Introduction
Weeds are commonly managed by directing a herbicide spray to plant foliage. The ultimate goal is to contact the target plant in a way to maximize both spray coverage and droplet retention on the leaf (Dorr et al. Reference Dorr, Kempthorne, Mayo, Forster, Zabkiewicz, McCue, Belward, Turner and Hanan2014). In terrestrial pesticide applications, spray solution retention on plants can range from 10% to 100% (Zabkiewicz Reference Zabkiewicz2007). This large range is due to the multiple factors that affect spray retention, which include, but are not limited to, target plant leaf angle, size, shape, density, surface area, roughness, and wettability, as well as spray solution properties and spray droplet physics (Dorr et al. Reference Dorr, Kempthorne, Mayo, Forster, Zabkiewicz, McCue, Belward, Turner and Hanan2014; Furmidge Reference Furmidge1962; Gossen et al. Reference Gossen, Peng, Wolf and McDonald2008; Hess and Falk Reference Hess and Falk1990; Journaux et al. Reference Journaux, Simon, Destain, Cointault, Miteran and Piron2011; Massinon et al. Reference Massinon, Boukhalfa and Lebeau2014; Wirth et al. Reference Wirth, Storp and Jacobsen1991). In most weed management systems, spray solution that is not retained on the target plant is considered waste. However, spray runoff in aquatic systems enters the water column and can be a point of discussion for public stakeholders, regulatory agencies, and special interest groups. Consequently, maximized spray retention in aquatic foliar applications could improve efficacy and reduce aqueous herbicide levels during foliar treatments.
Spray coverage and retention can be altered by manipulating droplet size, carrier volume, and addition of an adjuvant (Bruns and Nalewaja Reference Bruns and Nalewaja1998; Knoche Reference Knoche1994). Adjuvants are nonpesticidal compounds used to improve herbicide effectiveness. Though there are many types, ranging from surfactants to oils, they primarily work to improve herbicide efficacy by increasing penetration of the leaf cuticle and decreasing droplet surface tension and thereby increasing area overage per unit volume and decreasing droplet bounce or roll-off (Hartzler and Foy Reference Hartzler and Foy1983; Monaco et al. Reference Monaco, Weller and Ashton2002). However, not all adjuvants affect herbicide deposition and efficacy equally, as some herbicides have been shown to provide differential levels of weed control depending on the adjuvant used (Jordan et al. Reference Jordan, Vidrine, Griffin and Reynolds1996; Scott et al. Reference Scott, Shaw, O’Neal and Klingaman1998). Considering the diversity of commercially available adjuvants, additional research is required to better understand how these products impact spray retention from foliar applications. Most state regulatory agencies limit the number of adjuvants approved for use in aquatic sites, based on ingredients that may be harmful to nontarget aquatic biota, and the list of such adjuvants is much shorter than those used in terrestrial applications (Haller and Stocker Reference Haller and Stocker2003).
Carrier volume, also referred to as spray volume by applicators, is another critical application parameter that can influence spray retention and, ultimately, efficacy. In general, spray coverage will increase as carrier volume increases, which is often noted on aquatic herbicide labels. Knoche (Reference Knoche1994) reported that spray volumes up to 100 L ha−1 improved herbicide efficacy, while decreased efficacy was observed when volumes increased to 400 L ha−1 in terrestrial weed species. This is likely due to concentrated herbicide spray solutions at lower carrier volumes, whereas more carrier (i.e., water) dilutes the herbicide and potentially increases runoff of spray solution. Previous research on carrier volume effects on herbicide efficacy for aquatic plant species has shown that decreased carrier volumes can increase efficacy, depending on the target species and herbicide being used (Van et al. Reference Van, Vandiver and Conant1986; Willard et al. Reference Willard, Shilling, Haller and Langeland1998; Nelson et al. Reference Nelson, Glomski and Gladwin2007; Sperry and Ferrell Reference Sperry and Ferrell2021). Additionally, Van et al. (Reference Van, Vandiver and Conant1986) reported that glyphosate applications to waterhyacinth exhibited 33% greater spray retention at a carrier volume of 467 L ha−1 compared to 935 L ha−1. Therefore we hypothesized that spray retention could be increased when applied across floating aquatic plant species by reducing carrier volume.
The floating invasive plants waterhyacinth [Eichhornia crassipes (Mart.) Solms], waterlettuce (Pistia stratiotes L.), and giant salvinia (Salvinia molesta D.S. Mitchell) are problematic in the United States and worldwide (Gettys et al. Reference Gettys, Haller and Petty2020). These invasive species primarily spread by vegetative reproduction, which forms extensive free-floating mats (not rooted to the sediment) and often interferes with water use, alters water quality (pH, dissolved oxygen, and light availability), and impacts fish and wildlife habitats (Weldon and Blackburn Reference Weldon and Blackburn1966; Mitchell and Tur Reference Mitchell and Tur1975; Harley et al. Reference Harley, Forno, Kassulke and Sands1984; Owens et al. Reference Owens, Smart, Honnell and Dick2005; Nelson Reference Nelson, Gettys, Haller and Petty2014; Horst and Mapes Reference Horst and Mapes2000). In Florida alone in 2019, more than 113,000 ha of waterhyacinth and waterlettuce received foliar herbicide treatments (FFWCC 2019), and approximately 7,200 ha of giant salvinia was chemically managed by the Louisiana Department of Wildlife and Fisheries during the same year (D. Hill, personal communication, February 4, 2020). Owing to their rapid growth, these three species likely account for the majority of foliar aquatic herbicide applications in the United States, as they are constantly managed at the lowest feasible level to minimize the negative impacts of these plants (FFWCC 2019; Joyce Reference Joyce1985). Focusing research efforts on the aforementioned target species will provide valuable information on and understanding of the occurrences of spray retention when treating these floating species.
