INTRODUCTION
Malaria caused by Plasmodium vivax, after decades of research neglect, is being re-assessed as a major contributor to morbidity and mortality in the widespread regions where it is endemic (Price et al. Reference Price, Tjitra, Guerra, Yeung, White and Anstey2007; Galinski and Barnwell, Reference Galinski and Barnwell2008). However, large gaps still exist in knowledge of the epidemiology, entomology and ecology of this parasite (Mueller et al. Reference Mueller, Galinski, Baird, Carlton, Kochar, Alonso and del Portillo2009; Gething et al. Reference Gething, Elyazar, Moyes, Smith, Battle, Guerra, Patil, Tatem, Howes, Myers, George, Horby, Wertheim, Price, Mueller, Baird and Hay2012). One of these gaps is the phenomenon of extended incubation periods (>28 weeks) (Warrell and Gilles, Reference Warrell and Gilles2002). These phenotypes have been observed in modern parasite strains from diverse global settings including Brazil and the Korean peninsula (Brasil et al. Reference Brasil, de, Costa, Pedro, da, Bressan, da Silva, Tauil and Daniel-Ribeiro2011; Kim et al. Reference Kim, Kim, Jo, Gwack, Youn and Jang2013) and antimalarial drug prophylaxis has also been implicated in prolonged latency (Schwartz et al. Reference Schwartz, Parise, Kozarsky and Cetron2003). The biological basis of delayed onset infections after sporozoite inoculation remains unclear; whether the persisting parasites are hypnozoites, quiescent merozoites or both is unknown (Markus, Reference Markus2012). Persistent and infective dermal sporozoites have also been suggested (Guilbride et al. Reference Guilbride, Guilbride and Gawlinski2012; Ménard et al. Reference Ménard, Tavares, Cockburn, Markus, Zavala and Amino2013).
The incubation period is a key parameter in epidemiological and clinical studies; in malaria, it is defined as the time from exposure to infected anopheline vectors to febrile illness. The pre-patent period is similarly calculated to the time when parasites are first visible in the peripheral blood. Gaps in these areas have been highlighted as key research needs for P. vivax human vaccine development (Targett et al. Reference Targett, Moorthy and Brown2013).
Historical human experimental challenges during malariotherapy for terminal neurosyphilis (1920s–1950s) and prison volunteer experiments for antimalarial prophylaxis suggested an inverse relationship between the size of sporozoite inocula and time-to-infection; modern reviews have supported this view (Krotoski et al. Reference Krotoski, Garnham, Cogswell, Collins, Bray, Gwadz, Killick-Kendrick, Wolf, Sinden and Hollingdale1986; Glynn, Reference Glynn1994; White, Reference White2011; Vanderberg, Reference Vanderberg2014).
Specifically, it has been reported that small sporozoite doses (10–100 sporozoites) of P. vivax strains isolated from temperate regions resulted in the primary attack generally being delayed for 9–10 months or longer, whereas illness occurred after a ‘normal’ incubation period of about 2 weeks (White, Reference White2011) when larger inocula (⩾1000 sporozoites) were used. Conversely, for tropical parasite strains in these historical studies, no relationship between sporozoite doses and the latent period was observed.
However, there is also considerable ambiguity in relation to dose-dependence in the literature; some authors suggest no differences by latitude of parasite origin, with dose dependence of time-to-infection being a general feature of P. vivax (Vanderberg, Reference Vanderberg2014). Pampana reported that long-latency was due to small inocula, but also quoted Russian researchers who described four strains that invariably showed long-latency (Pampana, Reference Pampana1969). Other researchers reported that the length of incubation of a North Korean strain was dependent on the number of mosquito bites (Tiburskaja and Vrublevskaja, Reference Tiburskaja and Vrublevskaja1977). Finally, recent research has suggested that parasite strain itself may independently influence incubation period (Herrera et al. Reference Herrera, Fernandez, Manzano, Murrain, Vergara, Blanco, Palacios, Velez, Epstein, Chen-Mok, Reed and Arevalo-Herrera2009).
These historical studies utilized analytical methods that were very limited and inappropriate for time-to-event data, including simple linear regressions, differences in means between groups, or qualitative reporting of trends between groups. For example, in foundational work James reported that in a large series of 700+ cases analysed by linear regression the incubation period was inversely correlated with the number of mosquitoes biting (James, Reference James1931); other researchers suggested an inverse relationship using mosquito-transmitted St. Elizabeth strain infections based on a visual examination of the trend, writing ‘Correlation is apparent and all of the 3 greatly delayed primary attacks occurred after relatively small inocula’ (Coatney et al. Reference Coatney, Cooper, Ruhe, Young and Burgess1950a ). These studies, while limited by experimental design and reporting, form the core evidence for the alleged dose-dependent basis of long-latency in both experimental and natural P. vivax infections, but their validity has heretofore not been formally investigated.
