Significant outcomes
-
This study showed increased C1q and decreased C4BP, C4d and C3a levels in narcolepsy, pointing to the fact that complement system factors in narcolepsy might predict disease severity.
-
Complement breakdown products may have a negative impact on sleep functions. Especially, C1q may be used as a potential indicator of worsening sleep quality and increased daytime sleepiness.
-
The role of other complement system factors in neuroinflammation and sleep physiology needs to be investigated.
Limitations
-
A limitation of our study is the absence of hypocretin level measurements and analysis of putative correlations between hypocretin, C4BP and anti-C4BPA levels.
-
Similarly, measurement of autoantibodies that are frequently observed in narcolepsy patients and correlation with complement levels could have provided informative insights.
Introduction
Narcolepsy is a chronic sleep disorder characterised by abnormal transition to rapid eye movements (REM) sleep phase and increased daytime sleepiness (Kornum et al., Reference Kornum, Knudsen, Ollila, Pizza, Jennum, Dauvilliers and Overeem2017). Hypocretin/orexin deficiency due to loss of hypocretinergic neurons is the main aetiology of the disease (Siegel, Reference Siegel1999; Mahoney et al., Reference Mahoney, Cogswell, Koralnik and Scammell2019). In narcolepsy type 1 (NT1), cataplexy and/or low levels of hypocretin in cerebral spinal fluid must be observed; whereas for narcolepsy type 2 (NT2) diagnosis, cataplexy must be absent (American Academy of Sleep Medicine, 2014).
Both humoral and cellular immune mechanisms seem to play a role in this selective loss of neurons in narcolepsy. Genetic evidences have emphasised the presence of human leukocyte antigen (HLA)-DQB1*06:02 allele for the differential diagnosis of narcolepsy (Manni et al., Reference Manni, Capittini, Pasi, De Silvestri, Terzaghi, Martinetti, Tinelli, Rebuffi and Scotti2018). In addition, many mutations in various immunity-related genes have been observed in narcoleptic patients (Singh et al., Reference Singh, Mahlios and Mignot2013; Degn et al., Reference Degn, Dauvilliers, Dreisig, Lopez, Pfister, Pradervand, Kornum and Tafti2017; Miyagawa & Tokunaga, Reference Miyagawa and Tokunaga2019; Pedersen et al., Reference Pedersen, Holm, Kristensen, Bjerregaard, Bentzen, Marquard, Tamhane, Burgdorf, Ullum, Jennum, Knudsen, Hadrup and Kornum2019; Postiglione et al., Reference Postiglione, Antelmi, Pizza, Vandi, la Morgia, Carelli, Nassetti, Seri and Plazzi2020). Autoreactive CD4+ and CD8+ T cells were also found to target-specific hypocretinergic neurons in HLA-associated narcolepsy cases (Luo et al., Reference Luo, Ambati, Lin, Bonvalet, Partinen, Ji, Maecker and Mignot2018; Pedersen et al., Reference Pedersen, Holm, Kristensen, Bjerregaard, Bentzen, Marquard, Tamhane, Burgdorf, Ullum, Jennum, Knudsen, Hadrup and Kornum2019). Recently, the critical importance of HLA characterisation in lower levels of effector memory CD4+ T cells observed in NT1 was mentioned in the literature (Viste et al., Reference Viste, Lie, Viken, Rootwelt, Knudsen-Heier and Kornum2021). Besides, proinflammatory cytokines, such as interleukin 6 and tumour necrosis factor-alpha, are effective in the sleep-wake cycle and are found at higher levels in patients with narcolepsy compared to healthy controls (Mohammadi et al., Reference Mohammadi, Mayeli, Saghazadeh and Rezaei2020). Moreover, increased hypocretin-1 reactive total immunoglobulin (Ig) G and IgM autoantibodies have been detected in NT1 patients’ sera (Deloumeau et al., Reference Deloumeau, Bayard, Coquerel, Déchelotte, Bole-Feysot, Carlander, De Cock, Fetissov and Dauvilliers2010; Nixon et al., Reference Nixon, Mavanji, Butterick, Billington, Kotz and Teske2015). This notion is supported by the presence of a plethora of additional autoantibodies in narcolepsy patients (Kornum, Reference Kornum2020). These findings suggest that different immune system mechanisms may partake in narcolepsy and the disease may have an autoimmune basis.
