Published online by Cambridge University Press: 13 May 2004
Two strains of Schistosoma mansoni were used to investigate the hereditary basis of species-specific host recognition by analysing behavioural responses of miracidia to snail-conditioned water. An Egyptian strain of S. mansoni, capable of distinguishing its host snail Biomphalaria alexandrina from other snails was cycled repeatedly through Biomphalaria glabrata, the intermediate host of a Brazilian strain known to respond even to non-susceptible snails with high intensity. After 5 cycles in the non-natural host, miracidia of the Egyptian strain still retained their preference for the original host snail. In a second experiment, host-finding behaviour of hybrids between these two parasite strains was studied. In the F1 generation, hybrids of both parental combinations showed the same low degree of specificity as the pure-bred Brazilian strain. Approximately one quarter of F2 hybrids proved to be as discriminatory as the Egyptian strain, confirming dominant Mendelian inheritance of non-specificity in schistosome miracidial host-finding behaviour. Moreover, hybrids seem to have lost the ability to develop in B. alexandrina, possibly suggesting a link between host recognition and host compatibility. The heredity of this behavioural trait is of evolutionary and epidemiological significance, since a shift to low host-finding specificity might have been a prerequisite for S. mansoni to acquire new host snails after being introduced to South America by the slave trade.
The specificity of parasite–host interactions has received great attention by parasitologists and evolutionary biologists. A high degree of host specificity can be considered as evidence for a long co-evolutionary history of a parasite–host system, but is also found as a consequence of locally dynamic co-evolution on the level of different genotypes within a host population. In trematode–snail interactions, which are generally regarded as highly specific (Wright, 1971), compatibility patterns of species or strains of parasites and hosts have been used for phylogenetic studies (Blair, Davis & Wu, 2001) as well as for investigations of parasite–host co-evolution on a local scale (Lively, 1999; Lively & Dybdahl, 2000). Especially in medically relevant blood flukes snail compatibility is also a topic of practical importance for epidemiological surveys and development of biological control methods. This is the reason why most work on snail compatibility and the snail's internal defence system against trematode infections was performed with Schistosoma mansoni (Basch, 1976; Morgan et al. 2001), the cause of intestinal schistosomiasis in man. Host snails of the genus Biomphalaria respond with humoral and cellular mechanisms to S. mansoni sporocysts (reviewed e.g. by Bayne & Yoshino, 1989; Adema & Loker, 1997; Bayne, Hahn & Bender, 2001). It was recently demonstrated by selection experiments under laboratory conditions with different snail lines that, in the system S. mansoni–B. glabrata, compatibility characteristics seem to be inherited with resistance being dominant over susceptibility (Webster & Woolhouse, 1998; Webster, 2001).
However, specific interactions between schistosomes and their snail hosts start prior to penetration and development in the snail. Like first larvae of most digeneans, schistosome miracidia locate and penetrate their intermediate hosts actively. After hatching and dispersal, they approach their specific host snails' preferred microhabitat, guided by environmental stimuli (Saladin, 1979). Once miracidia have reached the area, where an encounter is likely, they search for a suitable host snail by chemo-orientation in response to glycoconjugates derived from the snail's mucous surface layer (Haberl et al. 1995). These ‘miracidia attracting glycoconjugates’ (MAGs) also stimulate the parasite's behaviour after contact with the snail host (Haberl & Haas, 1992). In nature, these parasite–host interactions preceding the actual penetration are essential for transmission success of miracidia within their life-span of only a few hours. Furthermore, Combes & Moné (1987) pointed out that previous contact of miracidia with non-host organisms may reduce their ability to further develop in a suitable snail species. To avoid such detrimental failures trematodes should, therefore, have evolved precise species-specific mechanisms of host finding and host recognition. In fact, we found that an Egyptian (laboratory) strain of S. mansoni discriminated between its host snail B. alexandrina and other snail species and strains, already during approach and after contact (Kalbe, Haberl & Haas, 1996; Haberl et al. 2000; Hassan et al. 2003). Also miracidia of the liver fluke Fasciola hepatica, and of the bird schistosomes Trichobilharzia ocellata and T. franki, respond exclusively to MAGs from their respective hosts and not to those from even closely related snail species (Kalbe, Haberl & Haas, 1997, 2000; Kock, 2001). Several infection