Introduction
Adult mating is a key part of offspring production in most insects (Thornhill and Alcock, Reference Thornhill and Alcock1983), and mating systems have a great impact on the reproductive output (e.g., Ono et al., Reference Ono, Hayakawa, Matsuura, Shiraishi, Yasui, Nakamura and Arakawa1995; Opp and Prokopy, Reference Opp and Prokopy2000; Torresvila and Jennions, Reference Torresvila and Jennions2005; Blyth and Gilburn, Reference Blyth and Gilburn2006). Mating systems have been defined as the number of mates that one sex can accumulate, and the precise form depends on which sex is limiting and on the manner and the degree to which the limited sex controls the resource base or monopolizes mates (or both) (Emlen and Oring, Reference Emlen and Oring1977). Mating systems found in insects (Matthews and Matthews, Reference Matthews and Matthews2010) include monogamy, in which there is fidelity between sexual partners; polygamy (including polygyny and polyandry), in which one sex has two or more sexual partners; and polygynandry, in which both males and females mate multiple times (Túler et al., Reference Túler, Silva-Torres, Torres, Moraes and Rodrigues2018). Because of the asymmetry in the number of partners in certain mating systems, an individual's fitness is a function of both its own mating strategy and its partner's mating strategy (Parker, Reference Parker2006). Furthermore, the mating propensity is a crucial aspect of the mating system that depends on the costs and benefits of copulating, which may vary between the sexes and across mating opportunities (Marieorleach et al., Reference Marieorleach, Janicke and Scharer2013; Gress et al., Reference Gress, Waltzer, Lüpold, Droge-Young, Manier and Pitnick2014).
Most studies conducted on the effect of mating propensity on the mating and reproductive success of insects focused on the operational sex ratio (OSR), adult physiological status, and repeated or multiple matings, among other factors (Allou et al., Reference Allou, Morin, Kouassi, Hala N'klo and Rochat2008; Stelinski and Gut, Reference Stelinski and Gut2009; Chinajariyawong et al., Reference Chinajariyawong, Drew, Meats, Balagawi and Vijaysegaran2010; Fritzsche et al., Reference Fritzsche, Booksmythe and Arnqvist2016; Avila et al., Reference Avila, Withers and Holwell2017; Túler et al., Reference Túler, Silva-Torres, Torres, Moraes and Rodrigues2018; Li and Zhang, Reference Li and Zhang2020). The OSR is the ratio of receptive males to receptive females that are ready to mate in a population at a given time (Clutton-Brock, Reference Clutton-Brock and Bendall1983; Emlen and Oring, Reference Emlen and Oring1977; Brown et al., Reference Brown, Laland and Mulder2009) and has an impact on the reproductive potential of both sexes as a result of the effects of intra- and inter-sexual competition (Kvarnemo and Ahnesjo, Reference Kvarnemo and Ahnesjo1996; Vargas et al., Reference Vargas, Shannon, Taveras, Soto and Hilje2001; Andrade and Kasumovic, Reference Andrade and Kasumovic2005; Soffan et al., Reference Soffan, Yousif and Aldawood2012; Moura and Peixoto, Reference Moura and Peixoto2013). Changes in OSR can occur in a population from a variety of factors (e.g., births, deaths, parasitism, and changing abiotic conditions), whereby both sexes may need to adjust their mating strategies to optimize their reproductive success and survival (Wang et al., Reference Wang, He, Yang, Duncan and Lorraine2009). The competition from OSR may result in creating a delay in the time in which moths mate (Adler and Bonduriansky, Reference Adler and Bonduriansky2011). Delayed mating is defined as a condition in which male and/or female mating happens at later stages of life (Waqas et al., Reference Waqas, Shoaib, Elabasy, Cheng, Zhang and Shi2020). The age of mating is usually related to time when the insect reaches sexual maturity, which varies among species, and reductions in fecundity and egg viability, presumably because of physiological changes associated with age (Jiménez-Pérez and Wang, Reference Jiménez-Pérez and Wang2003; Stelinski and Gut, Reference Stelinski and Gut2009; Mori and Evenden, Reference Mori and Evenden2013; Kawazu et al., Reference Kawazu, Shintani and Tatsuki2014), especially in moths (Zhang et al., Reference Zhang, Li, Zeng, Wu and Liu2016). The effects of delayed mating by both sexes yield variable results among species (Huang and Subramanyam, Reference Huang and Subramanyam2003; Wang et al., Reference Wang, Fang and Zhang2011; Kawazu et al., Reference Kawazu, Shintani and Tatsuki2014). Mating history is defined as the accumulated matings of one sex over the individual's lifetime (Emlen and Oring, Reference Emlen and Oring1977; Safonkin, Reference Safonkin2011) and has an important influence on pair formation when one sex is exposed to the same sexual partner or different sexual partners (Chinajariyawong et al., Reference Chinajariyawong, Drew, Meats, Balagawi and Vijaysegaran2010; Li and Zhang, Reference Li and Zhang2020). Repeated mating and particularly multiple mating directly affect reproductive fitness, especially in moths (Zhang et al., Reference Zhang, Zeng, Wu, Peng and Liu2015). The mating propensity of individual species will affect population fluctuation and therefore control measures in the case of insect pests.
The oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae), is a fruit pest widely distributed (Cardé and Minks, Reference Cardé and Minks1995). G. molesta larvae feed on stone or pome shoots and fruits, mostly from the genera Prunus L., Malus Mill., and Pyrus L. (all Rosaceae) (Rothschild and Vickers, Reference Rothschild, Vickers, van der Geest and Evenhuis1991; Kong et al., Reference Kong, Wang, Liu, Guo, Zhao and Fan2018, Reference Kong, Wang, Guo, Chai, Li and Ma2020b). Recently, insect sex pheromone control technology has provided two approaches including mass trapping that seeks to directly attract and kill pests (usually males), and mating disruption that seeks to disorient or misdirect pests (usually males) searching for mates for controlling G. molesta (Kong et al., Reference Kong, Hu, Zhao, Li, Zhang, Li and Ma2014a, Reference Kong, Li, Fan, Li and Mab; El-Shafie and Faleiro, Reference El-Shafie, Faleiro and Shields2017). However, the control effect of this technology decreases with increasing G. molesta population size (Kong et al., Reference Kong, Wang, Guo, Chai, Li and Ma2020a, Reference Kong, Wang, Guo, Chai, Li and Mab, Reference Kong, Wang, Guo, Chai, Niu, Li, Li and Mac). G. molesta is reported to be a polygamous species (Baker et al., Reference Baker, Nishida and Roelofs1981; de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). The possible effects of polygamy in G. molesta on reproductive performance have been studied (Fraser and Trimble, Reference Fraser and Trimble2001; de Morais et al., Reference de Morais, Sant'Ana, Redaelli and Lorscheiter2011, Reference de Morais, Redaelli and Sant'Ana2012; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). In male and female G. molesta moths, mating occurs throughout the entire adult life stage, but with greater occurrence in younger adults; and the first mating can occur on the first night after emergence when females can also produce fertile eggs (Fraser and Trimble, Reference Fraser and Trimble2001; de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). Because females seldom mate more than once, age-delayed mating of females does not affect the daily oviposition rate (Fraser and Trimble, Reference Fraser and Trimble2001; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). Because males can copulate multiple times, male mating history is inversely proportional to spermatophore production but has no effect on progeny egg production (de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). Furthermore, adult sex ratios were observed to differ between peach, apple, and pear larval hosts in the laboratory (Wang et al., Reference Wang, Kong, Zhao, Xiang, Zhang, Li, Ridsdill-Smith and Ma2018; Kong et al., Reference Kong, Wang, Guo, Chai, Li and Ma2020b).
In this study, we investigated the effects of manipulating the number (OSR) and age of reproductive partners by sex on lifetime total mating opportunities and fertile egg production. Then we examined the comparative effects of repeated single mating (same partner) vs multiple mating (many partners) of both sexes under different male mating histories to determine the preferences and discrimination of virgin females and males. The elucidation of G. molesta mating propensity will be helpful for clarifying the plasticity in mating systems and thus improving the efficacy of sex pheromone control technology.
Materials and methods
Insect rearing
G. molesta moths used in the experiments were from a colony established with larvae collected from infested peach (Prunus persica L. cultivar ‘Dajiubao’) orchards (Taigu, Shanxi, North China) in 2010 and maintained for >40 generations. Approximately 30% of this laboratory population was renewed each year with new larvae collected from the same orchards. To do this, larvae from infested shoots or fruits were collected and taken to the laboratory, removed from the plant material, and placed in glass tubes (3.6 cm diameter × 8 cm high). These tubes were filled with an artificial diet (D et al., Reference Du, Wang and Wu2010) and plugged with absorbent cotton balls, where larvae remained until pupation. The larvae were reared under 26 ± 0.8°C, 75 ± 5% relative humidity, and a photoperiod of 15:9 (L:D) h following the protocol used by Wang et al. (Reference Wang, Kong, Zhao, Xiang, Zhang, Li, Ridsdill-Smith and Ma2018). Moths used in experiments were collected on the morning of their emergence and were designated on that day as 1-day-old moths.