As one of the humoral mechanisms, complement system is particularly important in many autoimmune metabolic and central nervous system (CNS) diseases such as neuromyelitis optica, autoimmune encephalitis, myasthenia gravis (MG) and systemic lupus erythematosus (SLE) (Dalakas et al., Reference Dalakas, Alexopoulos and Spaeth2020). Anti-complement antibodies have also been shown in MG and SLE (Tüzün et al., Reference Tüzün, Saini, Ghosh, Rowin, Meriggioli and Christadoss2006; Kallenberg, Reference Kallenberg2008). It is known that autoantibodies can cause cell damage by complement activation (Alexander et al., Reference Alexander, Anderson, Barnum, Stevans and Tenner2008). Therefore, changes in peripheral blood complement and complement inhibitor levels, and the presence of serum anti-complement antibodies may be indicators of complement-mediated mechanisms in the CNS. Furthermore, complement factor alterations have been reported in association with sleep dysfunction (Shivshankar et al., Reference Shivshankar, Fekry, Eckel-Mahan and Wetsel2020). Despite its significance in sleep, the complement system has not been examined in detail in narcolepsy. Normal complement levels in narcolepsy have been mentioned only in a few early studies (Matsuki et al., Reference Matsuki, Honda, Satake and Juji1988). However, C4A3, C4B1 polymorphisms and C2 and C4 complement gene variants have been identified for narcolepsy (Matsuki et al., Reference Matsuki, Honda, Satake and Juji1988). These variants are also known to localise in between HLA-B and HLA-DR loci, which is close to narcolepsy-associated HLA allele region (Shiina et al., Reference Shiina, Hosomichi, Inoko and Kulski2009). Moreover, it has been shown that the level of complement factors C3 and C5, and circulating IgG, IgM and IgA increase when sleep deprived (Hui et al., Reference Hui, Hua, Diandong and Hong2007). Besides, C3a levels are known to decline during nocturnal awake time compared to nighttime sleep. In a recent proteomic profiling study, humoral immune response, complement and coagulation cascades were also found to be the main molecular mechanisms for insomnia (Wang et al., Reference Wang, Ren, Zhang, Wang, Liu, Deng and Yan2021). In sum, accumulating evidence suggests that sleep deprivation, daytime sleepiness and sleep quality change various complement factors which may as well explain the pathophysiology of narcolepsy (Hui et al., Reference Hui, Hua, Diandong and Hong2007; Besedovsky et al., Reference Besedovsky, Lange and Haack2019; Pak et al., Reference Pak, Butts, Hertzberg, Collop, Quyyumi, Cox, Rogers and Dunbar2020).
Overall, classical complement pathway factors are genetically linked to narcolepsy, and the presence of anti-neuronal antibodies in narcolepsy indicates antibody-dependent classical pathway activation as a potential disease mechanism (Matsuki et al., Reference Matsuki, Honda, Satake and Juji1988; Besedovsky et al., Reference Besedovsky, Lange and Haack2019). In order to gather more information about the classical complement pathway-mediated immunopathogenesis of narcolepsy, we investigated serum levels of classical pathway complement factors (C1q, C4d) and the classical pathway inhibitor (complement component 4 binding protein, C4BP), which are known to be elevated in serum and accumulated on target tissue in typical antibody and complement-mediated disorders (Segawa et al., Reference Segawa, Hisano, Matsushita, Fujita, Hirose, Takeshita and Iwasaki2010; Mihlan et al., Reference Mihlan, Blom, Kupreishvili, Lauer, Stelzner, Bergström, Niessen and Zipfel2011). These factors are also activated by acute-phase reactant C-reactive protein and thus might reflect acute inflammation activity (Agrawal et al., Reference Agrawal, Shrive, Greenhough and Volanakis2001; Sjöberg et al., Reference Sjöberg, Trouw, McGrath, Hack and Blom2006). To assess the common complement pathway activity, we measured levels of C4BP-independent C3-split product C3a, rather than C3b, since C4BP promotes the conversion of C3b to iC3b in the presence of Factor H (Sjöberg et al., Reference Sjöberg, Trouw and Blom2009; Ramos-Sevillano et al., Reference Ramos-Sevillano, Urzainqui, Campuzano, Moscoso, González-Camacho, Domenech, de Córdoba, Sánchez-Madrid, Brown, García and Yuste2015). Levels of breakdown products were measured since they might more efficiently indicate complement pathway activity (Davies et al., Reference Davies, Nasaruddin, Alhaq, Senaldi and Vergani1988; Lechner et al., Reference Lechner, Chen, Hogg, Toth, Silvestri, Chakravarthy and Xu2016). We also sought for potential correlations between complement factor levels and clinical parameters of narcolepsy. Our results suggest that complement pathway activation might be involved in the physiopathology of narcolepsy.