Because increased herbicide efficacy is one of the primary goals of aquatic weed management, factors like adjuvant type and carrier volume manipulation should be investigated to determine the role of these factors in improving control of floating and emergent species. Efficacy has been determined using various carrier volume trials on multiple aquatic plants (Van et al. Reference Van, Vandiver and Conant1986; Willard et al. Reference Willard, Shilling, Haller and Langeland1998; Nelson et al. Reference Nelson, Glomski and Gladwin2007; Sperry and Ferrell Reference Sperry and Ferrell2021), but to minimize spray deposition into the water column, further evaluations are required on specific growth habits of various floating plants. Therefore the objectives of these studies were to investigate (1) the effect of adjuvant type on spray retention in waterhyacinth and (2) the influence of carrier volume spray retention in waterhyacinth, waterlettuce, and giant salvinia.
Materials and Methods
Site Description and Plant Establishment
A series of outdoor mesocosm experiments were conducted and repeated from May to August 2020 at the University of Florida Center for Aquatic and Invasive Plants in Gainesville, FL (29.721542°N, 82.417300°W) and at the Louisiana State University AgCenter Aquaculture Research Facility in Baton Rouge, LA (30.36805556°N, 91.183333°W). All plants were sourced from local cultures and transferred to mesocosms consisting of 96-L high-density polyethylene (HDPE) containers (61-cm diameter) at Gainesville or 76-L HDPE containers (50-cm diameter) at Baton Rouge. Aqueous growth media in mesocosms were prepared to provide healthy growth of floating plants. Mesocosms at Gainesville were filled with well water (pH 7.8) and were amended with water-soluble fertilizer (24-8-16, Miracle-Gro® All Purpose Plant Food, The Scotts Company, Marysville, OH) at 100 mg L−1 plus 10% chelated iron (Grow More Iron Chelate 10%, Grow More Inc., Gardena, CA) at 200 mg L−1. At Baton Rouge, mesocosms were filled with pond water (pH 7.5 to 8.0) and amended with water-soluble fertilizer (24-8-16, Miracle-Gro®) at 30 mg L−1. Giant salvinia mesocosms in Baton Rouge were amended with sphagnum moss (200 mg L−1 dry material) to lower water pH to <7.0 (Cary and Weerts Reference Cary and Weerts1984; Owens et al. Reference Owens, Smart, Honnell and Dick2005). Zeta-cypermethrin (GardenTech Sevin Insect Killer Concentrate, TechPac LLC, Atlanta, GA) was applied to all plants as needed for herbivorous insect control.
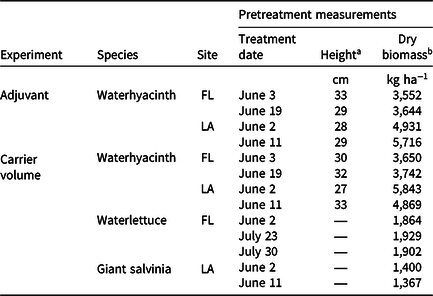
All experiments were set up as completely randomized designs with four replications. Regardless of species, mesocosms were filled with sufficient plants to provide 100% cover of the water surface, which resulted in densities similar to what is found under field conditions (Center and Spencer Reference Center and Spencer1981; Dewald and Lounibos Reference Dewald and Lounibos1990; Julien et al. Reference Julien, Center, Tipping, Van Driesche, Blossey, Hoddle, Lyon and Reardon2002; Table 1). Average plant densities for waterhyacinth, waterlettuce, and giant salvinia were 80, 18, and 120 plants m−2, respectively. Giant salvinia, which tends to form stacked layers of plants, was established and maintained as a single plant layer throughout the duration of the experiments.
Table 1. Dates, sites, and pretreatment measurements from mesocosm experiments conducted in 2020 investigating the effects of adjuvant types or carrier volume on rhodamine WT tracer dye spray retention in applications to floating plants in Gainesville, FL, and Baton Rouge, LA.
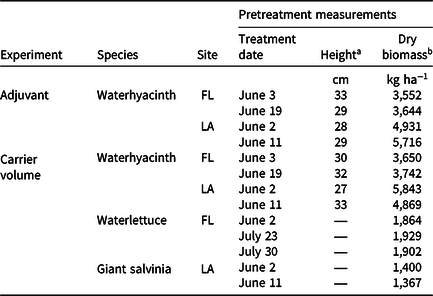
a Heights from five random waterhyacinth petioles within each experimental container were measured from the water surface to the leaf tip (tallest point). Waterlettuce and giant salvinia heights were not measured.
b Average plant densities for waterhyacinth, waterlettuce, and giant salvinia were 80, 18, and 120 plants m−2, respectively.
In all experiments, RWT (Rhodamine WT Liquid, Keystone Aniline Corp., Chicago, IL) was included in all spray solutions at 0.1% v/v to serve as a herbicide surrogate for rapid fluorometric tracing. The inert fluorescent dye RWT has been used for decades to simulate and trace herbicide movement in terrestrial and aqueous environments (Barber and Parkin Reference Barber and Parkin2003; Everts and Kanwar Reference Everts and Kanwar1994; Foster et al. Reference Foster, Sperry, Reynolds, Kruger and Claussen2018; Fox et al. Reference Fox, Haller and Getsinger1991a, Reference Fox, Haller and Shilling1991b, Reference Fox, Haller and Getsinger1993, Reference Fox, Haller, Getsinger and Petty2002; Fox and Haller Reference Fox and Haller1992; Getsinger et al. Reference Getsinger, Skogerboe, Madsen, Wersal, Nawrocki, Richardson and Sternberg2013; Langeland et al. Reference Langeland, Fox, Laroche, Martin, Martin, Norris and Wang1994; Roten et al. Reference Roten, Hewitt, Ledebuhr, Thistle, Connell, Wolf, Sankar and Woodward2013; Turner et al. Reference Turner, Getsinger and Netherland1994). Despite ranges in water pH among species and sites, Feuerstein and Selleck (Reference Feuerstein and Selleck1963) reported that RWT fluorescence was stable at pH 5 to 10. Additionally, containers at both sites were filled and maintained with the maximum water volume allowable to ensure minimal spray deposition on the container walls, which could lead to artifacts in spray retention data. In each experiment, observed dye concentrations in reference mesocosms were ±3% to 5% of the theoretical maximum concentration. This level of precision was considered acceptable because limited information is available on recovery rates in similar research.