experiments, where miracidia had the choice between different snail species, support our assumption that species-specific host-finding is the normal case rather than an exception in digenean miracidia (Christensen, Nansen & Frandsen, 1976; Chipev, 1993; Toledo et al. 1999). Although high specificity in miracidial host finding is obviously advantageous in endemic areas, where the parasite's specific host snails occur permanently within a diverse mollusc fauna, it is clear that, on the other hand, this trait hinders the acquisition of new host species. Especially long-persisting digeneans of migratory, long-living definitive hosts may be faced with a situation where the required snail species is not available and a host switch is the only chance for further reproduction. Under these circumstances, parasites should adjust their host-finding behaviour and respond to a broad range of potential new host organisms. In fact, contrary to S. mansoni from Egypt, miracidia of two Brazilian strains showed no preference for their host B. glabrata over several other snails (Kalbe et al. 1996). This result concurs with observations that various aquatic organisms interfere with successful infection of B. glabrata snails by miracidia of Carribean or Latin American schistosome strains (Barbosa & Carneiro, 1965; Chernin & Perlstein, 1971; Upatham, 1972; Moné & Combes, 1986). This lack of specificity might be a consequence of the fact that S. mansoni was introduced to America by the slave trade and only recently acquired B. glabrata as a new intermediate host (Files & Cram, 1949; Després, Imbert-Establet & Monnerot, 1993; Morgan et al. 2001).
We used our model system of an Egyptian S. mansoni strain whose miracidia respond highly specifically to snail host cues, and a Brazilian strain with less distinctive host-finding behaviour, to investigate the hereditary background of a shift in the degree of specificity. In order to determine whether a sudden switch from high to low specificity occurs, we analysed miracidial behaviour of the Egyptian strain after repeated cycling through the non-host snail B. glabrata. To obtain information about the genetic basis of this species-specific recognition, we produced hybrids of these two S. mansoni strains and compared their capability to discriminate between different snail species.
The Brazilian S. mansoni strain (BR) was isolated in Belo Horizonte (Brazil) together with its host snail B. glabrata in 1967. Since 1996, this parasite was cycled through an albino strain of B. glabrata. The Egyptian S. mansoni strain (ET) originated from Egypt and was maintained at the University of Massachusetts, Lowell, USA. In 1990, it was re-imported to Egypt and maintained together with its intermediate host B. alexandrina at the Schistosome Biological Supply Program (SBSP), Theodor Bilharz Research Institute, Cairo. Lymnaea stagnalis as experimental non-host snails were originally collected from ponds near Erlangen, Germany, and reared in the laboratory for several years.
Snails were kept under a day–night rhythm of 12[ratio ]12 h at 27 °C (L. stagnalis at 18 °C) in aerated tap water and fed on lettuce and fish food. Parasites were cycled through female CD-1 mice infected by the paddling method (Webbe & James, 1971) and eggs were obtained from mouse faeces, which were washed with cold saline and transferred to tap water. The hatched miracidia were concentrated in side-arm flasks by illumination and used for the experiments within 1–3 h after hatching.
In this study, two different methods were used to infect snails: for repeated cycling in different species of intermediate hosts, batches of 20–35 young snails (diameter 3–6 mm) were incubated overnight in glass beakers containing 300 ml of tap water and 5 S. mansoni miracidia per snail. For crossing experiments, single miracidia were pipetted into 5 ml glass vials. Before a snail was added, it was confirmed under a dissecting microscope that the vials contained not more than 1 miracidium. In the case of these monomiracidial infections, snails were left in the vials for 24 h to make sure that non-penetrated miracidia were dead before the snails were transferred to aquaria in groups. After 6–8 weeks, surviving snails were checked for cercarial shedding by illuminating them individually in 40 ml glass beakers.
Schistosomes are dioecious, therefore offering the possibility to produce hybrids of different strains of the parasite. Freshly isolated cercariae of individual snails were washed in deionized water, and single larvae were transferred into PCR tubes and lyophilized. Cercariae were incubated in 25% ammonia solution for 10 min at 95 °C according to the method of Grevelding, Kampkötter & Kunz (1997). Determination of the gender of cercariae was performed by polymerase chain reaction (PCR) of the sex specific W1 sequence (Webster, Mansour & Bieber, 1989) employing the protocol and primers described by Gasser, Moharan & Mitchel (1991). Amplification products were analysed on Sybr Green-stained 1% TBE agarose gels.