Inherent issues with all methods of quantifying malaria parasite doses have been previously reviewed (Glynn, Reference Glynn1994). Briefly, the use of bite-based metrics assumes that all mosquitoes inject the same dose. The use of qualitative gland infection metrics is even more problematic, as a count of 6 ‘pluses’ could be a single heavily infective, 2 moderately infective, or 3 sparsely infective bites; with larger values of 20–30 ‘pluses’ this becomes even more uncertain. Lastly, even ‘quantitative’ doses are estimates; not all sporozoites may be viable, and dosing may be compromised by adhesion to glass syringes, among other experimental issues (Glynn, Reference Glynn1994).
In light of the severe limitations in these analyses, we sought to both critically review and to re-analyse these historical studies using appropriate statistical methods, with the intent to provide a solid evidence base for associations between sporozoite dosage and incubation/pre-patent period in P. vivax infections.
METHODS
Data inclusion
A comprehensive search was performed in Medline and Google Scholar, using [‘vivax’ AND (‘sporozoite’ OR ‘inoculation’) AND (‘latent’ OR ‘incubation’ OR ‘pre-patent’)] to identify all publications and grey literature reports that examined the relationship between sporozoite exposure and either incubation or pre-patent period. The references in these papers were then consulted, and un-indexed papers were identified. Inclusion criteria were malaria-naïve subjects (except for a set of studies in non-human primates); explicit follow-up without chance of re-infection; and inclusion of only primary infections (‘latencies’ due to presumed hypnozoite activation were not included). Exclusion criteria were poorly documented studies with insufficient experimental detail. Tropical and temperate strains were separated at 27·5°N/S, based on the reported origin of the parasites. All available covariates were extracted for multivariate analysis.
Analysis
A full meta-analysis was not possible due to wide variations in reported dose measurements, times-to-events, and extensive data aggregation in many reports.
Three complementary sets of analyses are presented: those from studies that reported semi-quantitative biting-based exposure metrics; quantitative inoculations in humans; and finally quantitative inoculations in non-human primate models. Contingency tables (χ2 tests using Fisher's exact test) were utilized to assess if any relationship exists between dose and incubation period or pre-patent period, but this analysis was unable to assess the direction or magnitude of response. Because of these limitations, after examination of Kaplan–Meier plots we used Cox proportional hazard models to address both of these issues. Each of the identified studies was analysed with the optimal methods considering the available data; for aggregated data, only contingency tables could be analysed. These were calculated for all studies to allow comparison between them. For more detailed reports, both the log-rank test for trend in dose categories, and hazard ratios (HR) from Cox survival models are presented with survival times, with sub-group analyses where possible. In this study, survival model analyses are focused on consideration of the time span from inoculation to all observed (clinical or parasitological) infections. Each of the exposure categories has a rate of progression to infection within the time interval of clinical follow-up (the ‘hazard rate’); the hazard ratio follows as the ratio of any of these rates. A hazard ratio of 1·50 can be interpreted as a 150% greater likelihood of presenting with infection at all time points throughout the study relative to the reference (low-dose) group. These models allow for adjustment of covariates, and provide measures of effect size and associated confidence intervals. Lastly, for one study logistic regression was also utilized to examine a binary outcome of clinical infection.
We used the exposure categories presented in the original reports where possible; doses reported as continuous values were divided into tertiles for consistent analysis and event times were also divided into tertiles for contingency table analysis. In studies with non-explicit follow-up, unsuccessful infections were censored one day after the final reported infection (Collins et al. Reference Collins, Morris, Richardson, Sullivan and Galland1994). Cox survival models were tested for proportional hazard violations using Schoenfeld residuals; logistic regression model fit was assessed using the le Cessie-van Houwelingen-Copas-Hosmer unweighted sum of squares test (Hosmer et al. Reference Hosmer, Hosmer, Le Cessie and Lemeshow1997). Statistical analysis was performed using Stata 12.1 (College Station, Texas, USA) and all tests were two-tailed.
RESULTS
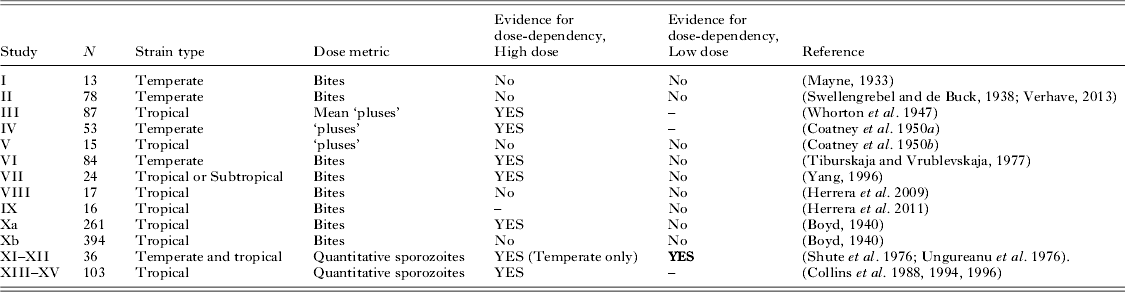
The majority of studies utilizing bite-based metrics showed no association between the number of infected bites and the incubation/pre-patent period in either contingency table or survival model analysis (Tables 1 and 2). We will therefore focus on those that showed evidence of statistically significant effects of dose on time-to-infection.
