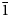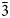Introduction
The majority of anhydrous vanadates in nature are endemics of oxidising-type volcanic fumaroles. The most diverse vanadate mineralisation of this origin is known in the active fumarole field located at the summit of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka, Russia. In the exhalations of two famous fumaroles belonging to this field, Yadovitaya and Arsenatnaya, 19 of 41 known anhydrous vanadate minerals occur. Vanadate mineralisation of the Yadovitaya fumarole includes 15 copper vanadates, most of which are endemic to this fumarole (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Britvin, Turchkova, Sidorov and Pushcharovsky2020). In the exhalations of the Arsenatnaya fumarole, four vanadates of Na, Ca and Mg occur, namely udinaite NaMg4(VO4)3 (Pekov et al., Reference Pekov, Zubkova, Koshlyakova, Belakovskiy, Vigasina, Agakhanov, Turchkova, Britvin, Sidorov and Pushcharovsky2018b), schäferite (Ca2Na)Mg2(VO4)3 (Koshlyakova et al., Reference Koshlyakova, Pekov, Zubkova, Agakhanov, Turchkova, Kartashov, Sidorov and Pushcharovsky2020), pliniusite Ca5(VO4)3F (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Krzątała, Galuskina, Belakovskiy, Galuskin, Britvin, Sidorov, Vapnik and Pushcharovsky2022), and reznitskyite CaMg(VO4)F, described in this paper.
Reznitskyite (Cyrillic – резницкиит) is named in honour of the outstanding Russian mineralogist Leonid Zinovievich Reznitsky (born 1938) who works in the Institute of the Earth's Crust of the Siberian Branch of the Russian Academy of Sciences, Irkutsk, Russia. Dr. Reznitsky has made a significant contribution to the mineralogy of vanadium; in particular, he is a senior author of descriptions of five new minerals with species-defining V, namely batisivite BaV8Ti6(Si2O7)O22, magnesiocoulsonite MgV2O4, natalyite NaV(Si2O6), oxyvanite V3+2V4+O5, and vanadio-pargasite NaCa2(Mg4V)(Al2Si6O22)(OH)2 and a co-author of first descriptions of three vanadian tourmalines: vanadio-oxy-chromium-dravite NaV3(Cr4Mg2)(Si6O18)(BO3)3(OH)3O, vanadio-oxy-dravite NaV3(Al4Mg2)(Si6O18)(BO3)3(OH)3O and oxy-vanadium-dravite NaV3(V4Mg2)(Si6O18)(BO3)3(OH)3O (the latter was described originally as vanadiodravite by Reznitsky with co-authors).
Reznitskyite is a new tilasite-group mineral and the first vanadate with titanite-type structure known not only in Nature but, to the best of our knowledge, also among synthetic compounds. Except reznitskyite, there is one titanite-type mineral with species-defining V, vanadomalayaite CaV4+(SiO–4)O; however, it contains tetravalent vanadium in the form of vanadyl cation (VO)2+ but not pentavalent vanadium forming vanadate anions (VO4)3–.
Both the new mineral and its name (symbol Rzs) have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (|IMA2021-067, Koshlyakova et al., Reference Koshlyakova, Pekov, Vigasina, Zubkova, Agakhanov, Britvin, Sidorov and Pushcharovsky2022). The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow under the catalogue number 97682.
Occurrence and mineral association
Reznitskyite was found at the Arsenatnaya fumarole, Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka peninsula, Far-Eastern Region, Russia, 55°41´N, 160°14´E, 1200 m asl. This famous fumarole, the type locality of 60 new minerals, was described in general by Pekov et al. (Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Schipalkina, Turchkova and Sidorov2018a) and Shchipalkina et al. (Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020).
The specimens with reznitskyite were collected by us during fieldwork in July 2018 from the deepest (depths of 3.5–4 m under the day surface) and hottest zone of the Arsenatnaya fumarole. The temperature measured during sampling with a chromel–alumel thermocouple was ~450°C. Thus, we assume that reznitskyite was formed at temperatures not lower than 450°C and that it was deposited directly from the gas phase as a volcanic sublimate or, more probably, formed as a result of the interaction between fumarolic gas and basalt scoria. The latter could be a source of Mg and Ca which have very low volatilities in such post-volcanic systems at temperatures up to 400–500°C (Symonds and Reed, Reference Symonds and Reed1993).
The new mineral was identified during scanning electron microscopy and electron microprobe studies of polymineralic sublimate encrustations covering the surface of basalt scoria altered by fumarolic gas. The major mineral of these crusts, open-work or, rarer, solid, is white anhydrite. Other common minerals are calciojohillerite, garnets of the schäferite–berzeliite series, minerals of the svabite–fluorapatite–pliniusite isomorphous system, hematite and diopside; less widespread minerals are members of the rhabdoborite group [rhabdoborite-(V), rhabdoborite-(W) and rhabdoborite-(Mo)] and the tilasite–isokite, wagnerite–arsenowagnerite, udinaite–arsenudinaite and powellite–scheelite series, as well as ludwigite, magnesioferrite, forsterite, titanite and baryte.
General appearance, physical properties and optical data
Reznitskyite forms zones (usually up to 0.05 mm thick) in crude prismatic crystals or in irregularly shaped grains of V- and P-containing tilasite or, rarer, occurs as homogeneous grains up to 0.1 mm across. Both tilasite and reznitskyite form intimate intergrowths with anhydrite, rhabdoborite-group minerals, V- and As-enriched fluorapatite, and magnesioferrite (Fig. 1).