Aims of the study
The aim of the study was to investigate complement-mediated immune mechanisms in narcolepsy by measuring the serum levels of classical pathway complement factors (C1q, C4d), the classical pathway inhibitor (C4BP) and common complement pathway activity (C3a).
Material and methods
Subjects
Consecutive 42 narcolepsy patients (26 men, 32.3 ± 11.8) diagnosed according to the International Classification of Sleep Disorders criteria and whose polysomnography (PSG) and multiple sleep latency test results were compatible with narcolepsy were selected (American Academy of Sleep Medicine, 2005). Eight narcolepsy patients were under modafinil treatment, whereas the remaining patients were not under treatment. Patients with a concomitant disease or other primary sleep disorder were excluded. For healthy control group, 26 age- and gender-matched volunteers (17 men, 31.0 ± 13.2-year-old), who had no infectious disease for the last 3 months, had not used immunosuppressive drugs, had no immunological or neurological disorders, had normal sleep study values and had not been diagnosed with primary sleep disorder were selected. The ethics committee approved the study protocol and all participants signed informed consent forms. Blood samples were collected venously from all participants between 8 and10 a.m. and sera were stored in a −80°C freezer until use.
Sleep studies
All participants had a whole-night PSG recording using the Embla a10 system (Flaga, Reykjavik, Iceland). Sixteen-channel electroencephalography recordings were used in PSG montage, and electrode placement was performed according to the 10–20 system. Other PSG montages included a left and right electrooculogram, chin electromyogram, left and right tibialis anterior electromyogram, electrocardiogram, nasal pressure, oronasal thermistor, thoracic and abdominal strain gauges, pulse oximetry and synchronised video recording. All recordings started at the subjects’ usual bedtime and continued until spontaneous awakening. The following conventional sleep parameters were evaluated: total non-REM/REM time, sleep onset latency, REM sleep latency, sleep activity, percentage of wakefulness after sleep onset and sleep onset REM. In addition, subjective sleep quality was evaluated by Pittsburgh sleep quality test (PSQT) and daytime excessive sleepiness was tested by Epworth sleepiness scale (ESS). Sleep parameters of NT1 and NT2 cases are reported in Table 1.
Table 1. Clinical information of NT1 and NT2 cases evaluated in the study

Parametric results are expressed as mean ± standard deviation.
BMI, body mass index; ESS, Epworth sleepiness scale; F, female; M, male; N, non-REM; PSQT, Pittsburgh sleep quality test; REM, rapid eye movements; SOREM, sleep onset rapid eye movement.
ELISA studies
Serum C1q (Thermo Fisher, Waltham, MA, USA), C3a (Thermo Fisher, USA), C4d (Quidel, San Diego, CA, USA), and C4BP (Abbkine, Wuhan, China) levels were determined using commercial ELISA kits, as per manufacturer’s recommendations. Plates were read at 450 nm and results were expressed in µg/ml or ng/ml. Anti-C4BPA was investigated by a home-made ELISA using a recombinant C4BPA protein (Novus Biologicals, Centennial, CO, USA). C4BPA (0.4 µg per well) was coated onto 96-well microtiter plates in 0.1 M carbonate/bicarbonate buffer overnight at 4°C. Sera (1:1000) were added and plates were incubated at 37°C for 90 min. After PBS washing, horseradish peroxidase-conjugated anti-human IgG (Abcam, Cambridge, UK) (1:5000) was added and plates were then incubated at 37°C for 90 min, followed by washing and incubation with a chromogen solution in the presence of H2O2. Plates were read at a wavelength of 450 nm and results were expressed as optical densities. Antibody positivity was defined as two standard deviations above the mean of healthy controls.
Statistics
Parametric variables were compared with Student’s t-test, whereas categorical variables were compared with Chi-square test. Pearson correlation test was used for correlation analysis. The significance level of the test parameters was accepted as p < 0.05 and the confidence interval as 95%. Statistical analyzes and graphs were performed with GraphPad Prism 5 programme.