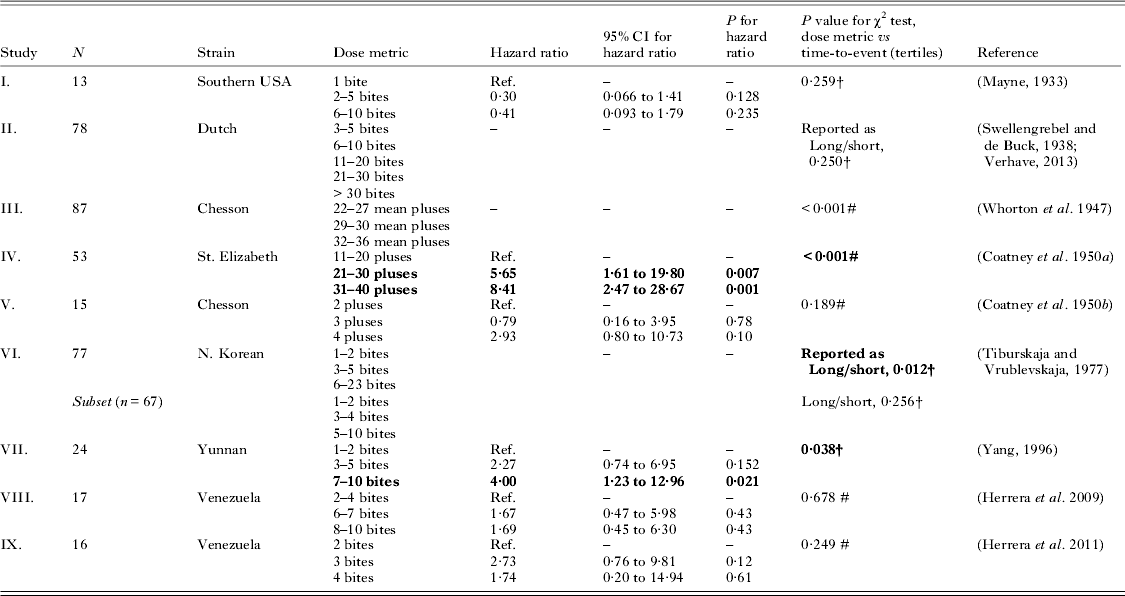
Table 1. (Studies I–IX) Contingency table and survival model analysis of historical human Plasmodium vivax malaria challenge studies using bite-based exposures, to assess the relationship between sporozoite dose and pre-patent or incubation period (entries in bold font are significant with P<0·05)

Notes: ‘Pluses’ refers to the grand total of the estimated sporozoite density (qualitative grading from 1 to either 4 or 6, depending on the study) for the infected mosquito or mosquitoes used in challenge, per volunteer. For χ2 tests not using tertiles, ‘short’ refers to incubation periods of less than 8 weeks, and ‘long’ as anything longer (generally, 6–9 months). Study end point: †=incubation period; #=prepatent period.
Table 2. (Study Xa) Contingency table and survival model analysis of historical human Plasmodium vivax malaria challenge studies with strains from the Southern US, to assess the relationship between sporozoite dose and incubation period (entries in bold font are significant with P<0·05) (N = 261)

Source: (Boyd, Reference Boyd1940).
Contingency table analysis of studies with the tropical Chesson strain (Table 1, study III) used a dose metric of ‘pluses’ to grade the salivary gland estimated sporozoite density in the infecting mosquitoes on a qualitative scale from 1 to 6. We found a significant association (P<0·001) between the tertiles of prepatent period and tertiles of the reported ‘mean pluses’; this remained unchanged when analysed for all explicit dose categories (mean pluses of 22, 25, 27, 29, 30, 32 and 36; range of 12–36). This range of ‘pluses’ would indicate 2 to 36 bites (Whorton et al. Reference Whorton, Kirschbaum, Pullman, Jones, Craige and Alving1947).
A study using the temperate St. Elizabeth strain (Table 1, study IV) showed significant dose-dependence in both contingency table and survival analyses. In Cox models, relative to 11–20 pluses, a dose of 21–30 pluses had a hazard ratio of 5·7 (95% CI 1·6 to 19·8; P = 0·007), and a dose of 31–40 pluses (∼3–10 infected bites) led to an hazard ratio of 8·4 (95% CI 2·5 to 28·7; P = 0·001) (Coatney et al. Reference Coatney, Cooper, Ruhe, Young and Burgess1950a ). That is, at all times throughout the study, the higher dose groups had a 5·7 and 8·4 times greater likelihood respectively of presenting with infection relative to the lowest-dose group. The relationship between dose classes and tertiles of the pre-patent period was also significant in contingency table analysis (P<0·001).
Analysis by contingency tables of long-term malariotherapy studies that used a North Korean strain (Table 1, study VI) showed evidence of dose-dependence for doses of up to 23 bites (P = 0·012); however, when limited to dose categories <11 bites, there was no evidence for an association between bites and the length of incubation periods (P = 0·256). More recent experimental infections with a P. vivax strain from Yunnan (Table 1, study VII) showed no significant difference between 1–2 bites (reference) and 3–5 bites (HR = 2·3, 95% CI 0·74 to 7·0; P = 0·152), but there was evidence for dose-dependence at higher doses of 7–10 bites (HR = 4·0, 95% CI 1·2 to 13·0; P = 0·021). Finally, the overall χ2 test and the log-rank test for trend for an association between bites and incubation period were both marginally significant (P = 0·038 and P = 0·032).