Fig. 1. Aggregate of zoned crystals with zones of reznitskyite (1) and V-enriched tilasite (2) in intimate association with rhabdoborite-(V) (3), magnesioferrite (4) and anhydrite (5). Polished section, bask-scattered electron image (specimen number Tolb6615-14).
Reznitskyite is colourless, transparent or semi-transparent, with white streak and vitreous lustre. It does not fluoresce in ultraviolet light. The cleavage was not observed, probably due to the small size of grains; it could be suggested, by analogy with the closely related mineral tilasite, that reznitskyite has a {10$\bar{1}$![]() } cleavage. The fracture is uneven. Density could not be measured because of the small size of individuals; the density value calculated for the holotype reznitskyite using the empirical formula and unit-cell volume determined from the single-crystal X-ray diffraction data is 3.453 g cm–3.
} cleavage. The fracture is uneven. Density could not be measured because of the small size of individuals; the density value calculated for the holotype reznitskyite using the empirical formula and unit-cell volume determined from the single-crystal X-ray diffraction data is 3.453 g cm–3.
Under the microscope, in reflected light the holotype reznitskyite is grey, non-pleochroic, with very weak bireflectance (ΔR 589 nm = 0.5%) and distinct anisotropy. Internal reflections were not observed. Reflectance values are given in Table 1.
Table 1. The reflectance data of reznitskyite, % (SiC standard, measured in air)*.

*The reference wavelengths (λ) required by the Commission on Ore Mineralogy (COM) are given in bold.
Analytical methods
The chemical data for samples from Tolbachik were obtained in the Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University using a JEOL JXA 8230 Superprobe instrument. Electron microprobe analyses (EMPA) were carried out in WDS mode (20 kV and 20 nA). Chemical data of the holotype sample (for five spot analyses of the crystal which was further used for the crystal structure determination) are given in Table 2. The contents of other elements with atomic numbers higher than carbon are below detection limits.
Table 2. Chemical composition of reznitskyite.