Since all cercariae from a monomiracidial-infected snail are genetically a clone, the sex of all larvae from a given snail was known when it was determined for one of them. Mice were infected with 60 male and 60 female cercariae originating from a single snail respectively. The possible hybrid combinations ET[male ]×BR[female] and BR[male ]×ET[female], as well as pure-bred controls (ET[male ]×ET[female] and BR[male ]×BR[female]) were used to produce F1 miracidia. F2 generations were raised from both pure-bred strains and from one of the hybrids (BR[male ]×ET[female])[male ]×(BR[male ]×ET[female])[female]. The whole crossing experiment was performed twice.
Snail-conditioned water (SCW) was prepared by incubating 1 Biomphalaria snail per ml of tap water (1 L. stagnalis per 3 ml) for 2 h at 27 °C (L. stagnalis at 18 °C). After molecular filtration in a stirred cell (Filtron, Northborough, Massachusetts, USA) the carbohydrate content of the fraction >30 kDa was determined colorimetrically by the sulphuric acid method (Dubois et al. 1956) with mannose serving as the standard. For experiments in which the effect of SCW from different snail species on the miracidial host-finding behaviour was compared, all of these substrates were adjusted to a uniform sugar content of 0·1 μg/ml corresponding to dilutions of 1[ratio ]10 in both Biomphalaria species and 1[ratio ]30 in the case of L. stagnalis. In a second experiment SCW of L. stagnalis was offered undiluted and in dilutions up to 1[ratio ]100 equivalent with a carbohydrate content of 3 to 0·03 μg/ml.
Chemo-orientation of miracidia was investigated in a one-arm chamber as described previously (Haas et al. 1995; Haberl et al. 1995). Test substrates were applied to the end of a straight chamber, and the behaviour of miracidia entering or leaving the substrate-containing section was recorded. In case of effective SCW, the normally straight-swimming miracidia show an increase in the rate of change of direction (RCD) when entering the point of inoculation and a turnback swimming response when leaving it. The responses of 10–50 individual miracidia per replicate were recorded and the percentages of these two chemokinetic responses were calculated independently. Experiments were performed at 27 °C by placing the chamber on a precision heating plate and keeping all substrates in a water bath. The test substrates, the water containing the miracidia, and the tap water for filling the chamber, were adjusted to pH 7·5 with Tris–HCl (9 mM). Two or three of the S. mansoni strains/crossings were compared on each experimental day and, for all investigations, aliquots of identical SCW samples were offered. For all experiments a blind protocol was used. The observer never knew which type of substrate and which strain or hybrid was under study.
The arcsin-square root transformed data from behavioural experiments and monomiracidial infections were analysed with the help of the statistical analysis software SPSS. Standard errors (‘SE’) were retransformed after computation. Means were compared using the TUKEY multiple t-test procedure with a significance level of 0·05 (indicated by *).
Data from behavioural experiments with miracidia of the hybrid F2 (BR[male ]×ET[female])[male ]×(BR[male ]×ET[female])[female] were used to test if host-finding specificity follows a Mendelian segregation pattern. Hypothetical values for a 3[ratio ]1 ratio were calculated for miracidial responses to non-host SCW of L. stagnalis in that particular dilution, where the greatest differences between the S. mansoni ET strain and the BR strain occurred. Within this range, hypothetical percentages for a 3[ratio ]1 ratio in the miracidial responses, ‘Increase of RCD’ and ‘Turnback swimming’, were calculated according to the formula
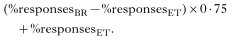
On the basis of these values and the total number of observations, the theoretical number of responding miracidia for a 3[ratio ]1 were determined. Subsequently, a chi-square analysis (χ2) was used to compare the actually observed and the predicted numbers of responses.
Infection rates of S. mansoni ET cycled through the Brazilian snail B. glabrata albino ranged between 12·3 and 18·7%. No obvious change in infection rates was observed with repeated cycling through the non-natural host. Even after 5 cycles, the ET miracidia retained their specificity in host-finding behaviour: the responses to B. alexandrina SCW was not altered, and they responded to SCW of the experimental intermediate host with the same low intensity as to the non-host snail L. stagnalis or the control. The BR strain showed similarly high rates of responses towards SCW of all 3 snails (Fig. 1).