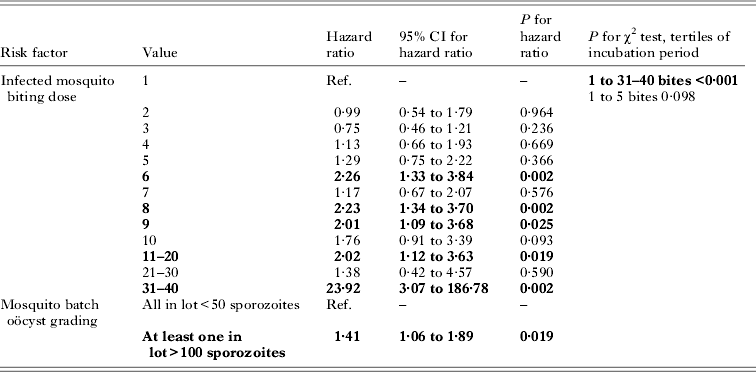
Analysis of data from a large series of studies reporting discrete (integer) bite exposures is presented in Table 2 (study Xa). Log-rank test for trend in survival curves from Kaplan–Meier analysis showed no difference between category doses of 1–5 bites inclusive (P = 0·486) in the incubation period; however, inclusion of the full data (reported as 1 to 10 individually and 11–20; 21–30; 31–40 bites) suggested a significant difference in incubation period (P<0·0001). In a multivariate Cox model adjusted for mosquito batch oöcyst grading (as reported in the original publication, as either: all in the infecting lot having <50 sporozoites; or at least one in infecting lot having >100 sporozoites) while there were no significant differences in the range of doses from a single bite to five inclusive, statistically significant differences in the hazard ratios were apparent from six infected bites onwards. The highest exposure category of 31–40 infected bites had an adjusted hazard ratio of 23·9 (95% CI 3·1 to 186·8; P = 0·002). These results were consistent with contingency table analysis using all the reported dose groups (P<0·001), while analysis of five bites and below did not show a significant association with incubation period (P = 0·098).
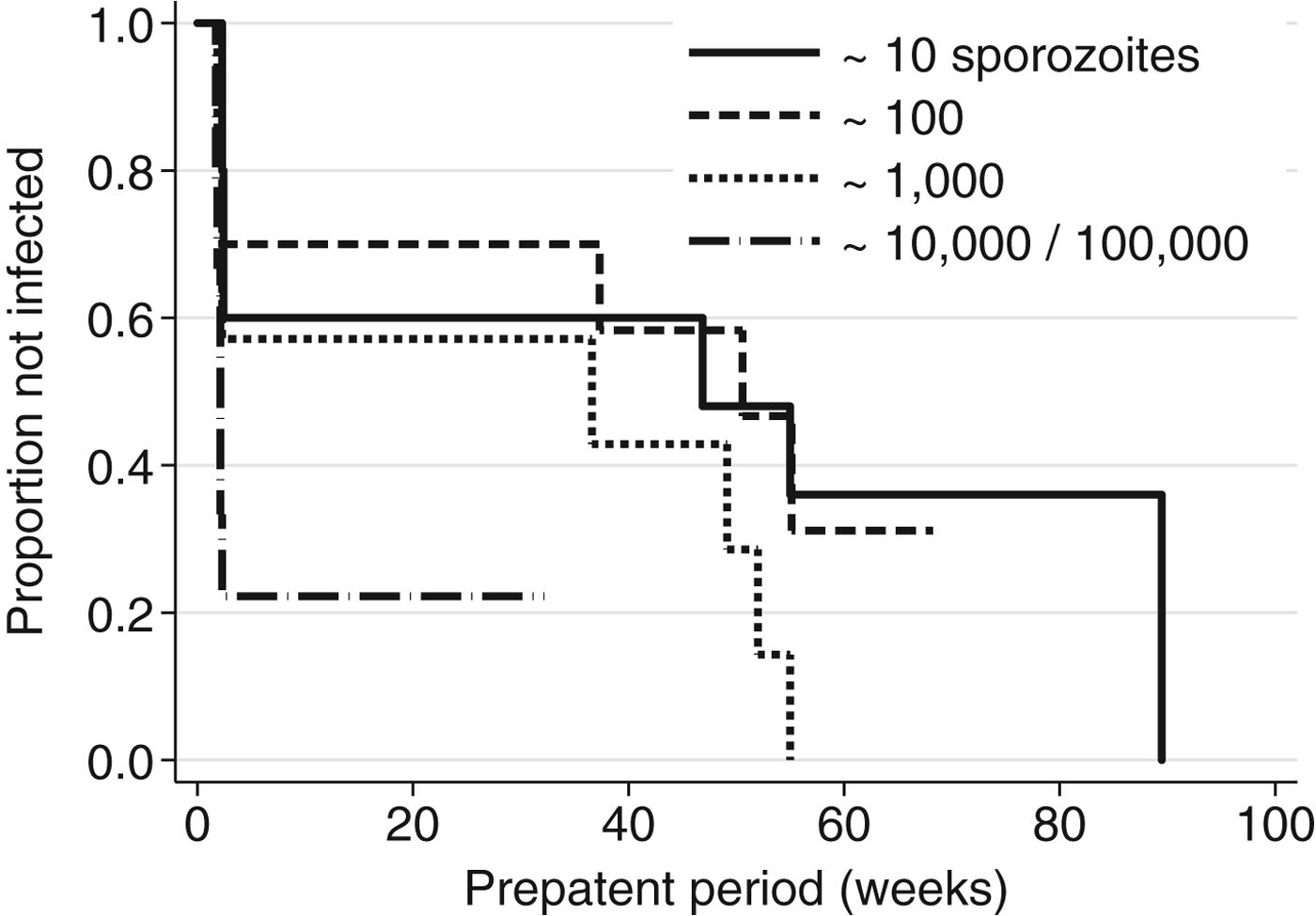