Adjuvant Type
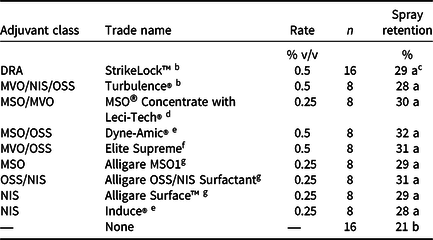
Waterhyacinth plants were arranged in each mesocosm to provide 100% coverage of the water surface, which equated to an average density of 80 plant m−2 in each experimental run. Treatment dates, average plant heights, and average dry biomass of experimental units are found in Table 1. Waterhyacinth at the Gainesville and Baton Rouge sites were sprayed with RWT dye (0.1% v/v) alone or in combination with one of nine commonly available adjuvants at the recommended use rate described in Table 2. All treatments at both sites were administered with a CO2-pressurized backpack sprayer equipped with XR11008 nozzles (TeeJet® nozzles, Spraying Systems Co., Wheaton, IL) at Baton Rouge and XR 11005 nozzles at Gainesville and calibrated to deliver 935 L ha−1. These application techniques simulated herbicide applications and are commonly used to apply herbicides in outdoor mesocosm settings. An additional treatment consisted of mesocosms without plants to serve as a control, which was used for maximum RWT concentrations. This experiment was conducted twice at each site for a total of four data sets.
Table 2. Adjuvants, rates, and numbers of observations from mesocosm experiments investigating the effect of adjuvant type on spray retention on waterhyacinth in Gainesville, FL, and Baton Rouge, LA. a
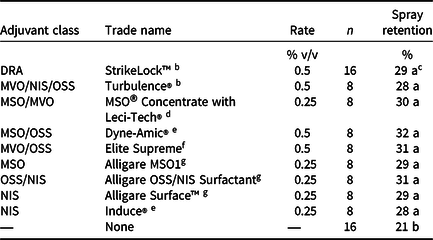
a Spray retention data are presented as a percentage of the applied rhodamine WT tracer dye rate (0.1% v/v). All treatments utilized a carrier volume of 935 L ha−1. Abbreviations: DRA, drift reducing agent; MVO, modified vegetable oil; MSO, methylated seed oil; NIS, nonionic surfactant; OSS, organosilicone.
b Winfield Solutions LLC, St. Paul, MN.
c Means followed by the same letter are not significant according to Fisher’s LSD test (α = 0.05).
d Loveland Products Inc., Greeley, CO.
e Helena Chemical Company, Collierville, TN.
f Red River Specialties Inc., Shreveport, LA.
g Alligare LLC, Opelika, AL.
Carrier Volume
The effect of carrier volume on spray retention on waterhyacinth was evaluated twice at each site. Waterlettuce was evaluated three times in Gainesville, whereas giant salvinia was evaluated twice in Baton Rouge (Table 1). Each species was assessed with identical treatments using the same methods, described later. Spray solution for every treatment consisted of RWT at 0.1% v/v plus a non-ionic surfactant at 0.25% v/v (Baton Rouge: Surface™, Alligare LLC, Opelika, AL; Gainesville: Induce®, Helena Chemical Company, Collierville, TN). Treatments consisted of application of the solution at 93, 234, 467, 935, or 1,870 L ha−1 to mesocosms with or without plants. Treatment of mesocosm without plants served as the control for each carrier volume. In Gainesville, treatments were applied with a CO2-pressurized backpack sprayer calibrated to deliver 93, 234, 467, 935, or 1,870 L ha−1 with XR 11001 (276 kPa), XR11003 (276 kPa), XR11003 (276 kPa), XR11006 (345 kPa), and XR11006 (345 kPa) nozzles and pressures, respectively. Likewise, treatments were applied in Baton Rouge with a CO2-pressurized backpack sprayer calibrated to carrier volumes of 93, 234, 467, 935, or 1,870 L ha−1 with XR11001 (276 kPa), XR11001 (276 kPa), XR11001 (276 kPa), XR11008 (207 kPa), and XR11015 (207 kPa) nozzles and pressures, respectively. According to the manufacturer, these spray nozzles likely resulted in fine to very coarse spray qualities (TeeJet Technologies 2014). Similar to the adjuvant experiment, plants were arranged to provide 100% cover of the water surface. This was equivalent to 80, 24, and 124 plants m−2 for waterhyacinth, waterlettuce, and giant salvinia, respectively.
Data Collection and Analysis
In all experiments prior to RWT dye application, background fluorescence was accounted for by measuring 15 cm below the water surface in each mesocosm using a calibrated handheld fluorometer (Cyclops-7F, Turner Designs Inc., San Jose, CA) at Gainesville or a multiparameter sonde (EXO1 Platform and Multiparameter Sonde, YSI Inc., Yellow Springs, OH) at Baton Rouge. In addition, heights of five random waterhyacinth petioles within each experimental container were measured from the water surface to the leaf tip (tallest point) prior to RWT dye application to collect baseline data on plant size across sites (Table 1).
Following dye applications, mesocosms remained undisturbed at least 1 h to allow time for the dye to dry prior to manual water column mixing. Water column mixing was achieved by inserting a submersible pump (MN404 MINI-Jet, Marineland, Spectrum Brands Pet LLC, Blacksburg, VA) halfway into the water column (ensuring to not disturb vegetation) and allowing the water to circulate for 1 min to ensure dye equilibrium. Immediately after mixing, dye concentrations were measured as previously described for pretreatment fluorescence. Both the submersible pump and fluorometer probes were thoroughly rinsed with clean water between experimental units to prevent cross-contamination. After all RWT dye concentrations were collected, all plants in each mesocosm were harvested and dried in an oven at 65 C to a constant weight to determine biomass for statistical analyses (Table 1).
Resultant RWT dye concentrations were adjusted for mesocosm volume and background fluorescence then normalized to the control treatment (treated mesocosms without plants), yielding percentage of applied dye not found in the water column (hereinafter referred to as % spray retention) using the following equation:
where Y is % spray retention, x is the posttreatment dye concentration in the water below treated plants, p is the background fluorescence, and c is the posttreatment dye concentration in mesocosms without plant material.