Experimental designs for all experiments
All pairings were performed in individual transparent mating cages (1.5-l bottles cut along the center; 15 cm diameter, 20 cm height) with absorbent cotton saturated daily with a 5% sugar–water solution. Courtship bouts were observed from the initial approach of a male until either copulation began, the female flew away, or 30 min had elapsed. The wings of males were under the wings of females, and their antennae were still put on the back (Zhang et al., Reference Zhang, Men, Peng, Li, Deng, Chen, Liu and Ma2017; Li et al., Reference Li, Jia, Xiang, Diao, Yan, Wang and Ma2019). All observations were conducted between 5 p.m. and 9 p.m., as this was found to be the time of maximal calling by females (Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019), and a diode red light emitter was used for insect observation during the scotophase (Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). We observed and recorded the start and end times of copulation, the number of days on which copulation occurred and the total number of copulations. The number of eggs laid on the surface of the mating cage was recorded daily, and each egg was marked with a small circle outside the mating cage using a marker pen until the female died. It normally takes three days for most fertile eggs to hatch (Wang et al., Reference Wang, Kong, Zhao, Xiang, Zhang, Li, Ridsdill-Smith and Ma2018; Kong et al., Reference Kong, Wang, Guo, Chai, Li and Ma2020b). After this time, eggs were considered fertilized if they were darker in color. The number of fertile eggs (those developing to the blackhead stage) vs infertile eggs (no darkening) were counted during the three-day observation period. The preoviposition, oviposition, and postoviposition periods of spawning females were recorded until the adult died. For males and females that died before an egg was laid, only the lifespan was recorded (Wang et al., Reference Wang, Kong, Zhao, Xiang, Zhang, Li, Ridsdill-Smith and Ma2018; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). All the containers were maintained in a climate chamber under the same conditions as the colony. If testing females or males died during the course of the experiment, the replicates were discarded. All the experiments were conducted from June to August of 2012–2015.
Experiment #1: OSR effects
The integrated effects of the numbers of males and females on mating and reproductive behavior were assessed in one experiment with four setting conditions (SCs) as follows.
The number of females varied, and the number of males was constant (with two numbers tested). In SC 1, a single 1-day-old virgin male was paired with variable numbers of 1-day-old virgin females at ratios of 1:1, 1:2, 1:3, 1:4, and 1:5 (male: female) and in SC 2, five 1-day-old virgin males were paired with variable numbers of 1-day-old virgin females at ratios of 5:5, 5:4, 5:3, 5:2, and 5:1 (male:female).
The number of males varied, and the number of females was constant (with two numbers tested). In SC 3, a single 1-day-old virgin female was paired with variable numbers of 1-day-old virgin males at ratios of 1:1, 2:1; 3:1, 4:1, and 5:1 (male:female), and in SC 4, five 1-day-old virgin females were paired with variable numbers of 1-day-old virgin males at ratios of 5:5, 4:5, 3:5, 2:5, and 1:5 (male:female).
When the ‘fixed-number’ sex was represented by one individual or five individuals, the sex ratio (♂:♀) was equal to both 1:5, 1:4, 1:3, 1:2, and 1:1 (male:female) for SC 1 and 5:1, 4:1, 3:1, 2:1, and 1:1 (male:female) for SC 3, or both 5:5, 5:4, 5:3, 5:2, and 5:1 (male:female) for SC 2 and 5:5, 4:5, 3:5, 2:5, and 1:5 (male:female) for SC 4, respectively. All treatments under each of SCs were repeated six or ten times. Overall, 400 cages were set up, and 400 males and 400 females were used for these observations.
Experiment #2. Age-shifted mating effects
The integrated effects of varying the age of males and/or females on mating and reproductive behavior were assessed in one experiment with three SCs as follows.
In SC 1 (shifts in both female age and male age at the time of mating), a single virgin female of different ages (1–10 days) were paired with a single virgin male of the same age.
In SC 2 (shifts only in the male age), a single virgin male of different ages (1–10 days) was paired with a single 1-day-old virgin female.
In SC 3 (shifts only in female age), a single virgin female of different ages (1–10 days) was paired with a single 1-day-old virgin male.
All treatments under each of SCs were repeated six times. Overall, 174 cages were set up, and 174 males and 174 females were used for these observations.
Experiment #3 mating history effects
The integrated effects of variation in the male mating history on mating and reproductive behavior in repeated single mating or multiple mating were assessed in one experiment with three SCs as follows.
In SC 1 (testing the effects of female and male repeated single mating), a single 2-day-old virgin female was paired with a single 1-day-old virgin male or males (<5 days old) that had previously mated one, two or three times, which were kept for the duration of the experiment.
In SC 2 (testing the effects of male multiple mating), a single 2-day-old virgin male or males (<5 days old) that had previously mated one, two, or three times was paired with a single 2-day-old virgin female, which were changed daily for the duration of the experiment.
In SC 3 (testing the effects of female multiple mating), a single 2-day-old virgin female was paired with a single 2-day-old virgin male or males (<5 days old) that had previously mated one, two, or three times, which were changed daily for the duration of the experiment.
For SCs 2 and 3, a new partner was provided daily based on a mating age of 8 days (Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019), and all partners were isolated in separate mating cages on the second day after pairing until death. All treatments under each of SCs were repeated seven or ten times. Overall, 624 cages were set up, and 284 males and 380 females were used for these observations.
Statistical analysis
All data sets were square-root transformed to stabilize their variances before analysis. The normality of the residuals was tested for all data using the Shapiro–Wilk test (Snedecor and Cochran, Reference Snedecor and Cochran1967).
To determine the differences of the mating and reproductive parameters among treatments under different SCs for either the OSR, mating age or male mating history, all data were analyzed by one-way analysis of variance. Post hoc comparisons involved Tukey's multiple range test or the Games−Howell test, depending on whether the treatment variances were equal (Shapiro–Wilks test, P > 0.05) or unequal (Shapiro–Wilks test, P < 0.05) (Kong et al., Reference Kong, Wang, Guo, Chai, Li and Ma2020a).
To determine the strength and direction of the linear relationship between all treatments under different SCs for either the OSR, mating age, or male mating history and the corresponding mating and reproductive parameters, the correlation was tested using the Pearson correlation or the Spearman correlation, depending on whether the transformed data were normally or non-normally distributed (Bolboacă and Jäntschi, Reference Bolboacă and Jäntschi2006).
The parental contribution to the progeny is a distrubtion of the number of surviving offspring among males and females in a mating system (Fiumera et al., Reference Fiumera, DeWoody, DeWoody, Asmussen and Avise2001; Parker and Tang-Martinez, Reference Parker and Tang-Martinez2005). To determine the possible effects of mating systems in G. molesta on offspring production, the relationship between all treatments under different SCs for either the OSR, mating age or male mating history and the corresponding values of the parental contribution to the progeny, was analyzed using non-linear regression.

Additionally, the means of repeated single mating and multiple mating of females or males were compared by the Mann–Whitney test and the data for the same OSR or age between the different SCs were analyzed using independent-samples t-test.
All analyses were performed with SPSS (Statistical Product and Service Solutions) for Windows version 16.0 software (SPSS Inc., Chicago, IL), and all figures were created with SigmaPlot for Windows version 12.5 software (Systat Software Inc., Germany).