Results
Identification of the levels of complement factors
Serum C1q levels of narcolepsy patients were significantly higher than those of healthy controls (p = 0.007), while C4d (p = 0.0001) and C4BP (p = 0.0012) levels of narcoleptics were significantly lower (Fig. 1). Narcolepsy patients showed trends towards displaying lower serum C3a levels than healthy controls, however this difference did not attain statistical significance (p = 0.053; Fig. 1). To investigate whether antibodies might have decreased C4BP levels and/or employed a neutralising action on C4BP, we measured serum levels of anti-C4BPA by ELISA. Narcolepsy patients showed trends towards exhibiting increased serum levels of C4BPA antibodies as compared to healthy controls (p = 0.061; Fig. 1). Moreover, 11 of 42 (26.1%) narcolepsy patients showed anti-C4BPA levels above the threshold level for positivity, while none of the 26 control sera exceeded this threshold (p = 0.004 by Chi-square test; Fig. 2). However, C4BPA antibody levels did not significantly correlate with levels of C4BP, C1q, C4d and C3a (not shown). Also, narcolepsy patients with and without C4BPA antibodies showed comparable levels of C4BP (p = 0.298), C1q (p = 0.155), C4d (p = 0.411) and C3a (p = 0.106). Likewise, no significant difference was detected between anti-C4BPA antibody positive and negative patients in terms of age, age of disease onset, gender, body mass index, narcolepsy type and sleep parameters measured by the PSG study (p > 0.05). No difference was observed between NT1 and NT2 patients in terms of levels of C4BP (p = 0.831), C1q (p = 0.571), C4d (p = 0.151), C3a (p = 0.259) and anti-C4BPA (p = 0.994; Fig. 3). Similarly, patients with and without modafinil treatment showed comparable levels of all measured complement parameters (not shown).
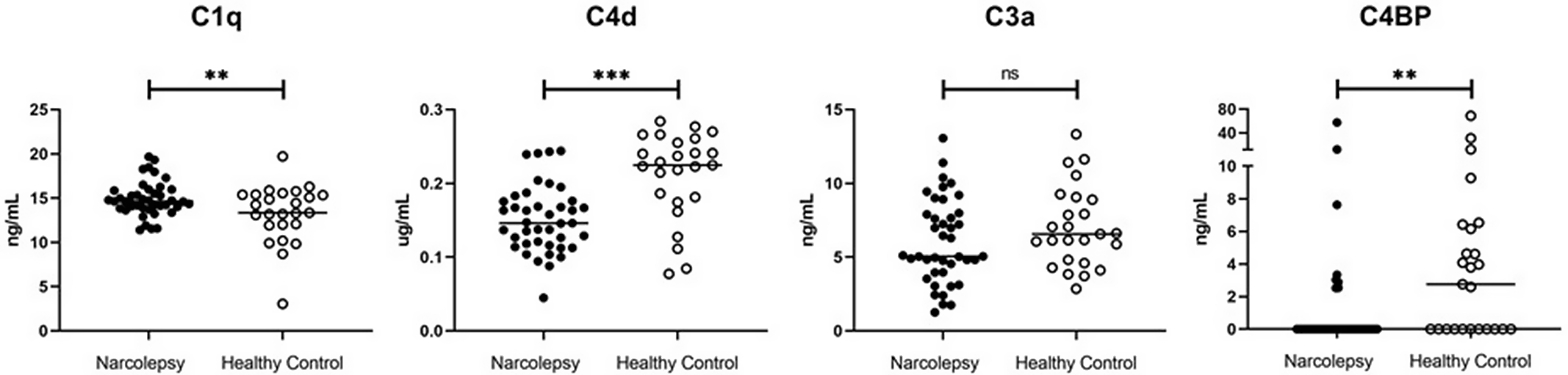
Fig. 1. Serum C1q, C4d, C3a and complement component 4 binding protein (C4BP) levels of narcolepsy patients and healthy controls. Horizontal lines indicate mean value of each group and lines at the top indicate statistical comparisons between groups. ** indicates p < 0.01, *** p < 0.001, ns= not significant by Student’s t-test.