Data from the adjuvant experiment were subjected to a mixed-model ANOVA under the lme4 package in R (version 3.6.1; R Core Team 2019) where site, experimental run, and replicate (nested in site) were considered random effects, while adjuvant type was considered a fixed effect (Bates et al. Reference Bates, Maechler, Bolker and Walker2015; Blouin et al. Reference Blouin, Webster and Bond2011). Where significant effects were detected, means were separated using Fisher’s LSD test (α = 0.05) under the multcomp and emmeans packages in R (Hothorn et al. Reference Hothorn, Bretz and Westfall2008; Lenth Reference Lenth2020). All model assumptions were met.
Data from carrier volume experiments were analyzed under the nlme package in R (Pinheiro et al. Reference Pinheiro, Bates, DebRoy and Sarkar2019). Percentage spray retention was regressed over carrier volume (L ha−1) using the asymptotic mixed model
where Y was the response variable (% spray retention), Y asym was the asymptotic Y value (fitted Y value where slope approaches 0), I was the explanatory variable (L ha−1), and a was the logarithmic rate constant (initial slope of the curve at low I values). Appropriateness of model fit was evaluated by lack-of-fit tests at the 95% level (Ritz and Streibig Reference Ritz and Streibig2005). Regression parameters were compared using 95% confidence intervals, and figures were constructed under the ggplot2 package.
Results and Discussion
Adjuvant Type
No differences in spray retention were observed among the nine different adjuvants tested when applied to waterhyacinth (Table 2). However, RWT applied without an adjuvant resulted in 6% to 11% less spray retention than when an adjuvant was included (P = 0.02). This was likely due to high surface tension of spray solutions without adjuvants, resulting in higher droplet contact angles and greater propensity for spray droplet roll-off (Hazen Reference Hazen2000). Some adjuvants have been shown to increase herbicide absorption and efficacy and reduce droplet drying time when applied at volumes of 140 to 374 L ha−1 and fine to medium spray qualities (Jordan et al. Reference Jordan, Vidrine, Griffin and Reynolds1996; Roggenbuck et al. Reference Roggenbuck, Penner, Burow and Thomas1993; Singh and Mack Reference Singh and Mack1993). However, the current study suggests that any differences in herbicide efficacy on waterhyacinth among the adjuvants would not likely be due to spray retention when treatments are applied at 935 L ha−1 with fine to medium spray qualities. Similar to the current study, little to no differences in spray retention were observed among adjuvants applied in a carrier volume of 200 L ha−1 to field bean (Vicia