Results
Experiment 1: effect of OSR on mating and reproductive behavior
Effect of varying the OSR at a fixed number of males
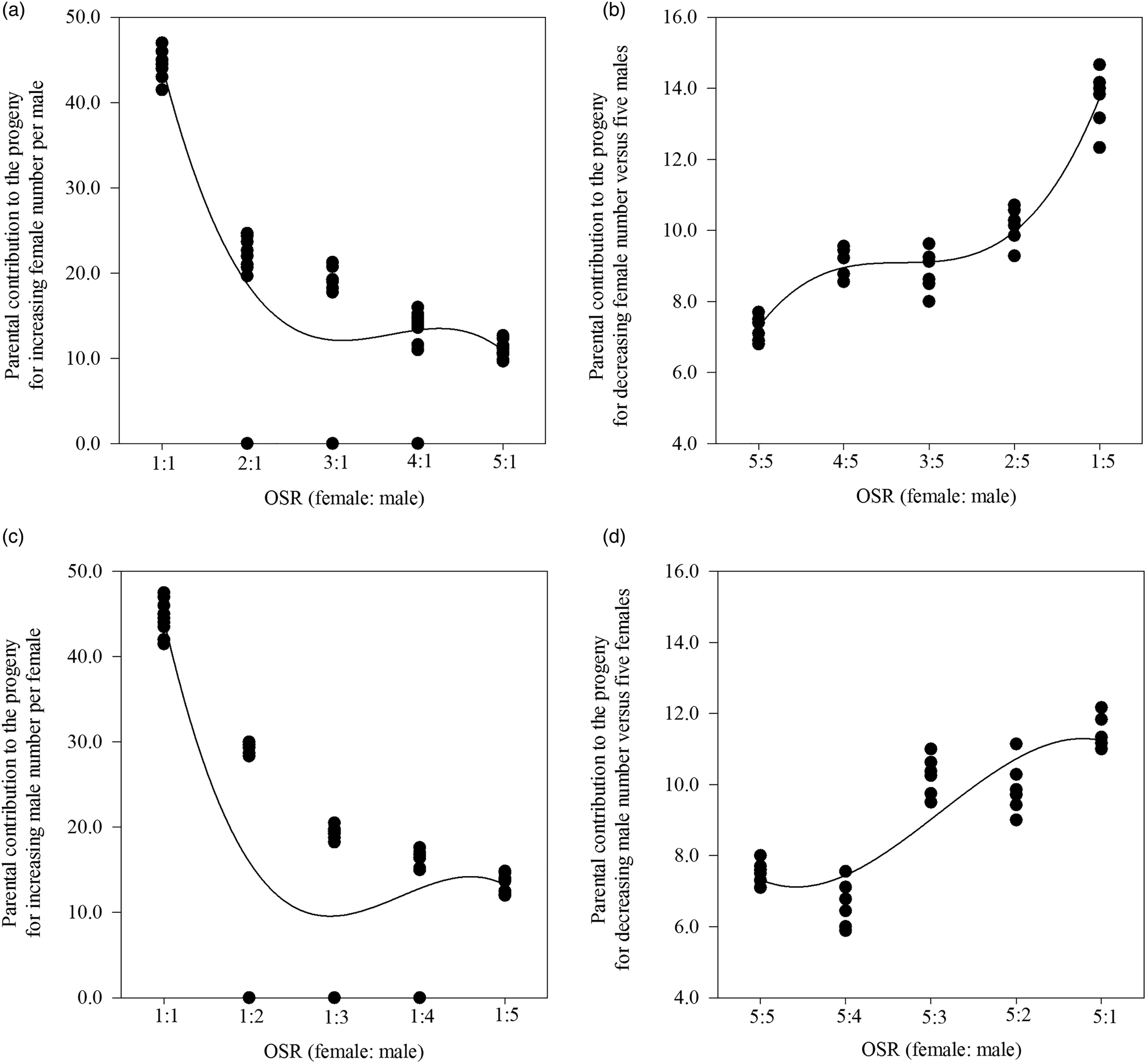
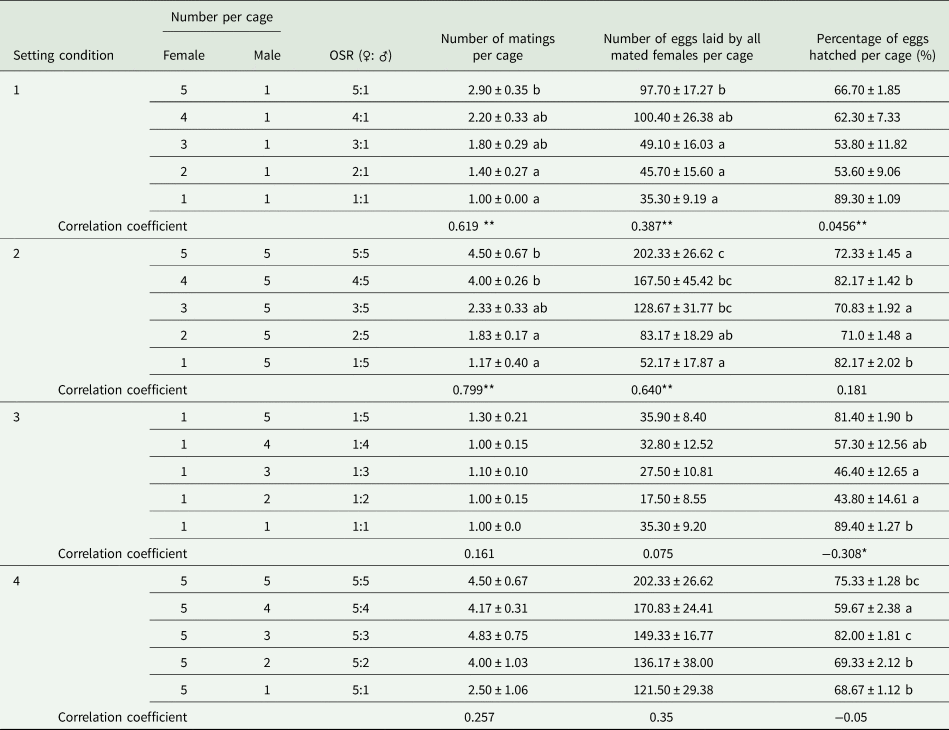
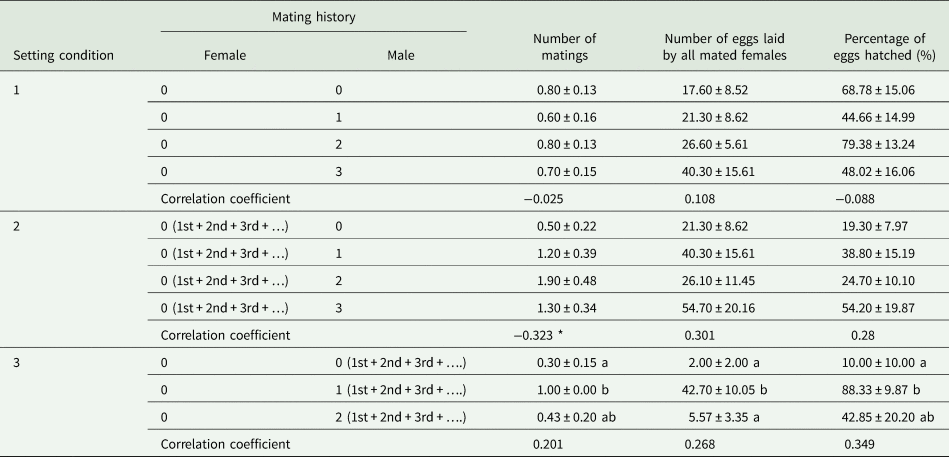
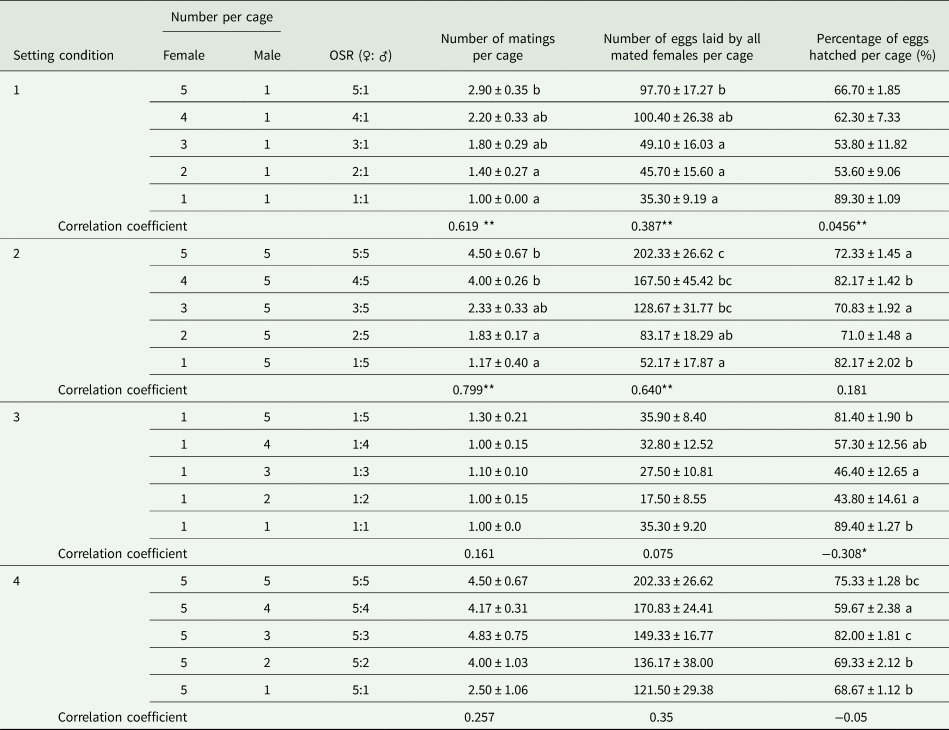
When the OSR became female biased based on one male per cage in SC 1, the greater availability of females significantly increased the number of matings per cage (F = 3.586, df = 49, 4, P < 0.05; r s = 0.619, P < 0.01, n = 50) and the number of eggs laid by all mated females per cage (F = 2.649, df = 49, 4, P < 0.05; r = 0.387, P < 0.01, n = 50), but not the percentage of eggs hatched per cage (F = 2.350, df = 49, 4, P = 0.068; r s = −0.456, P < 0.05, n = 50) (table 1). Additionally, we found a significant relationship between the less male-biased OSR (as the number of females increased and there was one male) (x) and the parental contribution to the progeny (y) (cubic regression: y = 100.6 − 75.2x + 20.9x 2 − 1.9x 3; F = 64.627; R 2 = 0.796; P < 0.05) (fig. 1A).
