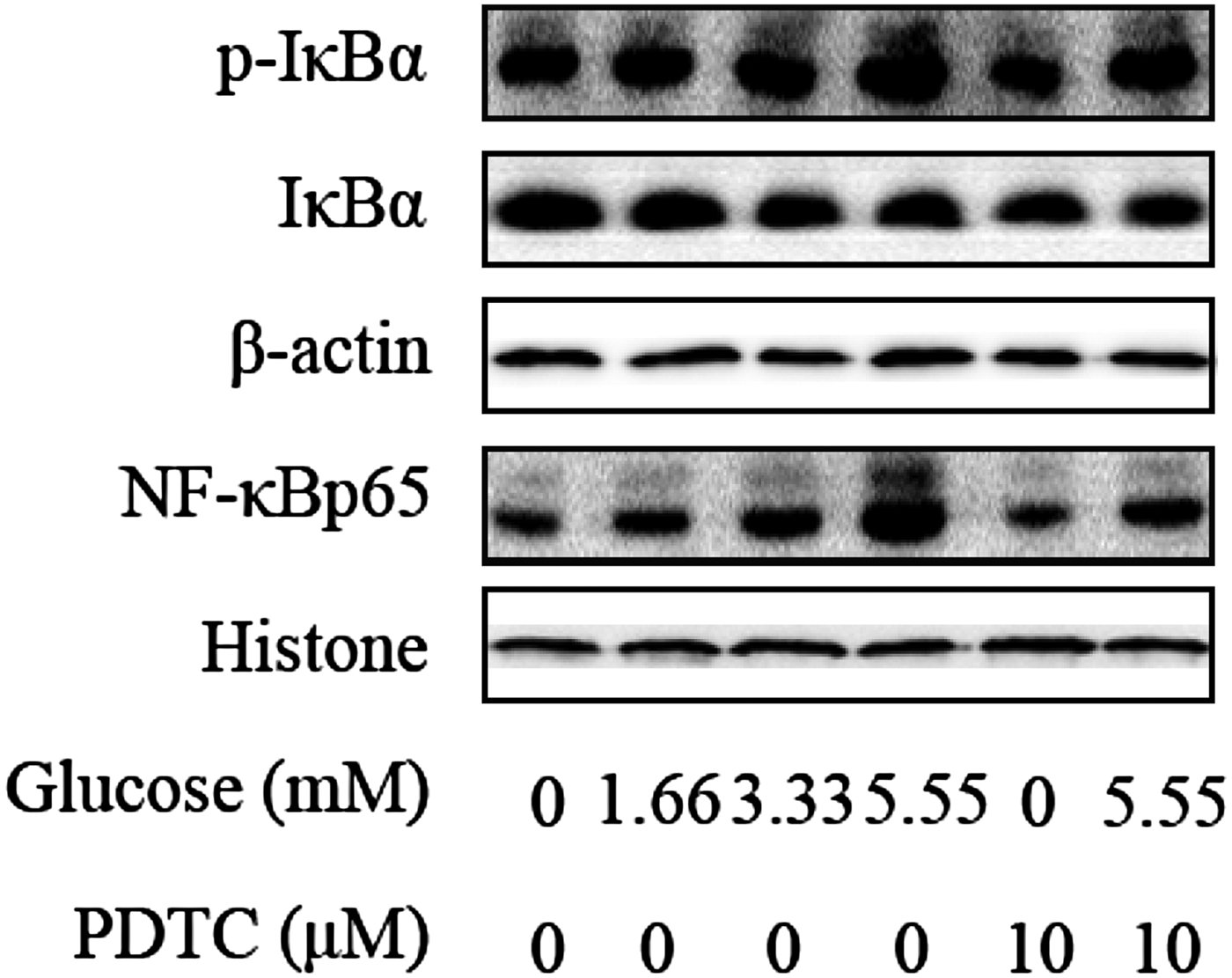
The protein expression levels of NF-κB and IκBα were detected by Western Blotting in order to examine the role of ACAC and glucose on the inflammatory response in dairy cows hepatocytes. Compared with control group, the IκBα phosphorylation levels were significantly increased in high concentrations of ACAC, glucose and lower in PDTC-ACAC (4·8 mm) and glucose (5·55 mm) treated groups (Figs 5, 6). Furthermore, the NF-κB p65 protein levels were also markedly higher in ACAC and glucose-treated groups than in control group. Compared with ACAC (4·8 mm) and glucose (5·55 mm) treated groups, the protein levels of NF-κB p65 were decreased significantly in PDTC-ACAC (4·8 mm) and glucose (5·55 mm) treated groups (Figs. 5, 6). These results show that high concentrations of ACAC and glucose could activate the NF-κB pathway in dairy cow hepatocytes.