faba L.), pea (Pisum sativum L.), or barley (Hordeum vulgare L.); however, without the use of an adjuvant, spray retention was much lower on these species as well (Holloway et al. Reference Holloway, Butler Ellis, Webb, Western, Tuck, Hayes and Miller2000). Yet treatments without an adjuvant only experienced 6% to 11% less spray retention in the current study. As described earlier, adjuvants are known to contribute to increased herbicide efficacy through mechanisms other than increased spray retention. Additionally, waterhyacinth was the only species tested in this experiment that has glabrous and upright leaves that are generally considered to have low wettability (Gaskin et al. Reference Gaskin, Steele and Forster2005; Hess and Falk Reference Hess and Falk1990; Penfound and Earle Reference Penfound and Earle1948). Future research should investigate these adjuvant effects on aquatic species with other leaf surface morphologies and plant architectures.
Carrier Volume
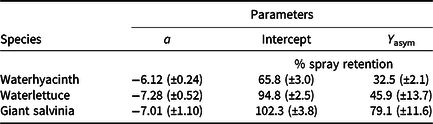
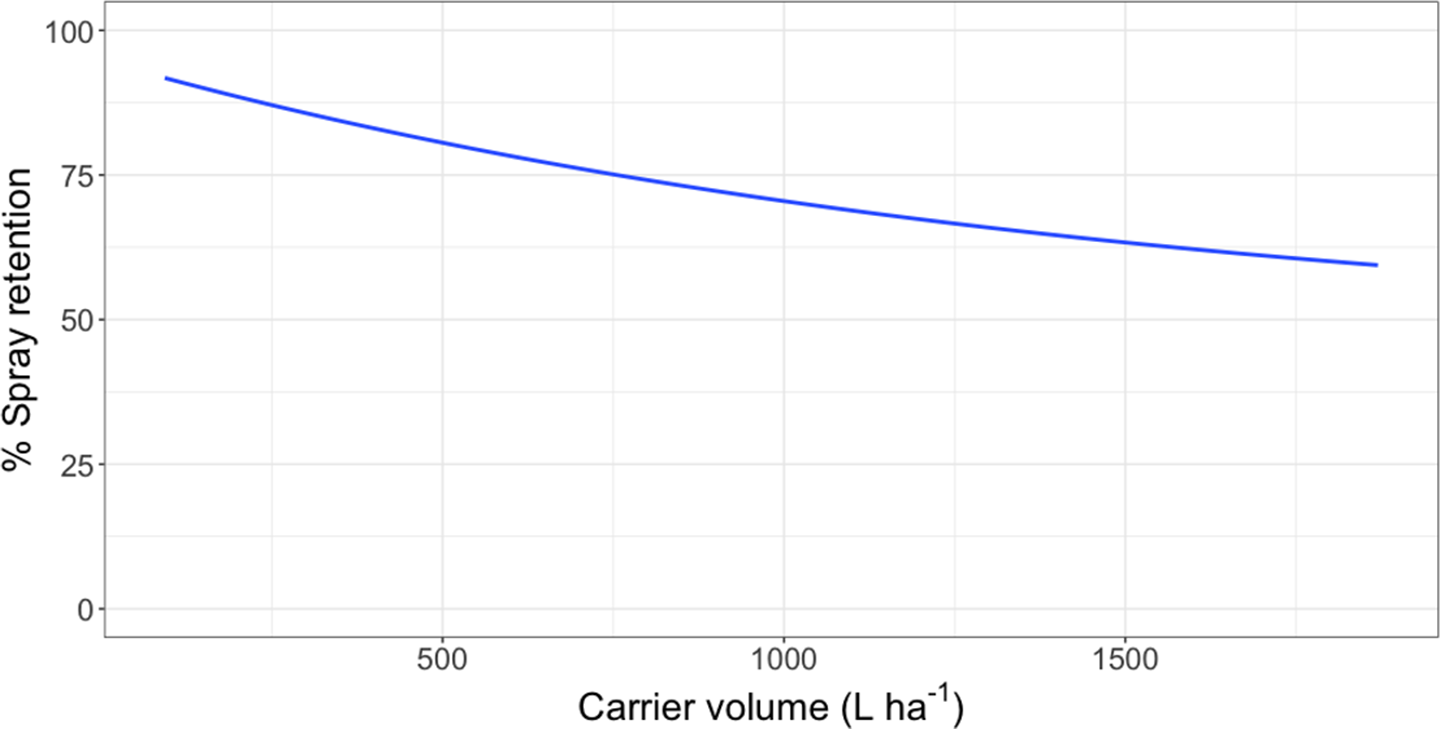
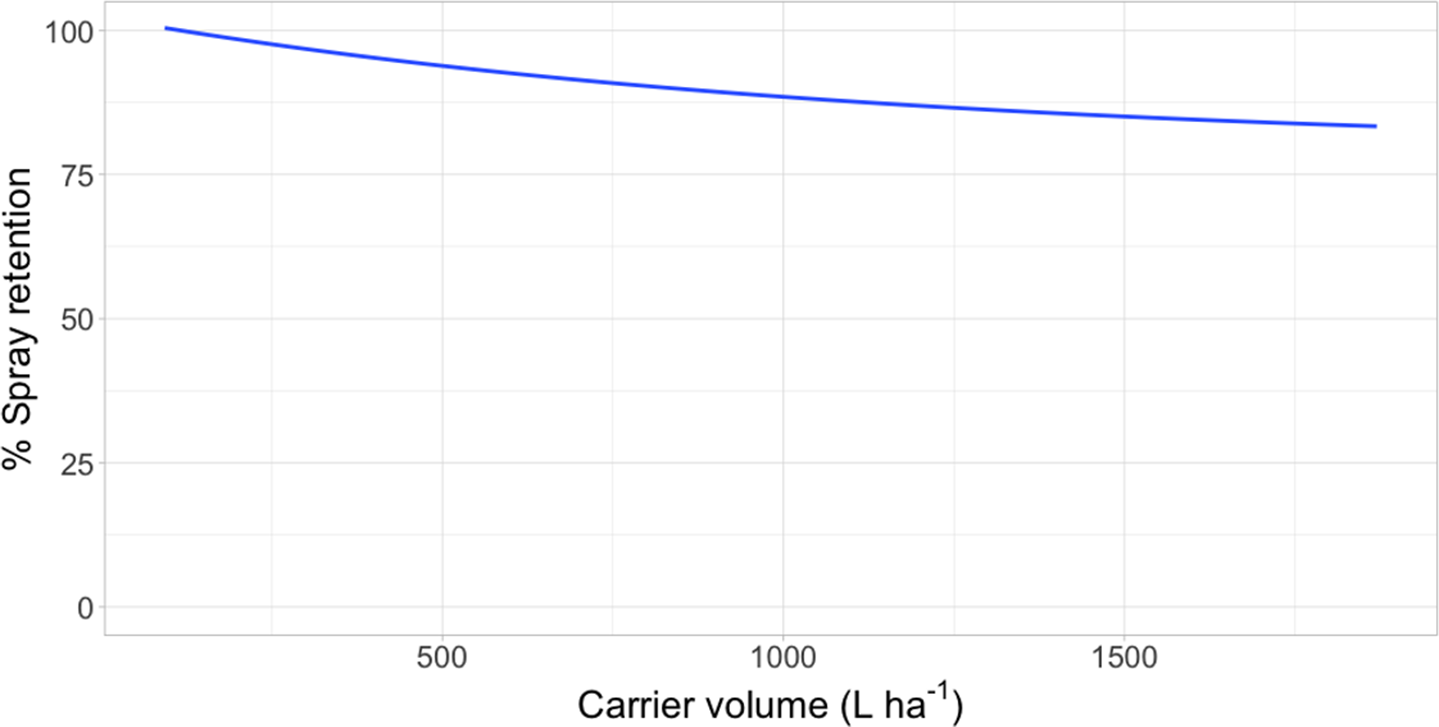
Lack-of-fit tests for all data were nonsignificant (P ≥ 0.32), indicating that model fit was appropriate. Logarithmic rate constants were similar for waterlettuce and giant salvinia; however, the logarithmic rate constant for waterhyacinth data was greater than for the two pubescent leaved species (Table 3; Figures 1 to 3). Owing to the type of regression model used, these logarithmic rate constants only reflect spray retention at low carrier volumes prior to approaching the asymptote on the fitted line (Equation 2; Figures 1 to 3). Furthermore, these rate constants are logarithmic, which equates to greater negative slopes that signify a flatter curve. The less negative values generated from waterhyacinth data likely reflect the glabrous leaf surfaces and more erect architecture, which would cause drastically reduced spray retention potential as carrier volumes increased (DPIRDAF 2020). Conversely, spray retention in giant salvinia and waterlettuce, which are both pubescent and comparatively less erect than waterhyacinth, was less affected by increases in carrier volume. The role of leaf pubescence in spray retention is 2-fold. First, pubescent leaves capture spray droplets more efficiently than glabrous leaves after impact due to a lowered tendency to rebound (bounce) or further fragment (Massinon et al. Reference Massinon, Boukhalfa and Lebeau2014). Second, spray droplets initially retained on pubescent leaves have increased sticking potential compared to those on glabrous leaves, from which they may roll off (Massinon et al. Reference Massinon, Boukhalfa and Lebeau2014).