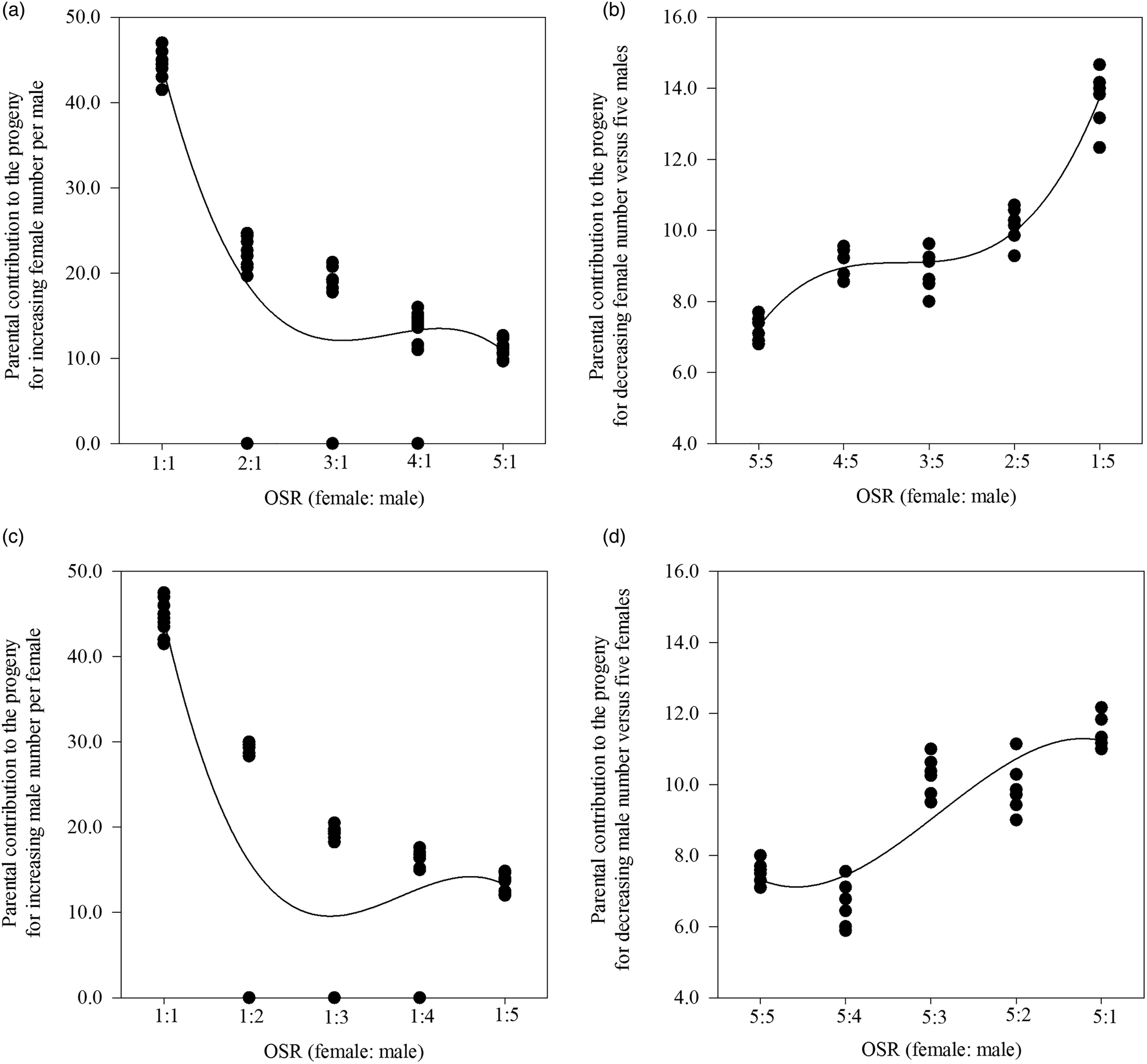
Figure 1. The four relationships between operational sex ratio (OSR) and the parental contribution to the progeny for a less male-biased OSR with the increasing female number per male (A), a more male-biased OSR with decreasing female number vs five males (B), a less female-biased OSR with increasing male number per female (C), and a more female-biased OSR with decreasing male number vs five females (D) in Grapholita molesta.
Table 1. Effects of either one (setting conditions 1 and 3) or five (setting conditions 2 and 4) individuals of a given sex of Grapholita molesta exposed to one to five mates of the opposite sex in individual mating cages on the mean number of matings per cage (±SE), number of eggs laid by all mated females per cage (±SE) and percentage of eggs hatched per cage (±SE) in the laboratory.
Different lowercase letters indicate significant differences among OSRs in each treatment at a P < 0.05 level, based on multiple comparisons (Tukey's multiple range test or Games-Howell test).
** and * indicate that the correlation coefficients between the OSR and measured parameters were significant at 0.01 and 0.05, respectively.
In contrast, as the OSR became more male biased based on five males per cage in SC 2, the progressive reduction in the number of females significantly decreased the number of matings per cage (F = 10.633, df = 29, 4, P < 0.05; r = −0.799, P < 0.01, n = 30) and the number of eggs laid by all mated females per cage (F = 4.414, df = 29, 4, P = 0.05; r = −0.640, P < 0.01, n = 30), but not the percentage of eggs hatched per cage (F = 12.169, df = 29, 4, P < 0.05; r = 0.181, P = 0.338, n = 30) (table 1). We also found a significant relationship between the more male-biased OSR (as the number of females decreased and there were five males) (x) and the parental contribution to the progeny (y) (cubic regression: y = 1.9 + 8.0x − 3.0x 2 + 0.4x 3; F = 138.516; R 2 = 0.934; P < 0.05) (fig. 1B).
Effect of varying the OSR at a fixed number of females
When the OSR became more male biased based on one female per cage in SC 3 or more female biased based on five females per cage in SC 4, the differences in the resulting number of matings per cage (SC 3: F = 0.802, df = 49, 4, P = 0.531; SC 4: F = 1.516, df = 29, 4, P = 0.228) and the number of eggs laid by all mated females per cage (SC 3: F = 1.370, df = 49, 4, P = 0.259; SC 4: F = 1.224, df = 29, 4, P = 0.326) were not significant. However, the OSR had a significant effect on the percentage of eggs hatched per cage (SC 3: F = 3.672, df = 49, 4, P < 0.05; SC 4: F = 20.79, df = 29, 4, P < 0.05) (table 1).
Moreover, a negative correlation in SC 3 was observed between more male-biased sex ratios and the percentage of eggs hatched per cage (r = −0.308, P < 0.05, n = 50) but not between such ratios and the number of matings per cage (r = 0.161, P = 0.264, n = 50) or the number of eggs laid by all mated females per cage (r = 0.075, P = 0.607, n = 50). There were no correlations in SC 4 between more female-biased sex ratios and the number of matings (r s = −0.257, P = 0.17, n = 30), the number of eggs laid by all mated females per cage (r s = −0.35, P = 0.058, n = 30), or the percentage of eggs hatched (r = −0.05, P = 0.792, n = 30) (table 1).
In addition, there were two significant cubic relationships between: (1) the less female-biased OSR (as the number of males increased and there was one female) (x) and the parental contribution to the progeny (y) (y = 107.2 − 84.3x + 23.4x 2 − 2.1x 3; F = 33.443; R 2 = 0.665; P < 0.05) (fig. 1C) in SC 3 and (2) the more female-biased OSR (as the number of males decreased and there were five females) (x) and the parental contribution to the progeny (y) (y = 10.0 − 4.5x + 2.0x 2 − 0.2x 3; F = 26.615; R 2 = 0.726; P < 0.05) (fig. 1D) in SC 4.
The differences in the mean number of matings per cage (5:1 (♀: ♂): t = 0.36, df = 6.104, P = 0.731; 1:5 (♀: ♂): t = −0.796, df = 14, P = 0.436), the number of eggs laid by all mated females per cage (5:1 (♀: ♂): t = −0.751, df = 14, P = 0.465; 1:5 (♀: ♂): t = 0.558, df = 14, P = 0.586) and the percentage of eggs hatched per cage (5:1 (♀: ♂): t = −0.767, df = 14, P = 0.456; 1:5 (♀: ♂): t = 0.273, df = 14, P = 0.789) were not statistically significant for the same OSR (5:1, ♀: ♂) between SCs 1 and 4, or for the same OSR (1:5, ♀: ♂) between SCs 2 and 3.