Analysis of the most rigorous experiments identified, which used quantitative intravenous/intradermal sporozoite dosing, is shown in Table 3 (studies XI and XII). The dose of 10 000 sporozoites failed due to experimental issues in the North Korean strain study and was not reported on by the original authors, and the dose of 100 000 was not included for the Chesson strain. The two highest doses of 10 000 and 100 000 have been combined for analysis after testing for heterogeneity of effect. Kaplan–Meier curves for these experiments are shown in Fig. 1. The two highest doses are evident at the far left, with lower doses tapering out to longer time intervals. The log-rank test for trend among doses was marginally significant in the full dataset (P = 0·03); inclusion of the lowest three doses (10, 100 and 1000) was significant for the Korean strain (P = 0·008), but not for the Chesson strain (P = 0·120).
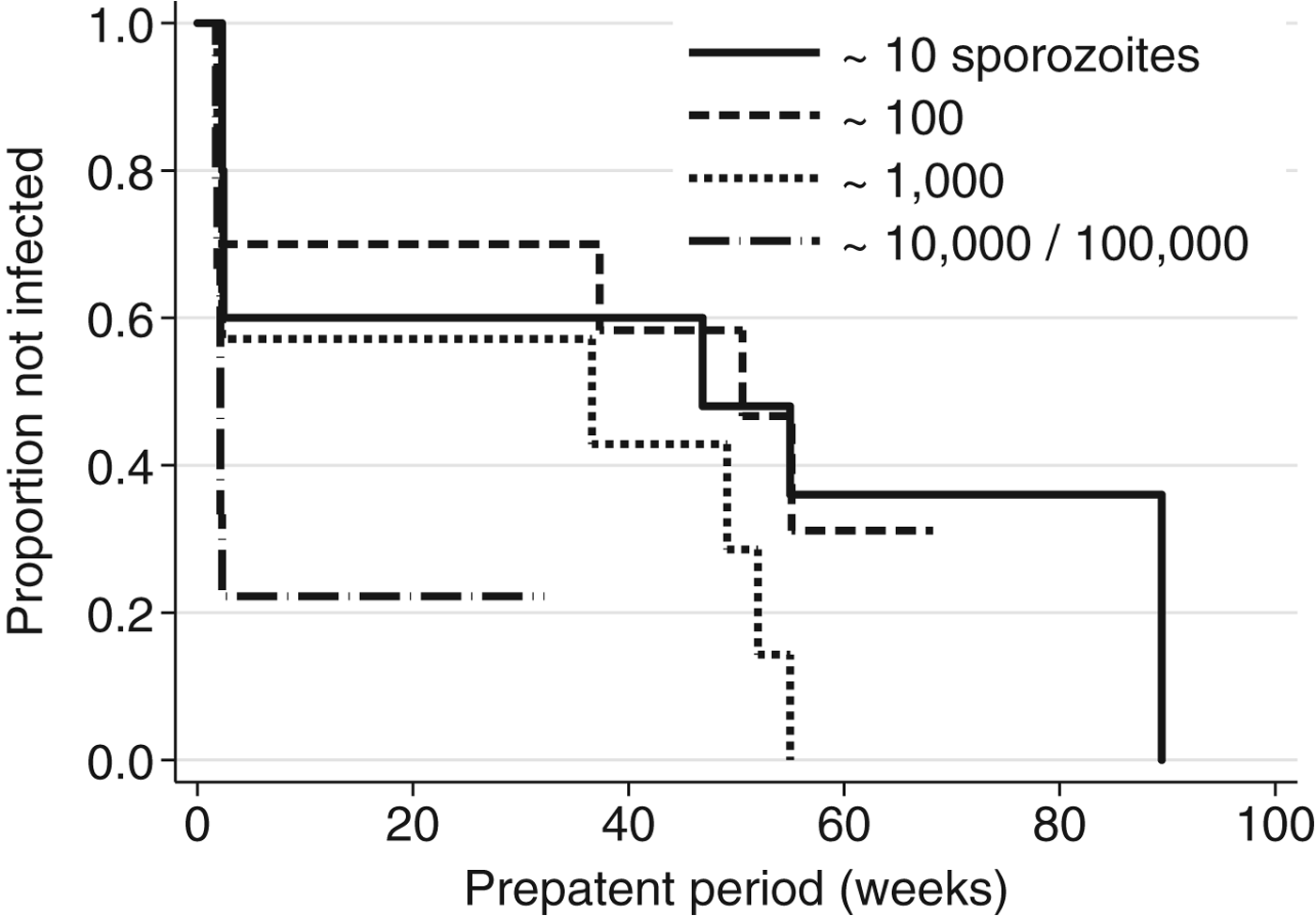
Fig. 1. Kaplan–Meier plot for the relationship between prepatent period and quantitative sporozoite doses in human Plasmodium vivax infections (N = 36). Source: (Shute et al. Reference Shute, Lupascu, Branzei, Maryon, Constantinescu, Bruce-Chwatt, Draper, Killick-Kendrick and Garnham1976; Ungureanu et al. Reference Ungureanu, Killick-Kendrick, Garnham, Branzei, Romanescu and Shute1976).