Fig. 1. Comparison of miracidial responses (%) to snail conditioned water from specific intermediate hosts, experimental hosts and the non-host Lymnaea stagnalis. (A) Brazilian Schistosoma mansoni cycled through its host snail Biomphalaria glabrata; (B) Egyptian S. mansoni cycled through its host snail B. alexandrina; (C) Egyptian S. mansoni cycled experimentally through B. glabrata (5 cycles). *P<0·05 vs control (t-test).
In both S. mansoni strains, high amounts of the sex-specific repetitive W1 sequence could be amplified by PCR only in female cercariae. Some males produced a very faint band at approximately 0·5 kb indicating a low number of W1 copies (not shown).
Crossing of the parasite strains produced viable offspring in both combinations of the parental sexes. Hybrids showed comparable low specificity in their host-finding behaviour as the pure-bred BR strain, with the hybrid ET[male ]×BR[female] apparently responding to a lower extent to SCW of B. glabrata and L. stagnalis than BR[male ]×ET[female]. Only the ET[male ]×ET[female] miracidia retained their specificity for their host snail B. alexandrina (Fig. 2). However, experiments with diluted SCW of the non-host L. stagnalis revealed that both hybrids (Fig. 3C,D) responded with a similar sensitivity as the pure-bred BR strain (Fig. 3A). The pure-bred ET strain responded only weakly to undiluted L. stagnalis SCW (a concentration 30 times higher than in the previous experiment), whereas the following dilutions elicited no behavioural changes at all (Fig. 3B). Comparable activity/viability in all 4 strains was indicated by the fact that all miracidia responded to SCW of B. alexandrina in a dilution of 1[ratio ]10 with similar high intensity (Figs 2 and 3).

Fig. 2. Miracidial responses (%) to snail conditioned water from two Biomphalaria species and the non-host Lymnaea stagnalis. (A) Pure-bred Brazilian Schistosoma mansoni strain; (B) pure-bred Egyptian S. mansoni strain; (C) F1 hybrid of male Brazilian and female Egyptian S. mansoni strain; (D) F1 hybrid of male Egyptian and female Brazilian S. mansoni strain. *P<0·05 vs control (t-test).

Fig. 3. Miracidial responses (%) to snail conditioned water from the non-host Lymnaea stagnalis in decreasing dilutions and from Biomphalaria alexandrina. (A) Pure-bred Brazilian Schistosoma mansoni strain; (B) pure-bred Egyptian S. mansoni strain; (C) F1 hybrid of male Brazilian and female Egyptian S. mansoni strain; (D) F1 hybrid of male Egyptian and female Brazilian S. mansoni strain.
The specificity of chemo-orientation in the F2 hybrid (BR[male ]×ET[female])[male ]×(BR[male ]×ET[female])[female] was studied with a similar experimental procedure. At a dilution of 1[ratio ]5, the highest differences between both pure bred parental strains in responses to L. stagnalis SCW were found. Within this range, 76·3% of the F2 hybrid miracidia would be expected to show an ‘increase of RCD’ in case of a 3[ratio ]1 ratio. This does not differ significantly (χ2=2·00, S.F.=1, P=0·65472) from the observed value of 73·3% (S.E. +3·17/−3·47; 120 observation, 4 replicates). Likewise, the observed 71·3% F2 hybrid miracidia performing ‘turnback swimming’ (S.E. +5·34/−6·06; 244 observations, 4 replicates) were approximately matched to the expected value for a 3[ratio ]1 ratio of 76·1% (χ2=1·281, D.F.=1, P=0·25763) at this particular concentration (Fig. 4). These proportions indicate that non-specific host-finding behaviour is genetically dominant following simple Mendelian inheritance.

Fig. 4. Miracidial responses (%) of a F2 hybrid of Brazilian and Egyptian Schistosoma mansoni to snail conditioned water from the non-host Lymnaea stagnalis in decreasing dilutions and from B. alexandrina as compared with the responses of the pure-bred parental strains. For clarity reasons, the behavioural patterns ‘Increase of RCD’ (A) and ‘Turnback swimming’ (B) are shown in separate graphs. Pure-bred BR strain, 89–696 observations (6–15 replicates); F2 hybrid BR[male ]×ET[female], 67–393 observations (4–9 replicates); pure-bred ET strain, 114–401 observations (6–15 replicates).