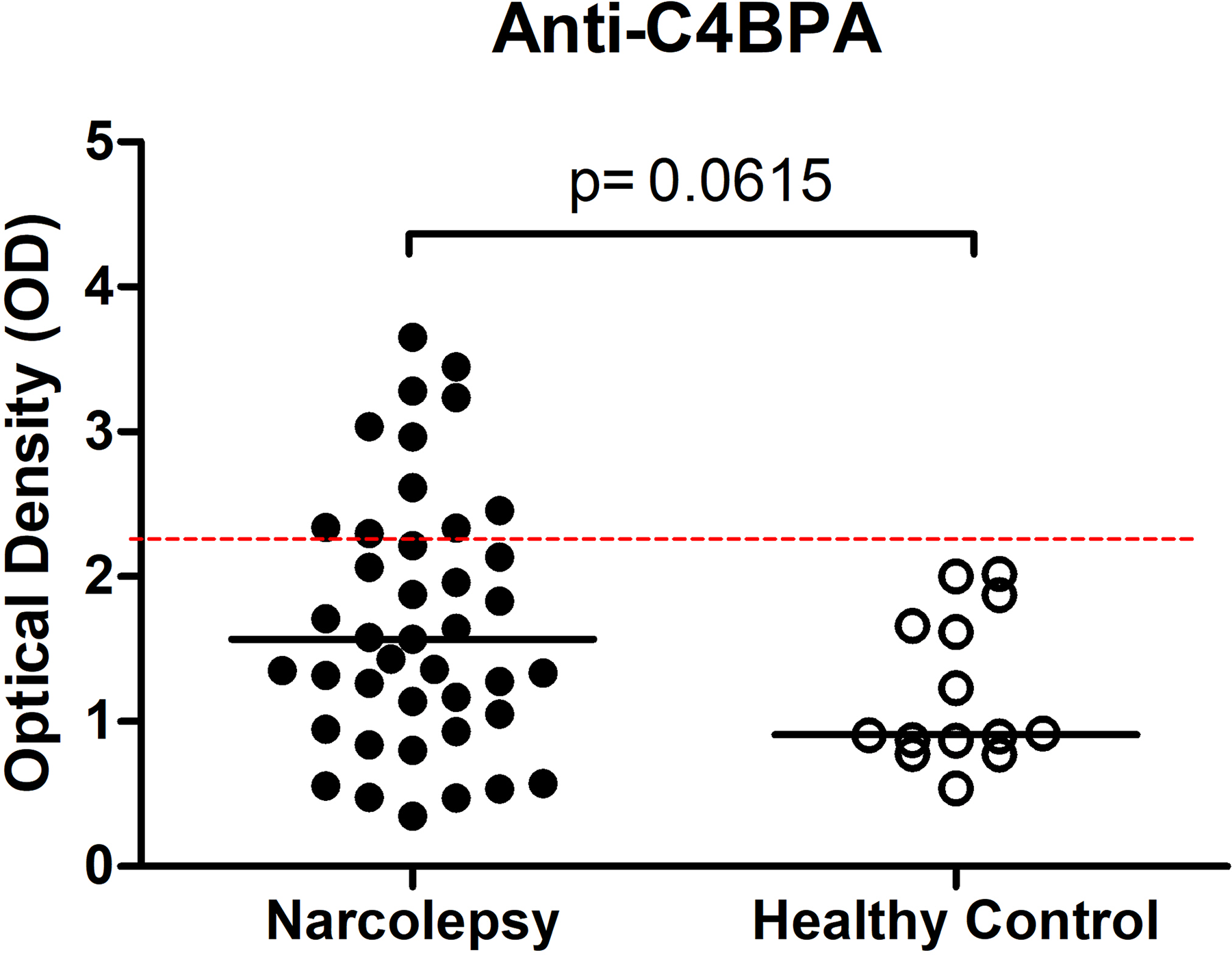
Fig. 2. ELISA detection of IgG antibodies directed against complement component 4 binding protein alpha (C4BPA) in sera of narcolepsy patients and healthy controls. The dashed lines represent two standard deviations above the mean of the healthy control samples (cut-off values for positivity). Horizontal lines indicate the mean value of each group.

Fig. 3. Complement pathway parameters are not significantly different between narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2) patients. Horizontal lines indicate the mean value of each group. p value is obtained by comparison of two groups by Student’s t-test and found non-significant (ns) for all comparisons.
Association of complement factors with clinical features
Clinical and demographic features of patients and complement factor levels were statistically compared. C1q levels were found to be positively correlated with ESS (r = 0.317, p = 0.048). On the other hand, C4d levels were positively correlated with REM sleep latency (r = 0.475, p = 0.006) and percentage of wakefulness after sleep onset (r = 0.491, p = 0.006), while both C4d and C3a levels negatively correlated with sleep activity (r = −0.361, p = 0.042; r = −0.526, p = 0.002, respectively) and total REM time (r = −0.389, p = 0.034; r = −0.402, p = 0.027, respectively). The levels C3a were also positively correlated with percentage of wakefulness (r = 0.533, p = 0.002) and PSQT (r = 0.451, p = 0.005). No significant correlation was found between anti-C4BPA and C4BP levels and any of the sleep parameters (p > 0.05; Fig. 4).

Fig. 4. Correlation matrix of complement pathway parameters and clinical features of narcolepsy patients. Positive correlations are represented in red, while negative correlations are represented in blue. The values are expressed as Pearson r scores. Darker colours mean higher correlation (p < 0.05). The significant correlation scores in line with our hypothesis are shown in red rectangles.