Experiment 2: effect of individual age on mating and reproductive behavior
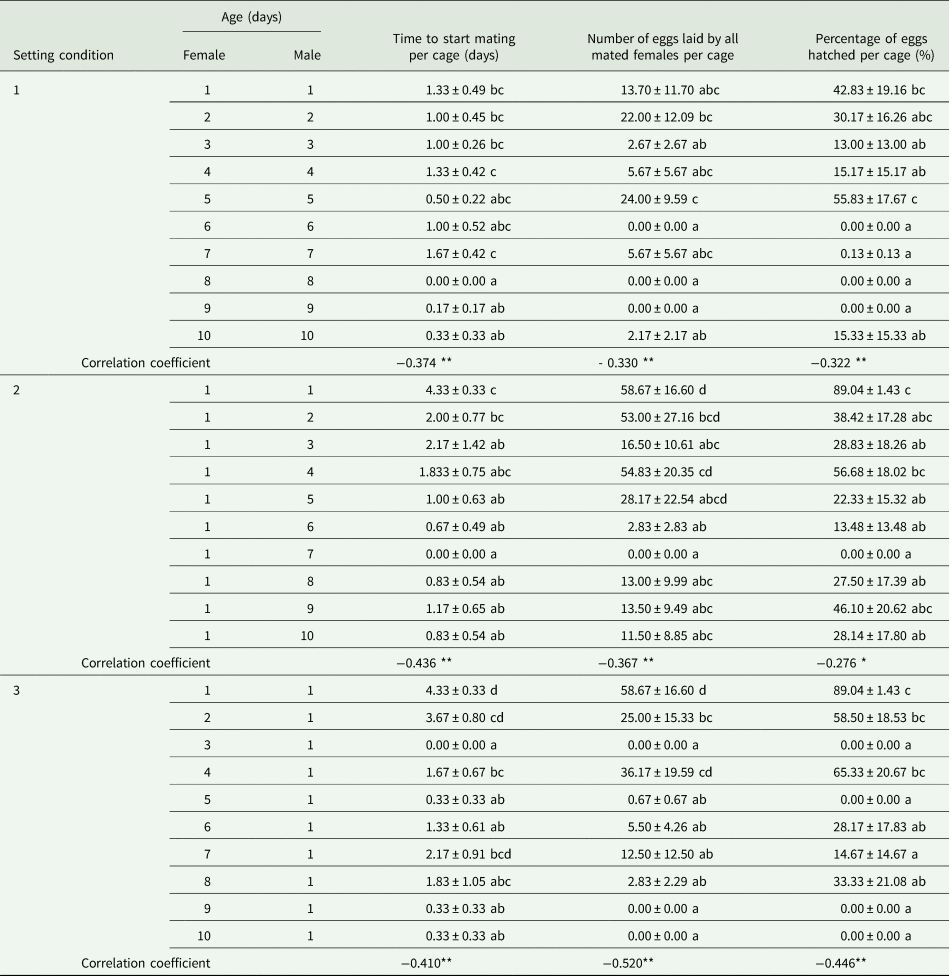
The difference in the mean percentage of eggs hatched per cage (t = −2.321, df = 5.013, P = 0.068) except for the start time per cage (t = −3.594, df = 5.722, P < 0.05) and the number of eggs laid by all mated females per cage (t = −3.002, df = 10, P < 0.05) were not statistically significant for 1-day olds between SC 1 and SCs 2 and 3.
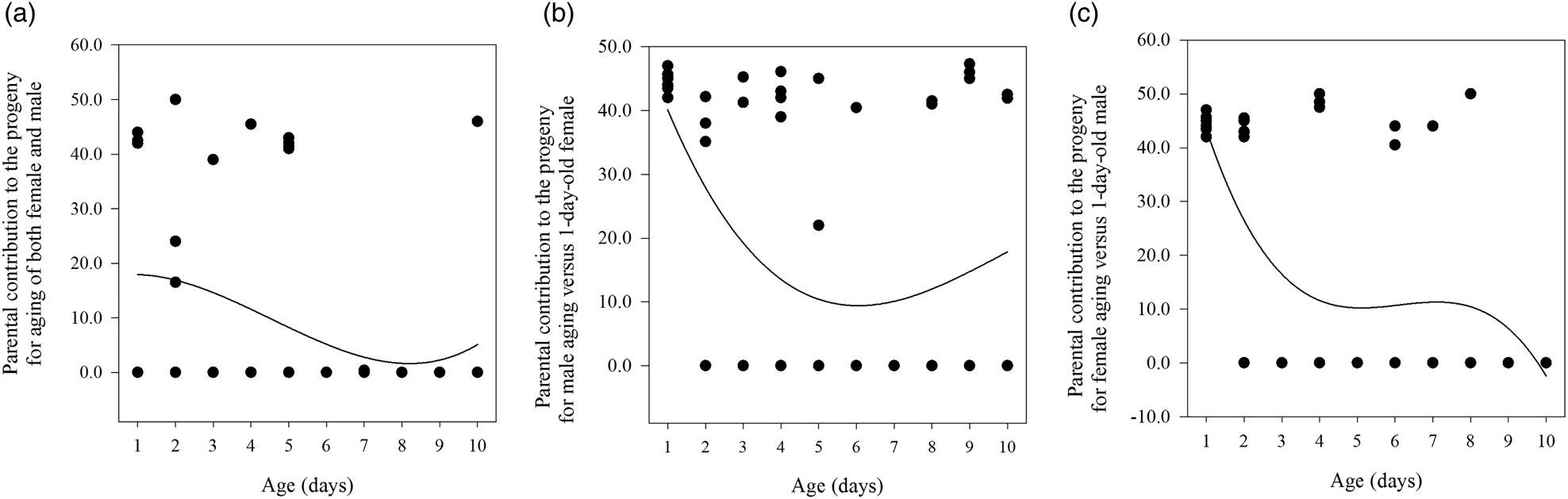
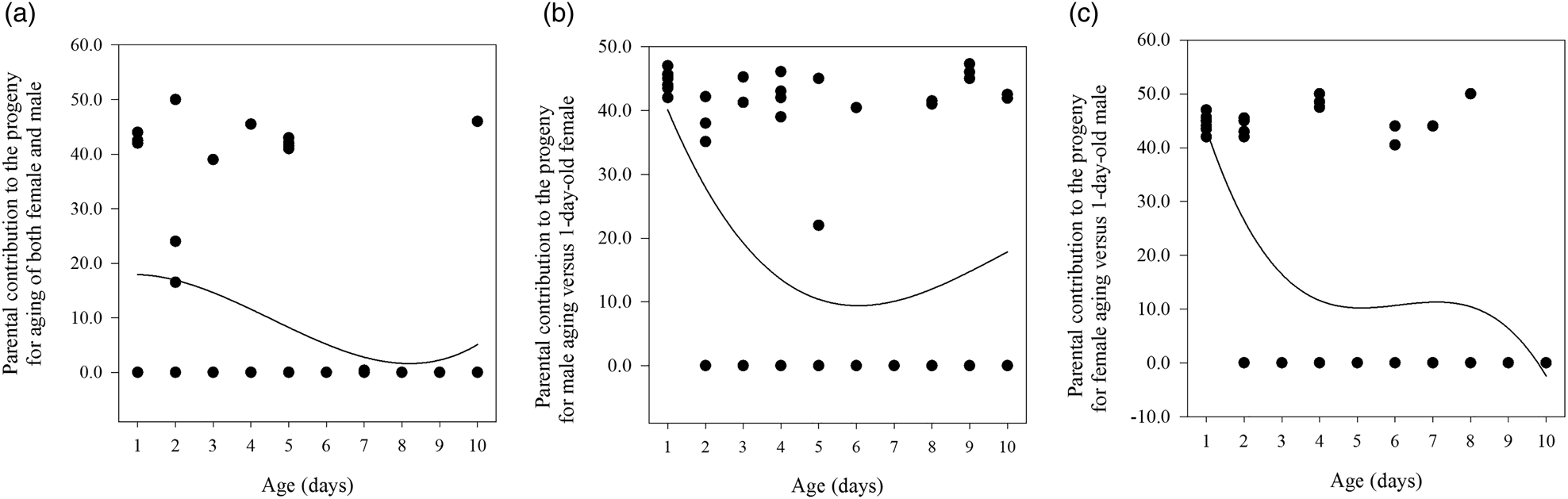
Figure 2. The three relationships between mating age and the parental contribution to the progeny for age-shifted mating of both male and female (A), male age-shifted mating (B), and female age-shifted mating (C) in Grapholita molesta.
Effect of varying age in both sexes
As the ages of both males and females increased in SC 1, the starting time of mating per cage (F = 2.777, df = 59, 9, P < 0.05; r s = −0.374, P < 0.01, n = 60), the number of eggs laid by all mated females per cage (F = 2.208, df = 59, 9, P < 0.05; r s = −0.330, P < 0.01, n = 60), and the percentage of eggs hatched per cage (F = 2.582, df = 59, 9, P < 0.05; r s = −0.322, P < 0.05, n = 60) all declined significantly (table 2). However, there were no significant relationships between aging of males and females (x) and the parental contribution to the progeny (y) (cubic regression: y = 17.2 + 1.8x − 1.1x 2 + 0.1x 3; F = 2.591; R 2 = 0.122; P = 0.062) (fig. 2A).
Table 2. Effects of age-shifted mating of both females and males (setting condition 1), males (setting condition 2) or females (setting condition 3) of Grapholita molesta on the mean mating start time per cage (±SE), number of eggs laid by all mated females per cage (± SE) and percentage of eggs hatched per cage (±SE) in the laboratory.