Fig. 5. ACAC activate NF-κB signalling pathway in cows hepatocytes. Cells were treated with 0, 1·2, 2·4 and 4·8 mm ACAC with or without 10 µm PDTC. Western blots results of p-IκBα, IκBα and NF-κBp65. β-actin and Histone were used as internal control, respectively.

Fig. 6. Glucose activate NF-κB signalling pathway in cows hepatocytes. Cells were treated with 0, 1·66, 3·33 and 5·55 mm glucose with or without 10 µm PDTC. Western blots results of p-IκBα, IκBα and NF-κBp65. β-actin and Histone were used as internal control, respectively.
Discussion
The period from 21 d before and after calving in dairy cows is defined as transition period (Wang et al. Reference Wang, Li, Zhao, Hu, Chen, Liu, Liu and Wang2012). This period is characterised by NEB caused by the lower DMI (intake of dry matter) and increased demand for foetal development and lactation, with increased risk of metabolic disorders. As a metabolic disease, ketosis has association with excessive NEB with high levels of ketone bodies (ACAC, BHBA and acetone) and low concentration of blood glucose (Grummer, Reference Grummer1995; Li et al. Reference Li, Li, Bai, Chen, Deng, Liu, Zhang, Liu and Wang2012). Previous studies have shown that concentrations of ACAC and glucose in dairy cows with clinical ketosis are above 1 mm and below 2·5 mm, respectively (Zdzisińska et al. Reference Zdzisińska, Filar, Paduch, Kaczor, Lokaj and Kandefer-Szerszeń2000; Xu et al. Reference Xu, Liu, Li, Xia, Zhang and Wang2010; Li et al. Reference Li, Li, Bai, Chen, Deng, Liu, Zhang, Liu and Wang2012). Therefore, 0, 1·2, 2·4 and 4·8 mm ACAC and 0, 1·66, 3·33 and 5·55 mm glucose were chosen to study the effects of high levels of ACAC and glucose on the inflammatory cytokines in bovine hepatocytes.
In transition cows, inflammation may be regarded as a missing link in the aetiology of metabolic disorders (Xu et al. Reference Xu, Liu, Li, Xia, Zhang and Wang2010). Recently, increasing attention has been given to the infectious or metabolic disorder which caused the release of proinflammatory cytokines in cows during parturition (Drackley et al. Reference Drackley, Dann, Douglas, Guretzky, Litherland, Underwood and Loor2005). Inflammatory cytokines plays an important role in response to infectious diseases as well as in catabolic effects on metabolism. They promote the breakdown of stored fat in a manner of decreasing feed intake, impairing insulin sensitivity and stimulating lipolysis directly. However, conditions mentioned above have association with ketosis. Studies have indicated that TNF-α activity in serum increased from moderate to severe fatty liver (Ohtsuka et al. Reference Ohtsuka, Koiwa, Hatsugaya, Kudo, Hoshi, Itoh, Yokota, Okada and Kawamura2001). Elevated concentrations of plasma TNF-α, IL-1β and IL-6 have been reported in dairy cows with mastitis (Bannerman et al. Reference Bannerman, Paape, Lee, Zhao, Hope and Rainard2004). In the present study, the secretion and mRNA of IL-6, IL-1β and TNF-α increased in a concentration-dependent manner and increased significantly when the ACAC concentrations exceeded 1·2 or 2·4 mm. These results indicate that high levels of ACAC in hepatocytes promote both the secretion and expression of these proinflammatory cytokines. Our results are similar to the report that 1 mm ACAC influenced lymphoproliferation (Targowski & Klucinski, Reference Targowski and Klucinski1983), which is consistent with our results that high concentrations ACAC promote the production of inflammatory factors in bovine hepatocytes. Moreover, both the mRNA and protein levels increased significantly in either milk or mammary tissues in the infected mammary glands (Hichem et al. Reference Hichem, Etienne, Pascal and Céline2007). These results are different from studies that ACAC have no significant influence on the production of bovine aorta endothelial cells (Zdzisińska et al. Reference Zdzisińska, Filar, Paduch, Kaczor, Lokaj and Kandefer-Szerszeń2000). It is likely that different reports result from the different cellular model or induction time. In the periparturient period, cows with a larger NEB had a more pronounced inflammatory status (Bertoni et al. Reference Bertoni, Trevisi, Han and Bionaz2008). However, immunocompetence of cows was impaired by higher ketones in early lactation (Zhang et al. Reference Zhang, Xue, Gao, Liu, Wang, Yao, Liu, Li, Li and Liu2013). These conflicting results indicate that the regulatory mechanism of ACAC on cytokines in dairy cows with ketosis is still unclear.