Discussion
Although the etiopathogenesis of narcolepsy is not fully understood, the autoimmune hypothesis related to narcolepsy has drawn attention for reasons such as strong relationship with the HLA haplotype DQB1*06:02, immunity-related gene polymorphisms, antibodies directed against hypocretin and other antigens expressed by hypocretinergic neurons, and influenza vaccine induced narcolepsy (Jennum et al., Reference Jennum, Sci, Pedersen, Maria, Bahl, Modvig, Fog, Holm, Kornum and Gammeltoft2017; Tanaka et al., Reference Tanaka, Honda, Honda, Yamada, Honda and Kodama2017; Latorre et al., Reference Latorre, Kallweit, Armentani, Foglierini, Mele, Cassotta, Jovic, Jarrossay, Mathis, Zellini, Becher, Lanzavecchia, Khatami, Manconi, Tafti, Bassetti and Sallusto2018; Luo et al., Reference Luo, Ambati, Lin, Bonvalet, Partinen, Ji, Maecker and Mignot2018; Pedersen et al., Reference Pedersen, Holm, Kristensen, Bjerregaard, Bentzen, Marquard, Tamhane, Burgdorf, Ullum, Jennum, Knudsen, Hadrup and Kornum2019; Kornum, Reference Kornum2020; Postiglione et al., Reference Postiglione, Antelmi, Pizza, Vandi, la Morgia, Carelli, Nassetti, Seri and Plazzi2020). Although these recent neurological investigations have increasingly focused on the role of immunity, there is not yet sufficient data regarding antibody-dependent complement system activation in narcolepsy. Due to previously reported association between classical complement pathway gene factors and narcolepsy (Matsuki et al., Reference Matsuki, Honda, Satake and Juji1988), in this study, we analysed classical pathway factors and the classical pathway inhibitor C4BP. We primarily looked for degradation products since they are often regarded as typical indicators of complement system activity (Ekdahl et al., Reference Ekdahl, Persson, Mohlin, Sandholm, Skattum and Nilsson2018).
As an element of innate immunity, complement system is the first line of defense against pathogens and triggers antibody production and T cell-mediated immune response. The main classical pathway element C1q binds to surface IgG and IgM and activates the system. Then, the common pathway factor C3 undergoes enzymatic cleavage in the early complement cascade and transforms into its main proteolytic fragment, C3b. C3b covalently binds to the pathogens or the body’s own cells, forms the membrane attack complex resulting in lysis of the pathogens/cells. In order to prevent damage to the body’s own healthy cells and tissues by the activated complement system, C4BP inhibits the classical and lectin complement pathways, prevents the formation of the C4bC2a complex known as C3-convertase and cleaves C4b into C4c and C4d fragments. While C4BP inhibits autoimmune processes, it can also lead to infectious conditions by preventing the elimination of pathogens by complement activity (Gordon, Reference Gordon2006; Sjöberg et al., Reference Sjöberg, Trouw and Blom2009; Abbas et al., Reference Abbas, Lichtman and Pillai2017).
In our study, we showed for the first time in narcolepsy, to our knowledge, significant alterations in levels of classical pathway factors. However, the complement level profile of narcolepsy patients was not compatible with the profile observed in a typical antibody and classical complement pathway-dependent autoimmune disease such as autoimmune glomerulonephritis (Segawa et al., Reference Segawa, Hisano, Matsushita, Fujita, Hirose, Takeshita and Iwasaki2010). In these disorders, autoantibodies lead to full complement activation by recruiting C1q to activate the classic complement pathway, ultimately giving rise to an elevation of C3 and C4 breakdown products and activation of C4BP to regulate the hyperactive complement system. As a result, target tissues typically show deposits of C1q, C4BP, C4d and C3 breakdown products (Segawa et al., Reference Segawa, Hisano, Matsushita, Fujita, Hirose, Takeshita and Iwasaki2010; Mihlan et al., Reference Mihlan, Blom, Kupreishvili, Lauer, Stelzner, Bergström, Niessen and Zipfel2011). By contrast, narcolepsy patients showed increased C1q but decreased C4BP, C4d and C3a levels. This pattern is congruent with activation of the classical pathway by factors that both stimulate the complement activator C1q and also interact with complement inhibitors and suppress the overall complement pathway activity (Sjöberg et al., Reference Sjöberg, Trouw and Blom2009). This pattern may be observed in rheumatoid arthritis and SLE patients and putative factors inducing this profile in narcolepsy might be dying cells and extracellular matrix proteins released due to ongoing orexinergic neuron loss (Sjöberg et al., Reference Sjöberg, Trouw and Blom2009). Reduced C4BP and C4d levels may also be explained by chronic activation and consumption of the classical complement pathway (Bergamaschini et al., Reference Bergamaschini, Miedico, Cicardi, Coppola, Faioni and Agostoni1999). Finally, NT1 patients, who are known to display a high prevalence of anti-neuronal antibodies, did not show an exceptionally higher complement activity than NT2 patients. This results further argue against the involvement of antibodies in complement level alterations observed in our study.