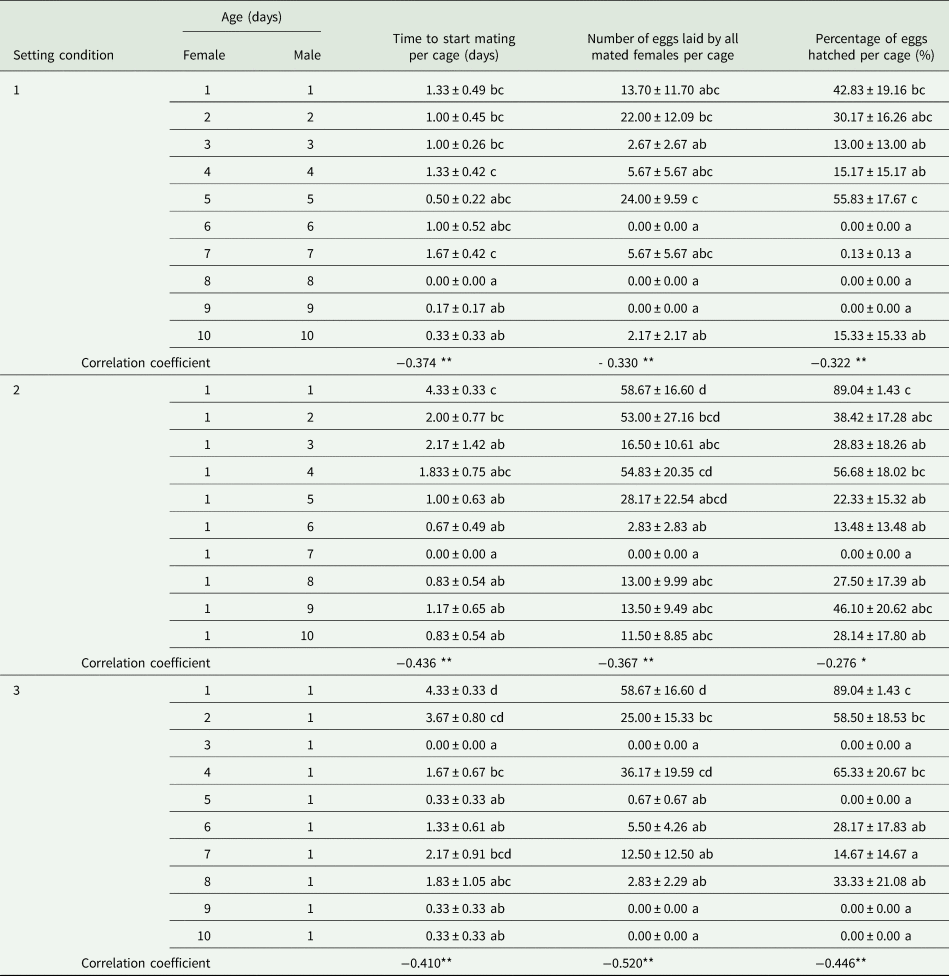
Different lowercase letters indicate significant differences among ages in each treatment at a P < 0.05 level, based on multiple comparisons (Tukey's multiple range test or Games-Howell test).
∗∗ and ∗ indicate that the correlation coefficients between age and the measured parameters were significant at 0.01 and 0.05, respectively.
Effect of varying male age
The time to start mating per cage (F = 2.808, df = 59, 9, P < 0.05; r s = −0.436, P < 0.01, n = 60), the number of eggs laid by all mated females per cage (F = 2.451 df = 59, 9, P < 0.05; r s = −0.367, P < 0.01, n = 60), and percentage of eggs hatched per cage (F = 2.362, df = 59, 9, P < 0.05; r s = −0.033, P < 0.01, n = 60) all decreased significantly with increasing male age in SC 2 (table 2). Moreover, we found a significant relationship between male aging (x) and the parental contribution to the progeny (y) (cubic regression: y = 56.1 − 18.1x + 2.2x 2 − 0.1x 3; F = 4.433; R 2 = 0.192; P < 0.05) (fig. 2B).
Effect of varying female age
The starting time of mating per cage (F = 5.161, df = 59, 9, P < 0.05; r s = −0.410, P < 0.01, n = 60), the number of eggs laid by all mated females per cage (F = 5.768, df = 59, 9, P < 0.05; r s = −0.520, P < 0.01, n = 60), and the percentage of eggs hatched per cage (F = 6.056, df = 59, 9, P < 0.05; r s = −0.446, P < 0.01, n = 60) all decreased significantly with increasing female age in SC 3 (table 2). We also found a significant relationship between female aging (x) and the parental contribution to the progeny (y) (cubic regression:y = 68.8 − 30.2x + 5.1x 2 − 0.3x 3; F = 8.375; R 2 = 0.310; P < 0.05) (fig. 2C).
Experiment 3: effects of repeated single mating or multiple mating on mating and reproductive behavior under different male mating histories
Repeated single mating of two sexes under different male mating histories
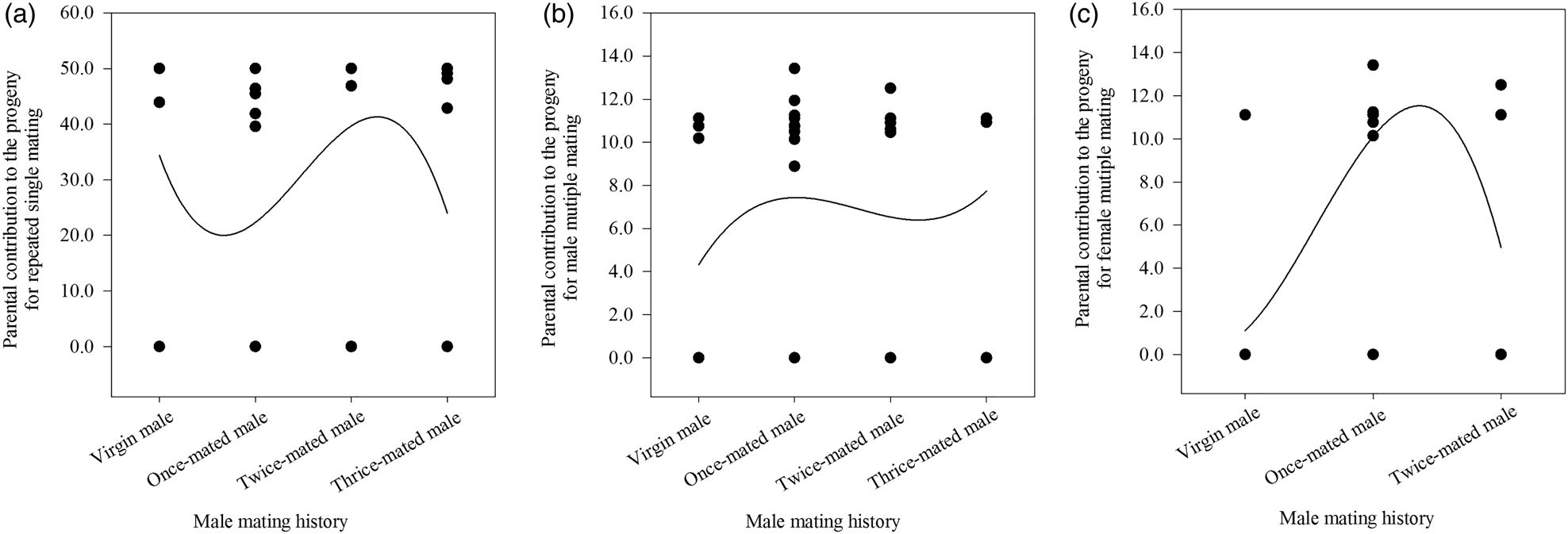
When males with different mating histories repeatedly mated with one virgin female in SC 1, the number of matings (F = 0.429, df = 39, 9, P = 0.734), number of eggs laid by all mated females per cage (F = 0.501, df = 39, 9, P = 0.806), and percentage of eggs hatched (F = 1.090, df = 39, 9, P = 0.366) were not significantly affected by the mating history of the males (table 3). Moreover, no correlation was observed between male mating history and the number of matings (r s = −0.025, P = 0.878, n = 40), the number of eggs laid by all mated females per cage (r s = 0.108, P = 0.505, n = 40) or the percentage of eggs hatched (r s = −0.088, P = 0.591, n = 40) (table 3). Additionally, we found no statistically significant relationship between repeated single mating among male mating histories (x) and the parental contribution to the progeny (y) (cubic regression:y = 0.8 + 1.1x; F = 1.253; R 2 = 0.095; P = 0.305) (fig. 3A).