S.D. – standard deviation
The Raman spectrum of reznitskyite was obtained on a randomly oriented crystal using an EnSpectr R532 instrument with a green laser (532 nm) at room temperature. The output power of the laser beam was ~4 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines⋅cm–1, spectral resolution was 6 cm–1. The diameter of the focal spot on the sample was ~10 μm. The back-scattered Raman signal was collected with 40× objective; signal acquisition time for a single scan of the spectral range was 1000 ms and the signal was averaged over 100 scans. The instrument was calibrated with the line of crystalline silicon at 520 cm–1.
A powder X-ray diffraction (XRD) study of reznitskyite was carried out using a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a curved image plate detector (Debye-Scherrer geometry, r = 127.4 mm) and CoKα radiation source. The data were integrated using the software package osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017).
Single-crystal XRD studies of the holotype reznitskyite were carried out using an Xcalibur S CCD diffractometer (MoKα radiation). A full sphere of three-dimensional data was collected. Data reduction was performed using CrysAlisPro Version 1.171.37.35 (Agilent Technologies, 2014). The data were corrected for Lorentz and polarisation effects. The crystal structure of reznitskyite was solved by direct methods with the SHELX software package (Sheldrick, Reference Sheldrick2015) and refined to R = 0.0370 for 463 unique reflections with I > 2σ(I).
Raman spectroscopy
The Raman spectra of reznitskyite and its isostructural arsenate and phosphate analogues, tilasite and isokite, respectively, are given in Fig. 2. The bands in the Raman spectrum of reznitskyite are assigned according to Nakamoto (Reference Nakamoto1986).

Fig. 2. The Raman spectra of reznitskyite (our data), tilasite and isokite (RRUFF database [Lafuente et al., Reference Lafuente, Downs, Yang, Stone, Armbruster and Danisi2015], R060618 and R070526.2, respectively).
Weak bands with frequencies at 1017 and 948 cm–1 correspond to stretching vibrations ν3(F2) and ν1(A1) of PO43– tetrahedra (compare to the isokite spectrum on Fig. 2), respectively. Strong bands in the region 750–900 cm–1 correspond to the same vibrational modes ν3(F2) and ν1(A1) of tetrahedra VO43– and AsO43– i.e. stretching vibrations of V5+–O and As5+–O bonds. Obviously the bands corresponding to these anions partially overlap each other, which led to the broadening of the lines. The bands in the region 300–500 cm–1 correspond to overlapped ν4(F2) and ν2(E) bending modes of AsO4 and VO4 tetrahedra. Bands corresponding to degenerate vibrational modes of tetrahedra are split in the spectrum of reznitskyite that can indicate deformation of tetrahedra due to a lower than (Td) site symmetry of anions (a similar situation is observed in the tilasite spectrum: see Fig. 2). The spectral region lower than 300 cm–1 corresponds to Ca and Mg translation modes and lattice modes.
The absence of bands with frequencies higher than 1030 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in the mineral.
Chemical composition
Electron microprobe analyses of the holotype specimen of reznitskyite were obtained from the polished section of the crystal later extracted for single-crystal XRD study. Chemical composition of this crystal, average based on five spot analyses, is given in Table 2. The empirical formula, calculated on the basis of O+F = 5 atoms per formula unit, is Ca0.97Mg1.03(V0.47As0.33P0.18S0.01)Σ0.99O3.99F1.01.
The simplified formula of reznitskyite is CaMg[(V,As,P)O4]F. The ideal formula is CaMg(VO4)F which requires MgO 20.32, CaO 28.28, V2O5 45.86, F 9.58, –O=F –4.04, total 100 wt.%.
X-ray crystallography and crystal structure
Powder XRD data of reznitskyite are given in Table 3. Parameters of the monoclinic unit cell calculated from the powder data are: a = 6.699(7), b = 8.938(4), c = 7.048(9) Å, β = 112.99(8)° and V = 388.5(9) Å3.
Table 3. Powder X-ray diffraction data (d in Å) of reznitskyite.