Reduced levels and/or attenuated function of C4BP might have led to the reduction in serum levels of C4d, which is produced by cleavage of C4b by the C4BP (Sjöberg et al., Reference Sjöberg, Trouw and Blom2009). C4BP also interacts with C3b (Blom et al., Reference Blom, Kask and Dahlbäck2003; Sjöberg et al., Reference Sjöberg, Trouw and Blom2009; Ramos-Sevillano et al., Reference Ramos-Sevillano, Urzainqui, Campuzano, Moscoso, González-Camacho, Domenech, de Córdoba, Sánchez-Madrid, Brown, García and Yuste2015) and thus to avoid the confounding effect of reduced C4BP, we assessed the common complement pathway activity via C3a level measurement. Although C3a is generally regarded as an anaphylatoxin with pro-inflammatory action, emerging anti-inflammatory impact of C3a is currently under scrutiny (Coulthard & Woodruff, Reference Coulthard and Woodruff2015). The correlation analysis showed that narcolepsy patients with higher C3a levels were more likely to have reduced total REM-sleep time, higher duration of wakefulness during sleep and worse sleep quality, as measured by PSQT scores. Likewise, patients with higher C4d levels were more likely to show increased REM sleep latency, increased wakefulness after sleep onset and decreased total REM time. These results suggest that these complement degradation products may have hazardous action on sleep functions and, presumably, reduction of C3a and C4d levels in narcolepsy might be a compensating measure to combat against sleep dysregulation. Intriguingly, reduced serum levels of C3 and C4 have been linked to longer sleep duration (Hui et al., Reference Hui, Hua, Diandong and Hong2007; Besedovsky et al., Reference Besedovsky, Lange and Haack2019; Pak et al., Reference Pak, Butts, Hertzberg, Collop, Quyyumi, Cox, Rogers and Dunbar2020).
Notably, narcolepsy patients exhibited significantly reduced C4BP levels. Expression analysis in a study reported decreased C4BPA expression in patients with obstructive sleep apnoea syndrome (Jurado-Gamez et al., Reference Jurado-Gamez, Gomez-Chaparro, Muñoz-Calero, Serna Sanz, Muñoz-Cabrera, Lopez-Barea and Gozal2012). In addition, decreased levels of C4BP have been reported to be associated with disease risk for schizophrenia, which can be comorbid with narcolepsy (Wang et al., Reference Wang, Lu, Ni, Zhang, Tang, Lu, Cai and Zhang2015). These results suggest that C4BP may be overconsumed in narcolepsy thus providing a hint about the inflammatory nature of the disease. To dissect whether C4BP reduction could have been due to neutralising action of antibodies, we developed an assay to measure serum IgG antibodies directed against C4BPA. This protein was selected since the alpha subunit appears to be the more functionally relevant component of the C4BP (Blom et al., Reference Blom, Kask and Dahlbäck2003). Previous studies have shown that antibodies are developed against immune system components with significantly altered expression and utilisation. As an example, anti-C1q antibodies have been detected in autoimmune diseases characterised with enhanced complement activation such as SLE and MG (Tüzün et al., Reference Tüzün, Saini, Ghosh, Rowin, Meriggioli and Christadoss2006; Orbai et al., Reference Orbai, Truedsson, Sturfelt, Nived, Fang, Alarcón, Gordon, Merrill, Fortin, Bruce, Isenberg, Wallace, Ramsey-Goldman, Bae, Hanly, Sanchez-Guerrero, Clarke, Aranow, Manzi, Urowitz, Gladman, Kalunian, Costner, Werth, Zoma, Bernatsky, Ruiz-Irastorza, Khamashta, Jacobsen, Buyon, Maddison, Dooley, Van Vollenhoven, Ginzler, Stoll, Peschken, Jorizzo, Callen, Lim, Fessler, Inanc, Kamen, Rahman, Steinsson, Franks, Sigler, Hameed, Pham, Brey, Weisman, McGwin, Magder and Petri2015). Also, multiple sclerosis patients show antibodies against switch-associated protein 70, expression of which is enhanced in lymphocytes upon activation (Türkoğlu et al., Reference Türkoğlu, Özyurt, Ulusoy, Erdağ and Tüzün2016). These antibodies are often associated with disease activity suggesting a functional role in disease mechanisms (Tüzün et al., Reference Tüzün, Saini, Ghosh, Rowin, Meriggioli and Christadoss2006; Türkoğlu et al., Reference Türkoğlu, Özyurt, Ulusoy, Erdağ and Tüzün2016; Shang et al., Reference Shang, Ren, Sun, Yu, Yao, Wang, Liu, Zhang, He and Liu2021). However, no correlation could be established between anti-C4BPA and levels of C4BP and other complement factors arguing against a putative impact of these antibodies on complement system activity.