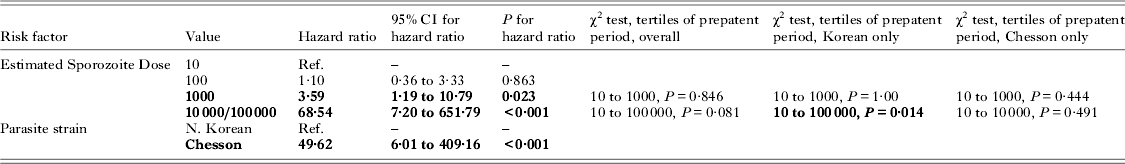
Table 3. (Studies XI and XII) Contingency table and survival model analysis of historical human Plasmodium vivax malaria challenge studies using quantitative sporozoite dosing, to assess the relationship between sporozoite dose and pre-patent period (entries in bold font are significant with P<0·05) ( N = 36)

In multivariate Cox survival models adjusted for parasite strain with 10 sporozoites as the references, doses of 100 sporozoites showed no significant impact on the pre-patent period, with a hazard ratio = 1·1 (95% CI 0·36 to 3·3; P = 0·863); dosing with 1000 sporozoites showed an increased HR of 3·6 (95% CI 1·2 to 10·8; P = 0·023); and a dose of 10 000/100 000 sporozoites showed a significant effect, with an HR = 68·5 (95% CI 7·2 to 651·8; P<0·001). Parasite strain itself was significant in this analysis: with the North Korean strain as the reference, the tropical Chesson strain showed an HR of 49·6 (95% CI 6·0 to 409·2; P<0·001). That is, at all time points, case-patients infected with the Chesson strain had 49·6 times greater risk of presenting with infection relative to those infected with the North Korean strain. However, when analysed using contingency tables, there was no evidence for a relationship between dose and time-to-event, including the highest dosages; the results were unchanged with inclusion of only the Chesson strain. Analysis of the North Korean strain alone in contingency tables showed no effect with analysis of the 10 to 1000 doses (P = 1·00), but a significant effect was evident with inclusion of the full range of up to 100 000 sporozoites (P = 0·014).
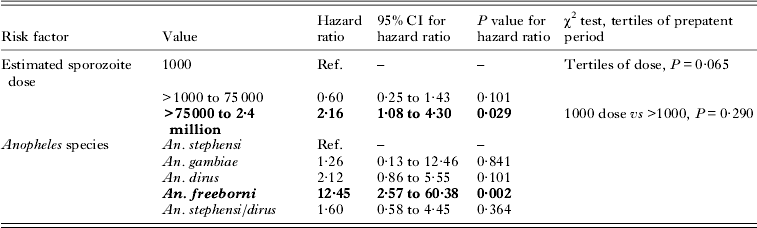
Analysis of data from a series of experiments carried out over several years using non-human primates and the US CDC-Salvador I P. vivax strain is shown in Table 4 (studies XIII–XV). The log-rank test for trend in unadjusted Kaplan–Meier analysis with the full dosing arms (which ranges from 1000 to 2·4 million sporozoites) was significant (P = 0·011), but not for doses ⩽10 000 (P = 0·083) or for doses ⩽100 0000 sporozoites (P = 0·500). In the full Cox multivariate model after adjustment for primate host species, previous malaria infections in individual non-human primates, and sporozoite vector source, with 1000 sporozoites as the references, doses of >1000 to 75 000 showed an adjusted HR of 0·60 (95% CI 0·25 to 1·4; P = 0·101), and doses from >75 000 to 2·4 million showed an HR of 2·2 (95% CI 1·1 to 4·3; P = 0·029).
Table 4. (Studies XIII – XV) Contingency table and survival model analysis of Plasmodium vivax malaria challenge studies in splenectomized Saimiri and Aotus non-human primate models to assess the relationship between sporozoite dose and incubation period (entries in bold font are significant with P<0·05) ( N = 105)

Note: US-CDC Salvador I strain used; models adjusted for primate host species and previous experimental malaria infections in individual non-human primates.