*For the calculated pattern only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
The unit-cell parameters obtained for reznitskyite from single-crystal XRD data are: a = 6.6912(7), b = 8.9395(7), c = 7.0587(8) Å, β = 113.078(13)° and V = 388.43(8) Å3. Crystal data, data collection information and structure refinement details are given in Table 4, atomic coordinates, equivalent displacement parameters and bond-valence sums are reported in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Crystal data, data collection information and structure refinement details for reznitskyite.

Table 5. Сoordinates, equivalent displacement parameters (U eq, in Å2) of atoms and bond-valence sums (BVS) for reznitskyite.

*Bond-valence parameters were taken from Gagné and Hawthorne (Reference Gagné and Hawthorne2015) (for oxygen anions) and Brese and O'Keeffe (Reference Brese and O'Keeffe1991) (for the fluorine anion).
**The refined s.o.f. values for the components of the mixed-cation tetrahedral site (T) are V 0.49(3), As 0.34(1) and P 0.17(2)
Reznitskyite adopts the well-known titanite-type structure. It contains infinite chains of MgO4F2 octahedra connected with one another via F– vertices and running along the c axis. The average cation–anion distances in these distorted octahedra are: Mg–O = 2.106 and Mg–F = 1.908 Å. Adjacent chains are connected to each other by isolated T 5+O4 tetrahedra forming a three-dimensional heteropolyhedral (octahedral–tetrahedral) framework. Calcium cations, coordinated by six O atoms and one F atom, centre the voids within the heteropolyhedral framework, with an average Ca–O distance of 2.508 Å and the Ca–F distance of 2.247 Å. The tetrahedrally coordinated T site is the only multicomponent position in reznitskyite, with the average T–O distance of 1.671 Å (Table 6, Fig. 3).

Fig. 3. The crystal structure of reznitskyite in three projections. The unit cell is shown by a dotted line.
Table 6. Selected interatomic distances (Å) in the crystal structure of reznitskyite