The monomiracidial infection series with both S. mansoni strains and their hybrids in the two Biomphalaria species revealed that cercarial shedding of the BR strain occurred only in its host snail B. glabrata (Table 1). The ET strain developed in both snail species, although with less success in B. glabrata. Interestingly, the infection rates of both hybrids in B. alexandrina were extremely low, similar to the BR strain. However, survival rates of these snails were lower than of B. glabrata, possibly indicating a higher mortality of B. alexandrina with pre-patent infections. Nevertheless, these results could be a first hint on a correlation between the ability to discriminate B. alexandrina from other snails during approach and the capability to develop within this snail.

In this study we demonstrated that unspecific miracidial host-finding behaviour of a Brazilian S. mansoni strain is genetically dominant over the ability to distinguish suitable hosts from non-host organisms in an Egyptian strain. This low host-searching specificity was obviously advantageous for S. mansoni after it had been transferred from Africa to America by the slave trade (Files & Cram, 1949; Després et al. 1993), where the parasite had to capture a new host snail species (Morgan et al. 2001).
There is one example showing that, in a population of S. mansoni introduced less than 400 years ago, heritable behaviour related to transmission has been adapted to a new host: in different isolates of the parasite from the Carribean island of Guadeloupe, Chassé & Théron (1988) discovered differences in the circadian emergence rhythms of the cercariae released daily from snail hosts. Cercarial shedding maximum of S. mansoni infecting humans took place at noon, whereas cercarial emergence of the same species obtained from rodents with nocturnal activity exhibited a peak later in the afternoon. Reciprocal crosses of both chronobiological races resulted in F1 offspring with a shedding maximum between the peaks of both parental lineages (Théron & Combes, 1988), indicating no clear genetical dominance of one of the two shedding patterns. Although limited genetic exchange is still possible, this sympatric alloxenic speciation (Théron & Combes, 1995) probably represents a continuous and ongoing process leading to distinct strains.
The behavioural adaptation investigated here is quite different: miracidia of the Brazilian S. mansoni strain did not shift their host-searching preference from an African snail to the American B. glabrata. Instead they seem to have abolished species-specificity in host-finding completely, as shown by the strong responses towards the allopatric non-host snail L. stagnalis. Although B. alexandrina appears to be almost insusceptible to the Brazilian strain, this snail was even more attractive to BR miracidia than their specific host B. glabrata. The natural distribution of B. alexandrina is restricted to the river Nile (Brown, 1994) and it does not occur in West and Central Africa, the geographical origin of American S. mansoni (Deprés et al. 1993). Therefore, the Egyptian parasites used here probably do not represent the ancestral population of the Brazilian strain in this study, but should be regarded as an example of an indigenous African S. mansoni. Hence, it would be very interesting to examine miracidial ability of West and Central African S. mansoni isolates to distinguish their intermediate hosts B. pfeifferi and B. sudanica from American host snails. Recent phylogenetic studies reveal that B. glabrata is more closely related to African Biomphalaria species than to other neotropical species of this genus (Woodruff & Mulvey, 1997; Campbell et al. 2000; DeJong et al. 2001). This close relationship might have contributed to the successful immunological adaptation of S. mansoni to B. glabrata. Nevertheless, it remains puzzling why the parasite did not also adjust its potentially highly specific host recognition mechanisms to this new host species. An explanation could be that changes in host-finding specificity take much more time than the indispensable ability to cope with the snail's internal defence system or that a reduction in specificity of the miracidial receptor(s) is an evolutionary one-way track. However, it is still not clear whether non-specifically responding miracidia have modified or lost receptors, or if they use additional receptors, recognizing more general characteristics of snail mucus. The latter scenario is likely since the Brazilian S. mansoni responds to a different fraction of B. alexandrina SCW than the Egyptian strain does (Haberl et al. unpublished observations) and to another fraction of L. stagnalis SCW than Trichobilharzia ocellata uses for specific host recognition (Kalbe et al. 2000). Additionally, the selection pressure for specificity in host finding, mainly determined by the abundance of host and non-host snails, could be different in the habitats in Africa and South America.