Table 3. Regression parameters from mesocosm experiments investigating the effect of carrier volume on spray retention in floating plants using rhodamine WT tracer dye in Gainesville, FL, and Baton Rouge, LA. a
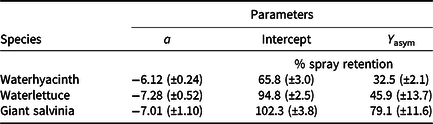
a Carrier volumes of 94, 234, 468, 935, and 1,870 L ha−1 were tested across each species. Regression model: Y = Y asym [1 − exp(−aI/Y asym)], where Y was the response variable (% spray retention), Y asym was the asymptotic Y value (fitted Y value where slope approaches 0 or the lowest spray retention observed at high carrier volumes), I was the explanatory variable (carrier volume in L ha−1), and a was the logarithmic rate constant (initial slope of the curve at low I values); 95% confidence intervals are given in parentheses.
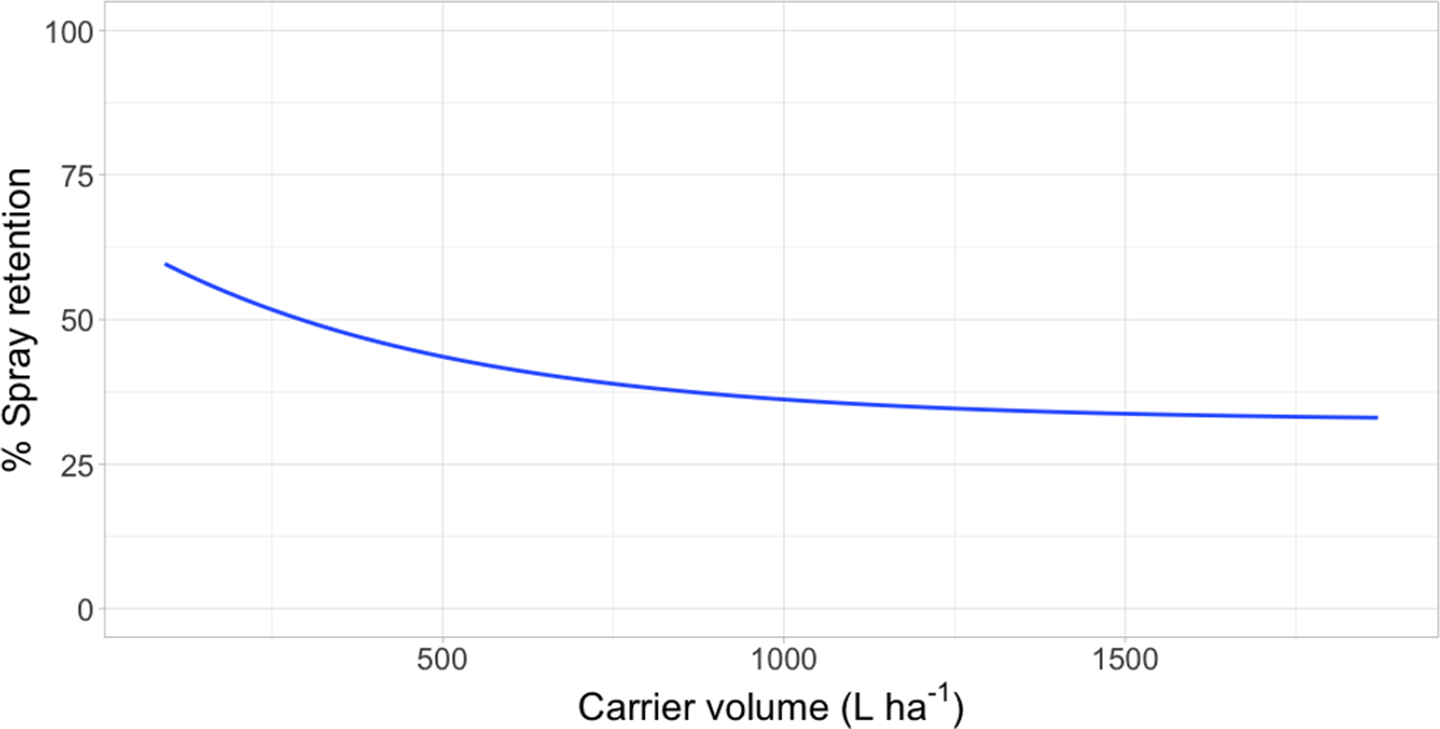
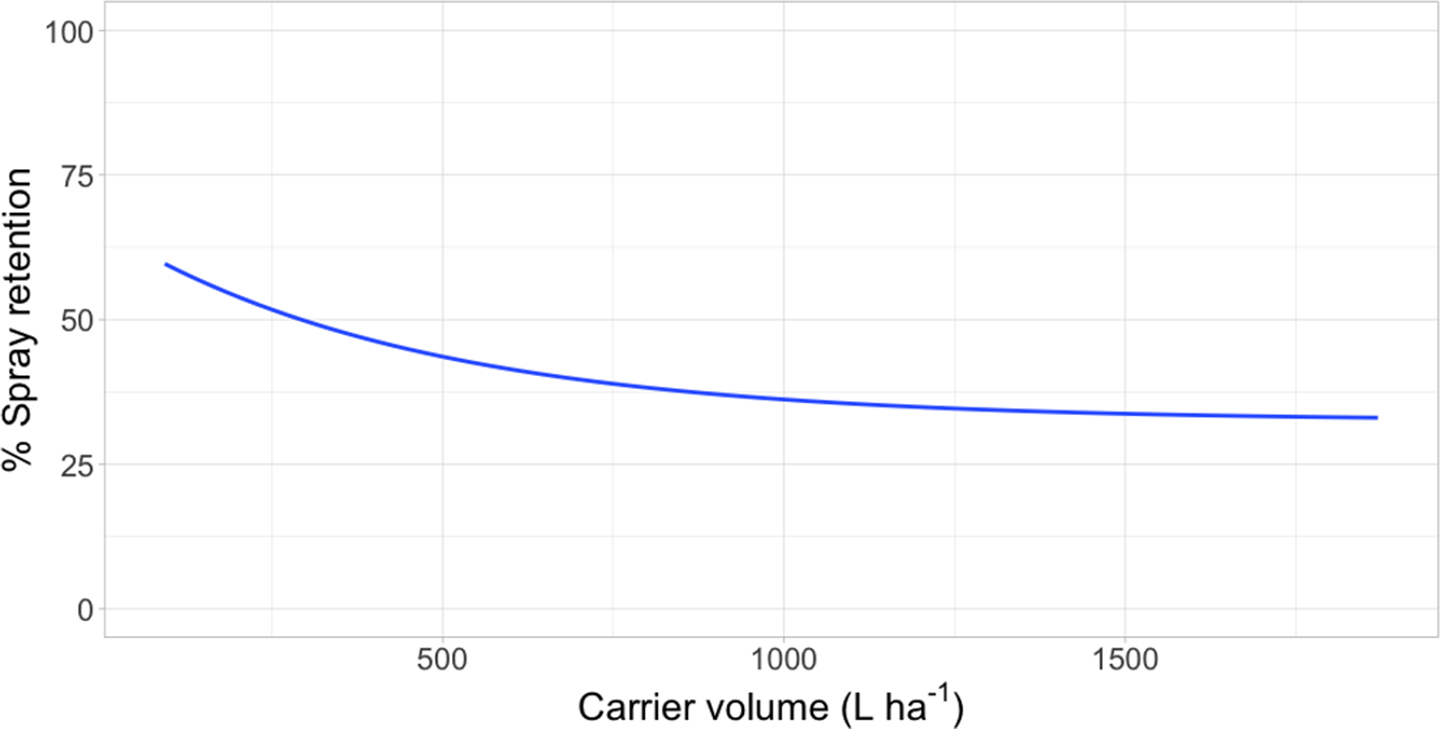