The refinement of site occupancies was performed considering the EMPA data obtained for the crystal studied from the polished specimen (Table 1). Minor admixture of sulfur was ignored, and three components, namely As, V and P, were assigned to the T site with the sum of site occupation factors (s.o.f.) restrained to be 1.00. The s.o.f. of Ca and Mg at the corresponding sites were also fixed at 1.00 each. The refined s.o.f. values for the tetrahedrally coordinated components are V 0.49(3), As 0.34(1), and P 0.17(2), which is in a very good agreement with the empirical formula calculated from the EMPA data: Ca0.97Mg1.03(V0.47As0.33P0.18S0.01)Σ0.99O3.99F1.01.
Discussion
Until recently, minerals with the titanite-type structure belonging to four chemical classes were known, namely silicates (titanite group), arsenates, phosphates (tilasite group), and sulfates (tilasite and kieserite groups) (Back, Reference Back2018). Reznitskyite is the first vanadate which adopts the titanite-type structure. Representatives of all these chemical classes were found by us in high-temperature exhalations of the Arsenatnaya fumarole: tilasite CaMg(AsO4)F, durangite NaAl(AsO4)F, arsenatrotitanite NaTi(AsO4)O (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019), kononovite NaMg(SO4)F (Pekov et al., Reference Pekov, Krzhizhanovskaya, Yapaskurt, Belakovskiy, Chukanov, Lykova and Sidorov2015), isokite CaMg(PO4)F, titanite CaTi(SiO4)O (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Schipalkina, Turchkova and Sidorov2018a; Shchipalkina et al., Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020), maxwellite NaFe3+(AsO4)F, and reznitskyite CaMg(VO4)F.
The diversity of titanite-type oxysalts in the Arsenatnaya fumarole is probably caused by both the chemical zonation of the exhalations and flexibility of the titanite structure. Kononovite was found in the sulfate-rich upper zone of the fumarole (see description of the zonation in: Shchipalkina et al., Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020) characherised by the abundance of langbeinite, aphthitalite-group members, krasheninnikovite, anhydrite and euchlorine. Titanite occurs in the paragenesis with alkali silicates – sanidine, anorthoclase and sodalite in the intermediate zone.
Tilasite is the most widespread titanite-type mineral in the Arsenatnaya fumarole. It is represented by two chemical varieties which occur in different mineral assemblages related to different zones. In the intermediate zone enriched with Cu, Fe3+ and Al arsenates (johillerite, badalovite, calciojohillerite, lammerite, urusovite and ericlaxmanite), the V- and P-poor variety of tilasite forms the complex solid-solution system with durangite, maxwellite and arsenatrotitanite (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Schipalkina, Turchkova and Sidorov2018a).
Reznitskyite CaMg(VO4)F, tilasite CaMg(AsO4)F and isokite CaMg(PO4)F form another solid-solution system in the deepest levels of the Arsenatnaya fumarole characterised by the presence of other minerals which demonstrate significant isomorphism between pentavalent P, As and V in tetrahedral coordination. There are, e.g. members of the solid-solution system between fluorapatite Ca5(PO4)3F, svabite Ca5(AsO4)3F, and pliniusite Ca5(VO4)3F (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Krzątała, Galuskina, Belakovskiy, Galuskin, Britvin, Sidorov, Vapnik and Pushcharovsky2022), solid-solution series udinaite–arsenudinaite NaMg4(VO4)3–NaMg4(AsO4)3, wagnerite–arsenowagnerite Mg2(PO4)F–Mg2(AsO4)F, schäferite–berzeliite (Ca2Na)Mg2(VO4)3–(Ca2Na)Mg2(AsO4)3, and the V- and P-rich variety of calciojohillerite NaCaMg3[(As,P,V)O4]3 (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Schipalkina, Turchkova and Sidorov2018a). Unlike the upper zone, here tilasite is represented by the V- and P-enriched variety.
All reznitskyite samples studied contain significant admixtures of As and P which substitute V5+ in a tetrahedrally coordinated position. Parameters of unit cells are predictably very close for reznitskyite and tilasite due to the closeness of ionic radii of IVV5+ (0.355 Å) and IVAs5+ (0.335 Å) (Shannon, Reference Shannon1976). It is noteworthy that the formation of solid solution between the arsenate and phosphate members of the system is more common in Nature, despite more significant difference between the ionic radii of IVAs5+ and IVP5+ (0.17 Å). Probably the isomorphism between the semi-metal As and the metal V (and, especially, between V and the non-metal P) is hampered due to the distinct difference of the structure of their electron shells. We believe that crystallisation in a non-stationary fumarole system, with the combination of high temperature with low (atmospheric) pressure favours the isomorphism between As5+, P5+ and V5+, like in apatite-type minerals (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Krzątała, Galuskina, Belakovskiy, Galuskin, Britvin, Sidorov, Vapnik and Pushcharovsky2022). Unit-cell parameters, the strongest reflections of the powder X-ray diffraction patterns and densities of reznitskyite, tilasite and isokite are compared in Table 7.
Table 7. Comparative data for Ca–Mg minerals of the tilasite group.

*For tilasite, the non-standard setting with the same space group was used in the cited papers (Bladh et al., Reference Bladh, Corbett, McLean and Laughton1972; Bermanec, Reference Bermanec1994). It can be transformed to the setting common for titanite-type compounds which is used, in particular, for isokite and reznitskyite, through the cell transformation matrix ($\bar{1}$![]() 01/0$\bar{1}$
01/0$\bar{1}$![]() 0/001) and translation matrix (0.25/0.25/0). In this common setting, the c' and β' values for tilasite are as follows: c' = 7.04–7.05 Å and β' = 113.1–113.2°.
0/001) and translation matrix (0.25/0.25/0). In this common setting, the c' and β' values for tilasite are as follows: c' = 7.04–7.05 Å and β' = 113.1–113.2°.
Acknowledgements
We thank Prof. Peter Leverett and two anonymous reviewers for valuable comments. This work was supported by the Russian Science Foundation, grant no. 20-77-00063. The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.16