Since low glucose is proposed as a significant sign of ketosis, we determined the effect of different concentrations of glucose on the inflammatory cytokine production. However, most studies focus on the effect of the high glucose concentrations on the cytokines. In vitro and in vivo studies have shown that high concentrations glucose promote inflammatory response in adipocytes (Lin et al. Reference Lin, Berg, Iyengar, Lam, Giacca, Combs, Rajala, Du, Rollman and Li2005). Moreover, hyperglycaemia induces the production of multiple inflammatory cytokines through key signalling pathway (Shanmugam et al. Reference Shanmugam, Reddy, Guha and Natarajan2003). In the present study, there are no significant changes of IL-1β, IL-6 and TNF-α in bovine hepatocytes treated with low concentrations of glucose (glucose concentration below 2·5). The IL-1β, IL-6 and TNF-α mRNA expression and secretion were higher when the glucose concentrations exceeded 1·66 or 3·33 mm, which may result from some signalling pathways being activated by the high glucose. These data are consistent with other reports (Jain et al. Reference Jain, Rains and Croad2007), implying that high concentrations of glucose can induce inflammatory injury in dairy cows. Glucose induced inflammatory responses depend on the toll-like receptor expression and nuclear factor-κB (NF-κB) transcription factor signalling pathway (Dasu et al. Reference Dasu, Devaraj, Zhao, Hwang and Jialal2008).
In order to further clarify the mechanism of increased inflammatory cytokines induced by high concentrations of ACAC and glucose, the protein expression levels of NF-κB and IκBα were detected in bovine hepatocytes. In our previous studies, we have found that high levels of BHBA and non-esterified fatty acids (NEFAs) promote inflammatory response by activating the NF-κB signalling pathway (Shi et al. Reference Shi, Li, Li, Li, Song, Deng, Wang, Zhang, Ding and Yin2014, Reference Shi, Li, Deng, Li, Sun, Yuan, Song, Wang, Li and Li2015). These results explain the inflammatory injury mechanism by BHBA and NEFAs in ketotic cows. ACAC and glucose are also the indicator of dairy cows ketosis. Therefore, inflammatory mechanism by ACAC and glucose was detected in the present study. Indeed, our results suggest that ACAC and glucose significantly increase NF-κB protein expression and IκBα phosphorylation levels which may provide, at least in in vitro studies, a more comprehensive explanation for the liver damage of ketotic dairy cows.
Conclusion
The present results suggest that high concentrations of ACAC and glucose increase the expression and secretion of inflammatory cytokines by activating NF-κB signalling pathway in bovine hepatocytes, thereby inducing the inflammatory injury in ketotic dairy cows. However, in vivo studies are needed to determine the mechanism of inflammatory liver damage in ketotic dairy cows.
This work was supported by the Program for New Century Excellent Talents in University (NCET-11-0199), the National Key Technology R&D Program (grant no. 2012BAD12B03) and the National Natural Science Foundation of China (Beijing, China; grant nos. 31201961, 31272621, 31402265, 31502136, 31472247 and 31460681).
None.