Figure 1. Spray retention on waterhyacinth as a function of carrier volume following foliar applications of rhodamine WR tracer dye in a mesocosm setting in Gainesville, FL, and Baton Rouge, LA. Regression model: Y = 32.5 [1 − exp(−6.12*I/32.5)], where Y was the response variable (% spray retention) and I was the explanatory variable (L ha−1).
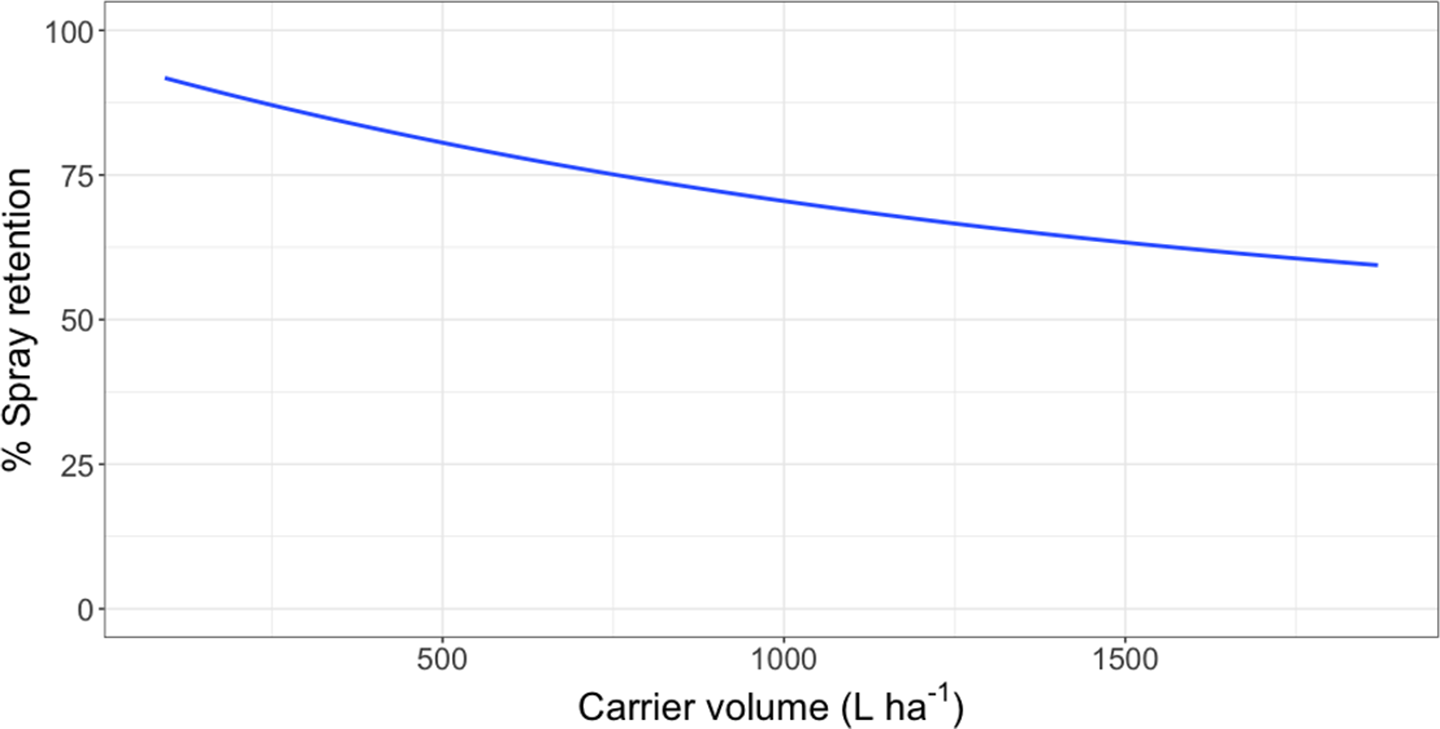
Figure 2. Spray retention on waterlettuce as a function of carrier volume following foliar applications of rhodamine WT tracer dye in a mesocosm setting in Gainesville, FL. Regression model: Y = 45.9 [1 − exp(−7.28*I/45.9)], where Y was the response variable (% spray retention) and I was the explanatory variable (L ha−1).

Figure 3. Spray retention on giant salvinia as a function of carrier volume following foliar applications of rhodamine WT tracer dye in a mesocosm setting in Baton Rouge, LA. Regression model: Y = 79.1 [1 − exp(−7.01*I/79.1)], where Y was the response variable (% spray retention) and I was the explanatory variable (L ha−1).