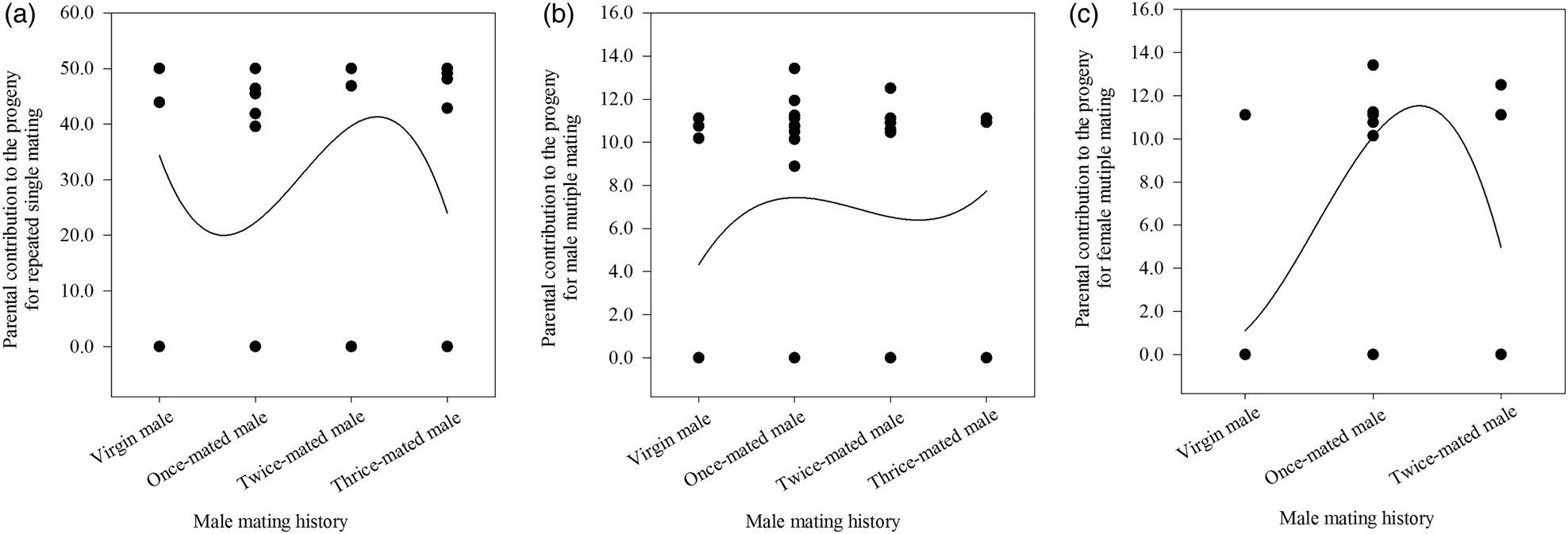
Figure 3. The three relationships between male mating history and the parental contribution to the progeny for repeated single mating (A), male multiple mating (B), and female multiple mating (C) in Grapholita molesta.
Table 3. Effects of different male mating histories in repeated single mating (setting condition 1), male multiple mating (setting condition 2), or female multiple mating (setting condition 3) in Grapholita molesta on the mean number of matings (±SE), number of eggs laid by all mated females (±SE), and percentage of eggs hatched (±SE) in the laboratory.
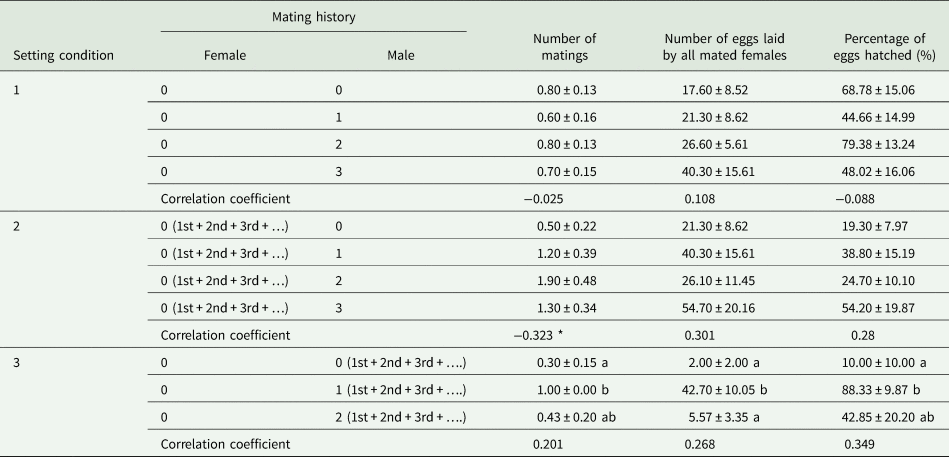
Different lowercase letters indicate significant differences among male mating histories in each treatment at a P < 0.05 level, based on multiple comparisons (Tukey's multiple range test or Games-Howell test).
* indicates that the correlation coefficients between male mating history and the measured parameters were significant at 0.05.
0, 1st, 2nd, and 3rd indicate virgin adults and adults mated one, two, and three times, respectively.
1st + 2nd + 3rd + ….indicates the daily replacement of one old adult with one new adult.
Multiple mating of males under different male mating histories
When males with zero (0♂), one (1♂), two (2♂), or three (3♂) prior mating experiences were paired with virgin females, there were no significant differences in the numbers of matings (0♂:U = 34, df = 1, P = 0.168; 1♂: U = 36, df = 1, P = 0.246; 2♂:U = 29, df = 1, P = 0.061; 3♂: U = 32.5, df = 1, P = 0.158), the number of eggs laid by all mated females (0♂:U = 37, df = 1, P = 0.303; 1♂: U = 48, df = 1, P = 0.876; 2♂:U = 50, df = 1, P = 1; 3♂: U = 41.5, df = 1, P = 0.507), or the percentage of eggs hatched (0♂:U = 31, df = 1, P = 0.119; 1♂: U = 32.5, df = 1, P = 0.171; 2♂:U = 31.5, df = 1, P = 0.228; 3♂: U = 31.5, df = 1, P = 0.139) between repeated single mating of SC 1 and male multiple mating of SC 2.
When males were mated with multiple different partners in SC 2, there were no significant effects of male mating history on the number of matings (F = 2.606, df = 39, 9, P = 0.067), the number of eggs laid by all mated females (F = 1.402, df = 39, 9, P = 0.258), or the percentage of eggs hatched (F = 0.794, df = 39, 9, P = 0.505) (table 3). Moreover, a positive correlation was observed between male mating history and the number of matings (r s = 0.323, P < 0.05, n = 40) but not between this history and the number of eggs laid by all mated females (r s = 0.301, P = 0.059, n = 40) or the percentage of eggs hatched (r s = 0.280, P = 0.080, n = 40) (table 3). Additionally, we found no significant relationship between male multiple mating among male mating histories (x) and the parental contribution to the progeny (y) (cubic regression: y = 4.3 + 7.2x − 5.1x 2 + 1.0x 3; F = 0.811; R 2 = 0.063; P = 0.496) (fig. 3B).
Multiple mating of females under different male mating histories
When virgin females were paired with virgin males, there were significant differences in the number of matings (U = 25, df = 1, P < 0.05), the number of eggs laid by all mated females per cage (U = 20.5, df = 1, P < 0.05), and the percentage of eggs hatched (U = 20.5, df = 1, P < 0.05) between repeated single mating of SC 1 and female multiple mating of SC 3. When virgin females were paired with males with one prior mating experience, there were significant differences in the number of matings (U = 30, df = 1, P < 0.05) and the percentage of eggs hatched (U = 14, df = 1, P < 0.05) but not in the number of eggs laid by all mated females (U = 31, df = 1, P = 0.145) between repeated single mating of SC 1 and female multiple mating of SC 3. When virgin females were paired with males with two prior mating experiences, there were significant differences in the number of eggs laid by all mated females (U = 12, df = 1, P < 0.05) but not in the number of matings (U = 22, df = 1, P = 0.126) or the percentage of eggs hatched (U = 12, df = 1, P = 0.196) between repeated single mating of SC 1 and female multiple mating of SC 3.
When females were exposed to different males with multiple mating experiences in SC 3, the number of matings (F = 8.508, df = 26, 2, P < 0.05), the number of eggs laid by all mated females (F = 16.199, df = 26, 2, P < 0.05), and the percentage of eggs hatched (F = 10.821, df = 26, 2, P < 0.05) were all significantly affected by male mating history (table 3). No correlation was observed between male mating history and the number of matings (r s = 0.201, P = 0.315, n = 27), the number of eggs laid by all mated females (r s = 0.268, P = 0.177, n = 27) or the percentage of eggs hatched (r s = 0.349, P = 0.074, n = 27) (table 3). However, we found a significant relationship between female multiple mating among male mating histories (x) and the parental contribution to the progeny (y) (cubic regression: y = 1.1 + 0.6x; F = 6.761; R 2 = 0.469; P < 0.05) (fig. 3C).
Discussion
In the case of polygamy, the ability of a portion of males to monopolize access to females is thought to be the fundamental determinant of mating systems (Thornhill and Gwynne, Reference Thornhill and Gwynne1986), whereas, the female's investment in reproduction is dictated by her physiological state, the expectation of future reproductive opportunities and the trade-off between the two (Nilsson and Svensson, Reference Nilsson and Svensson1996; Candolin, Reference Candolin1998). However, males generally seek promiscuous, multiple mating opportunities with minimal investment in offspring in order to maximize their reproductive success (Trivers, Reference Trivers and Campbell1972).