Examination of the anopheline source of the sporozoites shows that relative to those from An. stephensi, those harvested from An. freeborni had an adjusted HR of 12·5 (95% CI 2·6 to 60·4, P = 0·002), while all other vector sources were not significant. In contingency table analysis, no association was found between tertiles of dose and prepatent period (P = 0·065), and there was also no evidence of association between the 1000 and >1000 to 10 000 sporozoites dose categories and tertiles of event times (P = 0·29). All of these results were unchanged if only the larger sub-population of Saimiri monkeys (n = 65) was included; evidence has suggested that these primate species react differently to infection (Collins, Reference Collins and Doolan2002).
Finally, these survival analyses were complemented with a different series of studies from the same publication as study Xa (data not shown; study Xb), examining the number of ‘takes’; that is, bites from an infected vector which produced an infection (time span not specified) among 394 inoculations (Boyd, Reference Boyd1940). Logistic regression was utilized to examine the relationship between the number of infected mosquito bites and proportion of exposures producing an infection. Mosquito dose was not significantly associated with progression to infection when measured as either discrete exposure categories (1 to 10 individually and 11–20; 21–30; 31–40; all P>0·06) or for biting dose as a continuous variable (using the mid-point of the higher categories; P = 0·88).
DISCUSSION
Biologically plausible exposure to sporozoites
A crucial aspect of these historical studies that has not been previously considered is the plausible range of exposure to sporozoites under field conditions. Beyond informing experimental studies, these historical studies also serve as the basis for understanding natural infections (White, Reference White2011). A key measure of malaria exposure in field settings is the entomological inoculation rate (EIR, number of Plasmodium-infective mosquito bites per person per time period). The highest monthly P. vivax-specific EIRs during high transmission seasons we are aware of are 14·5 from Ethiopia (Animut et al. Reference Animut, Balkew, Gebre-Michael and Lindtjørn2013); 44·6 in Sri Lanka; and 46·8 from Thailand (authors’ calculations from hourly rates reported in Ramasamy et al. Reference Ramasamy, Ramasamy, Wijesundera, Wijesundera, Dewit, Ranasinghe, Srikrishnaraj and Wickremaratne1992; Rattanarithikul et al. Reference Rattanarithikul, Konishi and Linthicum1996).
Studies using Plasmodium falciparum have estimated the inocula of sporozoites per mosquito bite, with a median of 15 sporozoites (range 0–978) in three different studies reviewed (Rosenberg, Reference Rosenberg2008); remarkably similar estimates have been obtained with multiple rodent malarias (Frischknecht et al. Reference Frischknecht, Baldacci, Martin, Zimmer, Thiberge, Olivo-Marin, Shorte and Ménard2004; Medica and Sinnis, Reference Medica and Sinnis2005; Jin et al. Reference Jin, Kebaier and Vanderberg2007).
Taken together, these suggest that while the absolute maximum exposures under natural conditions (assuming maxima of 2 bites per night and inocula of 978 sporozoites) are ∼2000 sporozoites, likely exposures are less than 100 sporozoites. Historical work was predicated on vastly higher inocula; for example, 500 bites was assumed to inoculate ∼6 million sporozoites or 12 000 per bite (Shute et al. Reference Shute, Lupascu, Branzei, Maryon, Constantinescu, Bruce-Chwatt, Draper, Killick-Kendrick and Garnham1976).
Impact of sporozoite dose on incubation/prepatent periods
A summary of results from our analysis is presented in Table 5, with low dose defined as exposures with entomological plausibility and includes doses of ⩽5 infective bites, or ∼1000 sporozoites; while these values are not strictly comparable, they likely represent similar levels of exposure.
Table 5. Summary of evidence for association between sporozoite dose and incubation or prepatent period in Plasmodium vivax malaria challenge studies

Note: ‘Low’ dose refers to ⩽5 infected bites or ∼1000 sporozoites; ‘High’ dose is any greater exposure. ‘Pluses’ refers to the grand total of the estimated sporozoite density (qualitative grading from 1 to either 4 or 6, depending on the study) for the infected mosquito or mosquitoes used in challenge, per volunteer.
The studies that show strong evidence for an inverse dose-dependence utilize uncertain ‘plus’ metrics that correspond to very high sporozoite exposure: the highest exposures in studies III and IV corresponded to 10–40 and 32–36 bites respectively (Whorton et al. Reference Whorton, Kirschbaum, Pullman, Jones, Craige and Alving1947; Coatney et al. Reference Coatney, Cooper, Ruhe, Young and Burgess1950a ). A similar trend is observed in studies XIII–XV, with only doses of >75 000–2·4 million sporozoites having a significant inverse relationship with incubation period.
The quantitative dosing experiments in studies XI/XII suggest dose-response: the effects of dosing at 1000 sporozoites were significant relative to 10 or 100, and the combined 10 000/100 000 dose was highly significant. However, when examined by strains individually, the Chesson strain showed no evidence of dose-dependence in any of our analyses. The Korean strain also showed no dose-dependent effect at 100 sporozoites relative to 10; doses of 1000 and higher were associated with shorter pre-patent periods. However, as likely natural inocula lie in the range from zero to ∼1000 sporozoites, and there were no experimental challenge doses between 100 and 1000, it is unclear what the relevance of these data are to natural P. vivax infections.