Intercept values were different for each species (Table 3). Giant salvinia resulted in the greatest intercept (102% spray retention) of the species tested, and waterhyacinth resulted in the lowest intercept (66% spray retention). These intercept values reflect the maximum spray retention observed based on lowest carrier volumes tested. Therefore, in the case of giant salvinia, spray retention potential is greater than for the other two species tested. Similarly, Mudge et al. (Reference Mudge, Sperry and Getsinger2021) reported that spray loss to the water column was essentially undetectable at a high density of giant salvinia (i.e., 100% coverage) compared to waterhyacinth and waterlettuce. Consequently, spray retention potential is likely greater when giant salvinia reaches the tertiary growth stage, completely covers the water surface, and grows vertically into multiple layers. However, herbicides applied to the foliage of giant salvinia that consists of several plant layers may result in poor control due to the inability of spray solution to penetrate and contact all foliage (Mudge et al. Reference Mudge, Perret and Winslow2016; Mudge and Sartain Reference Mudge and Sartain2018; Thomas and Room Reference Thomas and Room1986). Alternatively, the relatively low intercept value from waterhyacinth data suggests that spray applications to this species may be highly dependent on the three-dimensional surface area above water. In other words, we would hypothesize that taller plants retain more spray solution than shorter plants; however, this hypothesis would need to be tested in future experiments. Regardless, waterhyacinth populations that exist in similar sizes and densities as tested in the current study would likely benefit from foliar applications of herbicides that contain both foliar and in-water activity, as spray retention was <70% even at low carrier volumes. In-water applications, also known as subsurface or submersed applications, target uptake through the roots or foliage positioned on or below the water surface. Spray loss of foliar-applied herbicides that are biologically active in the water column may still contribute to controlling target species. While this relationship has not been described in the literature, a good example is the operational use of flumioxazin for waterlettuce control. Flumioxazin is commonly included in foliar applications to manage mixed stands of waterhyacinth and waterlettuce; however, it is also utilized for submersed applications to control waterlettuce at concentrations of 12 to 50 μg L−1 (UF 2021).
Asymptotes, or the lowest spray retention levels observed at greater carrier volumes, did not differ between waterlettuce (46% spray retention) and waterhyacinth (33% spray retention) (Table 3). However, giant salvinia resulted in an asymptote of 79% spray retention. This suggests that greater spray retention on giant salvinia plants was sustained over greater carrier volumes than for waterlettuce and waterhyacinth. Massinon et al. (Reference Massinon, Boukhalfa and Lebeau2014) reported that increased leaf surface angle resulted in reduced surface area available for droplet interception from spray droplets with downward trajectories. This supports the findings of the current study, as waterhyacinth’s high leaf surface angle retained less spray solution compared to the lower leaf surface angles of waterlettuce and giant salvinia.
A component that was not directly measured or tested in these experiments but likely contributed to the results is the effect of spray quality and droplet size. Because not all treatments were applied with nozzles of the same orifice size and operating pressure, spray quality between treatments differed. In other words, lower carrier volume treatments likely consisted of sprays containing smaller droplets (e.g., XR11001 nozzles) compared to high-volume treatments (e.g., XR11006 nozzles). Fine droplets tend to retain on leaf surfaces more than coarse droplets (Feng et al. Reference Feng, Sammons and Ryerse2003; Salyani Reference Salyani1988). Unfortunately, there is little known of the droplet sizes found in operational foliar treatments in aquatic systems; therefore we are unsure how any of the droplet sizes that exist in field applications compare to these data. Further research is planned to address these data gaps.
These data support the reduction of carrier volumes in foliar herbicide applications if increased spray retention and decreased spray loss to the water column are desired. However, the goal of each application should consider the spray retention potential of each species, application technique, and in-water activity of the herbicide chosen. Although boat-based foliar applications commonly occur at 935 L ha−1, this number can vary between 467 and 1,870 L ha−1, depending on the target species, herbicide (contact vs. systemic), application equipment, treatment site, and spot versus broadcast application. Conversely, aerial applications rely on ultra-low- to low-volume applications and typically deliver a maximum carrier volume of approximately 140 L ha−1. The data in this study suggest that aerial applications would promote greater spray retention and reduced spray loss relative to a standard high-volume hand-gun application. Furthermore, there is evidence of decreased carrier volumes resulting in increased efficacy of systemic herbicides in some aquatic species (Sperry and Ferrell Reference Sperry and Ferrell2021; Van et al. Reference Van, Vandiver and Conant1986). It has been speculated that those herbicides in aquatics that possess in-water activity require a fraction of the intended herbicide rate to be absorbed by below-water structures; however, this theory has not been directly tested (Sperry and Ferrell Reference Sperry and Ferrell2021). For other herbicides that do not exhibit in-water activity, such as glyphosate or imazapyr (UF 2018), it is assumed that any spray solution that is not retained on above-water plant material does not contribute to plant uptake through the water column and, ultimately, to death. Consequently, the goal of foliar applications, without relying on in-water activity, is to maximize the amount of herbicide retained on the foliage to result in sufficient control and limit spray loss to the water column.
An important implication of data collected from these experiments is the detection of spray solution entering the water column using a tracer dye rather than herbicides. Rhodamine WT is not known to be significantly absorbed by aquatic plants, whereas most herbicides are absorbed by aquatic plants (Turner et al. Reference Turner, Netherland and Getsinger1991). Consequently, these data serve as proof of concept or relative trends without herbicide quantification. Future research will attempt to identify other potential parameters that affect herbicide spray retention and to confirm these findings at the operational scale.
Acknowledgments
This research was supported by the U.S. Army Engineer Research and Development Center’s Aquatic Plant Control Research Program. The authors thank Bennett Judice and J. P. Keller for assistance in conducting experiments. This document was reviewed in accordance with U.S. Army Engineer Research and Development Center policy and approved for publication. Citation of trade names does not constitute endorsement or approval of the use of such commercial products. The authors declare no conflicts of interest.