Our results showed that for specific-male, the less male-biased OSR (SC 1) caused more mating, eggs laid by all mated females per cage and thus higher percentage of eggs hatched per cage, while the more male-biased OSR (SC 2) only resulted in fewer mating and eggs laid by all mated females per cage (table 1). This may have been caused by a certain degree of male competition increasing the mating rate (Zhan et al., Reference Zhan, Liu and Liu2020). However, the percentage of eggs hatched per cage decreased with the less female-biased OSR (SC 3) for specific females (table 1). This may be because males are conservative in their release of sperm when sperm competition occurs (Weir et al., Reference Weir, Grant and Hutchings2011). These results thus indicated that mating opportunities and reproductive potential can vary with the OSR for virgin males but not virgin females. Further analysis of the relationship between the OSR and parental contribution to offspring showed that for OSRs from even (1:1) to more female (SC 1)/male (SC 3) biased at low density, the contribution gradually decreased with an increasing number of parents, and when the parental number was four to six, these contributions were kept to a minimum (fig. 1A, C). It may be resulted from lack of sperm based on the reported four copulations at most for males (de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019), or male competition based on the reported only mating once for females (de Morais et al., Reference de Morais, Sant'Ana, Redaelli and Lorscheiter2011; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019). For OSRs from even (5:5) to more female (SC 2)/male (SC 4) biased at high density, the contribution steadily increased with a decreasing number of parents to eight (fig. 1B, D). de Jong et al. (Reference de Jong, Forsgren, Sandvik and Amundsen2012) advocated that courtship propensity and copulation chance increase when the OSR become more biased toward competitors. Because of asymmetry of the two sexes in G. molesta, the parental contribution to the progeny based on OSR was relatively higher at low density than at high density. This result thus indicated that the effect of OSR was related to G. molesta population density. Our experimental design impeded us from recording this variable and therefore we recorded and analyzed the number of eggs per cage irrespective of the number of females involved. Taking these results together, virgin males prefer a female number that is less than three-fifths of the male number, whereas they discriminate against a female number that is more than three times the male number.
We also found that the age of the moths, of one or both sexes at the time of first copulation, was negatively associated with the time of mating per cage, the number of eggs laid by all mated females per cage, and the percentage of eggs hatched per cage. This relationship has also been found in many other moths (Mori and Evenden, Reference Mori and Evenden2013). Such a relationship may result from decreases in accessory gland secretions and hormones in older males (Huang and Subramanyam, Reference Huang and Subramanyam2003) and the associated significantly lower fecundity and fertility in older females (Pinto et al., Reference Pinto, Sant'Ana and Botton2005; Knight, Reference Knight2007; de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012). Although 5- and 8–10-day-old males and females required less than one day before mating, mating and reproduction successively stopped for 6- and 8-day-old males and females (SC 1; table 2), which was supported by previous reports (Wang et al., Reference Wang, Fang and Zhang2011; de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012) that the male can still fully inseminate the female even at a low level of fecundity for the same ages of both sexes. Mating and reproduction stopped for 7-day-old males (SC 2) or 3-day-old females (SC 3), while mating occurred from days 2–4 after pairing for 1–4-day-old males (SC 2) or 1–2-day-old females (SC 3) (table 2). A negative effect of a delay in mating on G. molesta fecundity was observed to be highest when the aging occurred in the female, followed by the male, while delays in mating for both sexes had the weakest effect on fecundity; this pattern is consistent with observations in many lepidopteron species that the aging of virgin females has a detrimental effect on reproduction (Jiménez-Pérez and Wang, Reference Jiménez-Pérez and Wang2003; Stelinski and Gut, Reference Stelinski and Gut2009; Kawazu et al., Reference Kawazu, Shintani and Tatsuki2014; Yang et al., Reference Yang, Zhang, Zhang, Zhang and Long2017). However, females mated preferentially with younger males but gained no apparent fitness benefits in a monandrous moth species, and a delay in the time of mating for both males and females is more detrimental to female fitness than a female delay alone (Stelinski and Gut, Reference Stelinski and Gut2009; Lai et al., Reference Lai, Zhang, Zhang and Liu2020). Further analysis of the relationship between mating age and the parental contribution to offspring showed that the contribution tended to decrease with the aging of both females and males (SC 1), with the values always low (fig. 2A); the contribution decreased and then increased with the aging of males (SC 2), with the contribution of 6-day-old males being the smallest (fig. 2B); and the contribution decreased with the aging of females (SC 3), but with two stages, including 5–8-day-old and 9–10-day-old females (fig. 2C). Overall, the ranking of the parental aging effect on offspring in G. molesta was as follows: both females and males > females > males. For 1-day-old between SC 1 and SCs 2 and 3, the start time per cage and the number of eggs laid by all mated females per cage differed because of different experimental time, but no difference in the percentage of eggs hatched per cage did not affect the parental contribution to offspring. It thus indicated that the mating opportunities and reproductive potential significantly decreased with partner's aging for virgin males but not virgin females. Taking these results together, virgin males prefer ≤2-day-old females and discriminate against ≥5-day-old females.
When we analyzed repeated single mating or multiple mating under different male mating histories in G. molesta, we found that the number of matings, the number of eggs laid by all mated females and the percentage of eggs hatched for males did not differ either among male mating histories in repeated single mating (SC 1) and male multiple mating (SC 2), or between repeated single mating (SC 1) and male multiple mating (SC 2) under a given male mating history (table 3), which was congruent with previous reports of no relationship between male mating history and female reproductive performance (Unnithan and Paye, Reference Unnithan and Paye1991; Sadek, Reference Sadek2001; Torresvila and Jennions, Reference Torresvila and Jennions2005). For repeated single mating (SC 1) and male multiple mating (SC 2), there were no relationships between parental contribution to offspring and male mating history (fig. 3A, B). Fertility, which was previously reported to be not altered by females with the constant presence of the same or different males, virgins or sexually mature in G. molesta (de Morais et al., Reference de Morais, Sant'Ana, Redaelli and Lorscheiter2011; Kong et al., Reference Kong, Wang, Jia, Gao, Fan, Li and Ma2019), may be related to female mass rather than male mass significantly responsible for the quantity of accessory gland secretions and apyrene sperm transferred (Marcotte et al., Reference Marcotte, Delisle and McNeil2005). The same pattern was observed in Plodia interpunctella (Pyralidae) (Cook, Reference Cook1999), Cnephasia jactatana (Tortricidae) (Jiménez-Pérez and Wang, Reference Jiménez-Pérez and Wang2003), Cydia pomonella (Knight, Reference Knight2007) and Choristoneura fumiferana (Marcotte et al., Reference Marcotte, Delisle and McNeil2005). This result thus indicated that pair formation in G. molesta was mainly affected by virgin females per se.
Comparing for repeated single mating (SC 1) and female multiple mating (SC 3) under different male mating histories, the number of eggs laid by all mated females and the percentage of eggs hatched for the same virgin male were higher than those for different virgin males (table 3), which was supported by a previous report (de Morais et al., Reference de Morais, Sant'Ana, Redaelli and Lorscheiter2011). However, the number of matings and the number of eggs laid by all mated females for different once-mated males (SC 3) were observed to be higher than those for the same once-mated male (SC 1) (table 3). Females may be required to trade-off male age against sperm age and mating history when choosing a mate (Jones and Elgar, Reference Jones and Elgar2004; Koppik et al., Reference Koppik, Ruhmanna and Fricke2018). Thus, the difference in pair formation in G. molesta from the virgin female perspective occurred between virgin and once-mated males.
For female multiple mating (SC 3), the number of matings, the number of eggs laid by all mated females and the percentage of eggs hatched of virgin females paired with different once-mated males were observed to be higher than those of virgin females paired with different virgin males (table 3), which was supported by a previous report (de Morais et al., Reference de Morais, Redaelli and Sant'Ana2012). Furthermore, the parental contribution to offspring from virgin females paired with different once-mated males peaked (fig. 3C), which possibly resulted from a male's prior mating regime (Wedell et al., Reference Wedell, Gage and Parker2002) and/or the age of his sperm (Siva-Jothy, Reference Siva-Jothy2000). Taking these results together, virgin females prefer once-mated males and discriminate against virgin males in multiple mating.
These results characterize G. molesta as a species with high reproductive potential, which is an important aspect to consider for pest control in Rosaceae orchards. The present study concluded that the mating system of G. molesta is modulated by the quantity, age, and prior mating status of either males or females. Most previous studies have reported that multiple mating in insects can significantly reduce the efficiency of synthetic female sex pheromones for managing field populations of pests (e.g. Sadek, Reference Sadek2001; Knight, Reference Knight2009). In G. molesta that display a combination of monandry and promiscuity, control strategies based on mating disruption and mass trapping should consider mating of virgin adults, and include the following measures: (1) virgin males paired with older or more females and (2) virgin females paired with virgin males. For example, confusing virgin males using mating disruption is equivalent to aging of virgin females or a more pseudo-female-biased sex ratio. Compromising the mating choice of virgin females using mass trapping for virgin males is equivalent to indirectly increasing virgin female number at the low density population. Future studies should work to further clarify the interaction of different ranges of mating age and history under variable OSRs in order to improve the effective suppression of insect pests.
Acknowledgements
The authors thank Ruiqing Ma and the undergraduate student assistants Wencui Liu, Jinghua Xie, Yujiao Dong, Ruixing Wang, and Bo Liao (Shanxi Agricultural University) for their help during the experiment.
Financial support
This work was supported by the Shanxi Key Research and Developmental Program (no. 201903D211001-1), the Support and Training of the Shanxi Academy of Agricultural Science of the National Nature Science Foundation of China (no. YGJPY2005), and the Special Export Platform for Fruit Trading of the Shanxi Academy of Agricultural Science (no. YCX2018304).
Conflict of interest
The authors declare that they have no conflicts of interest.