Alternatively, C4BP might be depleted by microorganisms, which widely utilise this protein for survival (Ermert & Blom, Reference Ermert and Blom2016). Bacteria carry IgG-like molecules on their surfaces to avoid complement-mediated elimination (Nordenfelt et al., Reference Nordenfelt, Waldemarson, Linder, Mörgelin, Karlsson, Malmström and Björck2012). Narcolepsy patients might be harbouring similar types of pathogens leading to depletion of C4BP. Another potential explanation of our results could be mild liver injury due to modafinil treatment, since C4BP levels are decreased in liver cirrhosis (Kusada-Funakoshi et al., Reference Kusada-Funakoshi, Sasaki, Takada, Soji and Arakawa1991) and the diminished factors C4BP, C4 and C3 are all produced in liver, whereas elevated C1q is mainly produced by macrophages and dendritic cells (Nagura et al., Reference Nagura, Hasegawa, Yoshimura and Watanabe1985; Kusada-Funakoshi et al., Reference Kusada-Funakoshi, Sasaki, Takada, Soji and Arakawa1991; Chen et al., Reference Chen, Tan, Teh and Lu2011). However, no significant difference was observed between patients with and without modafinil treatment in terms of complement factor levels.
C1q level increase was another intriguing finding of our study. Earlier studies have proposed that microglial phagocytosis of hypocretinergic neurons might be the leading cause of the disease (Nadjar et al., Reference Nadjar, Wigren and Tremblay2017). A striking feature of microglial cells is to produce C1q (Stephan et al., Reference Stephan, Barres and Stevens2012). In recent years, C1q has gained attention due to its role in synaptic elimination. The weak synapses are tagged with C1q, and subsequently downstream classical complement cascade is activated for the removal of the synapse (Stephan et al., Reference Stephan, Barres and Stevens2012). For example, brain tissue of schizophrenia patients is typified with disrupted synaptic elimination processes and enhanced C1q production (Druart & Le Magueresse, Reference Druart and Le Magueresse2019). It can thus be assumed that microglial activation due to exposure to dying neuronal byproducts might be the reason underlying C1q elevation in narcolepsy. The correlation between C1q and ESS is worth mentioning since it indicates worsening sleep quality and increased daytime sleepiness with increased levels of C1q. Thus, C1q may be used as a potential indicator of disease activity in narcolepsy.
A limitation of our study is absence of hypocretin level measurements and analysis of putative correlations between hypocretin, C4BP and anti-C4BPA levels. Similarly, measurement of autoantibodies that are frequently observed in narcolepsy patients and correlation with complement levels could have provided informative insights.
In brief, the complement system is a vastly uncharted field in narcolepsy and our results suggest that the significance of complement factor alterations needs to be further scrutinised. Our results provide reasonable doubt for involvement of the complement system in narcolepsy and also suggest that C1q, C3a and C4d may act as biomarkers in narcolepsy for prediction of disease severity. Therefore, further studies investigating the role of complement system factors in neuroinflammation, and sleep physiology are recommended.
Acknowledgements
None.
Authors contributions
CİK directed the project. ET was in charge of supervising the project and interpreting the results. DK and GBŞ helped sample selection, performing sleep studies and gathering clinical data of the subjects. DGA and ED were participated in planning the study. VY and HY were involved in planning and performing ELISA studies. HY wrote the manuscript in consultation with all authors providing critical feedback.
Financial support
The present work was supported by the Research Fund of Istanbul University (grant number 33590).
Conflict of interest
None.
Ethical standards
All procedures comply with the Helsinki Declaration and the ethical standards of Istanbul University, Faculty of Medicine, Clinical Research Ethics Committee.