The second major limitation of these data is that challenges in studies XI/XII utilized both intradermal and intravenous dosing; however, only aggregated data were reported. These routes have been shown to have divergent infectivity in experimental human P. falciparum infections (Sheehy et al. Reference Sheehy, Spencer, Douglas, Sim, Longley, Edwards, Poulton, Kimani, Williams, Anagnostou, Roberts, Kerridge, Voysey, James, Billingsley, Gunasekera, Lawrie, Hoffman and Hill2013). Additionally, the non-randomized nature of treatment arms, and uncertain inclusion/exclusion criteria suggest that we cannot discount the possibility that these findings were due to chance.
The studies included in this analysis were powered from 0·5–0·96 to detect a minimum hazard ratio of 2·0 for dose tertiles. The smallest study reported to have acceptable power of 0·83 in survival models was study VII (N = 24); the combined quantitative dosing experiment (XI/XII; N = 36) was adequately powered at 0·96. The very large confidence intervals for the hazard ratios in several of the analyses (e.g. studies X, and XI/XII, Tables 2 and 3) reflect the limited number of subjects per strata; however, the lower bounds of these CIs represent a lower limit of effect size for significant factors.
Our results suggest that the general theory of dose-dependence has exceedingly limited statistical support that does not meet modern standards of evidence. While broad dose-dependence was found in a subset of the studies, experimental details and the biologically implausible doses utilized make even these results highly suspect.
Threshold effects
Our analysis of data from studies VI and X suggests that mosquito challenge of ∼five bites may represent a fundamentally different biological response from greater sporozoite exposure (Boyd, Reference Boyd1940; Tiburskaja and Vrublevskaja, Reference Tiburskaja and Vrublevskaja1977). At doses lower than this threshold, there was no evidence of any dose-response, but a clear inflection point occurred at higher doses, where a dose-dependent response is apparent. Additional evidence comes from early malariotherapy studies where 6 bites led to incubation periods that were slightly longer than average, while those from 30+ bites were much shorter (James, Reference James1931).
The notable consistency of these results suggests a fundamental biological limit, and that dosing beyond this threshold potentially involves divergent biological pathways. If this inflection point occurs at 5–6 bites, and assuming a median of 15 and a maximum of 978 sporozoites (Rosenberg, Reference Rosenberg2008), then bites in this range would lead to doses of approximately (zero) to 90–5868 sporozoites. The data from the quantitative analysis in this work also suggests that doses above this range of ∼1000 sporozoites give rise to clinically divergent outcomes (studies XI/XII). Importantly, saturation of a biological pathway could also obviate the need for the postulated existence of several different types of sporozoites that have been suggested by multiple researchers (Shute et al. Reference Shute, Lupascu, Branzei, Maryon, Constantinescu, Bruce-Chwatt, Draper, Killick-Kendrick and Garnham1976; Collins et al. Reference Collins, Skinner, Pappaioanou, Broderson, Filipski, McClure, Strobert, Sutton, Stanfill and Huong1988).
CONCLUSIONS
Understanding the basis of long-latency in P. vivax malaria infections has important implications for the planning of control programmes, and both the design and long-term sustainability of surveillance programmes in the context of global malaria elimination (Mueller et al. Reference Mueller, Galinski, Baird, Carlton, Kochar, Alonso and del Portillo2009; Shanks, Reference Shanks, Hay, Price and Baird2012). Moreover, an improved understanding of dose-dependence is crucial for planning human vaccine studies – investigators in a recent study were surprised at finding no impact of increasing bite challenge on the pre-patent period (Herrera et al. Reference Herrera, Fernandez, Manzano, Murrain, Vergara, Blanco, Palacios, Velez, Epstein, Chen-Mok, Reed and Arevalo-Herrera2009). Our study strongly suggests that sporozoite dose is not a main driver of incubation period in naturally or experimentally acquired infections, and that the true causes remain to be discerned.
The extremely large effect size between the two strains (Chesson and North Korean) in this study suggests that parasite strain (and hence genetics) are far more important factors in long-latency than sporozoite dose itself. Additionally, the large differences by mosquito species in studies XIII–XV suggest important parasite-vector interactions; these results bolster the suggestion that Anopheles are far more than just simple vectors (Paul et al. Reference Paul, Diallo and Brey2004). Our results also provide effect estimates that reinforce earlier entomological and epidemiological studies suggesting sub-speciation within P. vivax by hemispheres (Li et al. Reference Li, Collins, Wirtz, Rathore, Lal and McCutchan2001; Lover and Coker, Reference Lover and Coker2013).
Our findings highlight research gaps that should be addressed to ensure that costly and technically demanding challenge experiments truly mirror natural infection pathways, thereby leading to accurate conclusions about the effectiveness of prophylaxis and vaccine candidates.
ACKNOWLEDGEMENTS
We are grateful for comments and suggestions from Alex Cook, Chris Drakeley and two anonymous reviewers who considerably strengthened this paper.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial source, or not-for-profit organization.