The extremely low infection rates of F1 hybrids between Egyptian and Brazilian S. mansoni strains for B. alexandrina in this study disagree with many observations in other hybrids of Schistosoma strains or species, where the offspring usually were compatible with both parental snail hosts (Wright & Southgate, 1976; Paraense & Corrêa, 1981; Mutani, Christensen & Frandsen, 1985; Imbert-Establet & Combes, 1986; Tchuem Tchuenté et al. 1997; Webster & Southgate, 2003). This loss of specificity for B. alexandrina in their host-finding behaviour together with their ability to develop successfully in this snail might be a hint for a genetical linkage between these two aspects of parasite transmission. This link could also explain why the high degree of specificity has been maintained by the Egyptian S. mansoni strain even after several years of cycling in the laboratory. As miracidia are exposed to snails in a few ml of water they do not need effective host-finding and host-recognition mechanisms for transmission, but larvae unable to overcome the snail's internal defence system would be outcompeted, even under these artificial conditions.
A striking conclusion from the simple dominant Mendelian inheritance of non-specificity in snail recognition is that this complicated behavioural process seems to be controlled by a single gene or gene complex. It is possible that the dominant allele for non-host-specificity was always present at low frequencies in natural African S. mansoni populations. Even a small number of individuals with this genotype might have been sufficient to become the founder generation after introduction to America, since a reduced precision in discriminatory host finding increases the likelihood of encounters with potential new host species. However, in their natural habitat, maintenance of this genotype requires a high abundance of susceptible host snails in the sympatric mollusc fauna to ensure sufficient transmission rates by random contacts with suitable snails. Numerous reports from epidemiological surveys in Africa show that even in areas with a high prevalence of schistosomes in humans, infection rates in snails are astonishingly low. This seems to indicate that infecting a host snail is a very critical phase, probably under high selection pressure. Therefore, it appears uncertain whether miracidia with the disadvantage of carrying the non-specific host-finding allele (homozygous or heterozygous) are competitive enough to persist within a population capable of precise host-finding behaviour.
Alternatively, if the loss of specific host-finding behaviour appeared de novo in American S. mansoni strains, possible explanations could be either a random mutation or that digeneans might have evolved a kind of genetic switch mechanism. Although we could not demonstrate such an effect within only five generations of a laboratory strain in a non-natural host snail, it is conceivable that occasionally (or triggered by physiological parameters of the definitive host) a certain proportion of the parasite's offspring with the characteristic ‘low host-finding specificity’ appears. In case of a breakdown of the sympatric snail host population, or migration of the final host, these miracidia may become the founder generation of a non-specific strain. This new physiological strain would easily spread and replace the original genotype because of the genetical dominance of this trait. On the other hand, if the specific host species is available, these larvae would be readily outcompeted by their specifically responding siblings, which gain advantage from their reduced likelihood of detrimental encounters with non-host organisms (Combes, 1991).
The evolutionary and epidemiological significance of species-specific miracidial host-finding behaviour is often overlooked in investigations focussing on parasite–host interactions inside the snail. Yet, host-finding preferences seem to be strongly conserved and probably reveal much more reliable information about phylogeny and biogeographic history than sole observation of cercarial shedding, especially in laboratory-adapted strains. Neglecting the behavioural ability of miracidia to discriminate host snails from non-host snails underestimates the potential of such organism in which the central nervous system occupies as much as 8% of the whole body mass (Wilson & Denison, 1970). Nevertheless, a switch from high to low host-finding specificity as suggested by the findings of this study would be an adequate behavioural adaptation in order to acquire new intermediate hosts.
We would like to thank C. Loy for technical assistance and taking care of mice and snails. Furthermore, we thank C. G. Grevelding for the introduction to the technique of cercarial sexing and B. Oberstadt for his help with PCR trouble shooting. Special thanks to R. Rübsam, K. M. Wegner and J. Kurtz for discussions. This work was funded by the Deutsche Forschungsgemeinschaft.

Fig. 1. Comparison of miracidial responses (%) to snail conditioned water from specific intermediate hosts, experimental hosts and the non-host Lymnaea stagnalis. (A) Brazilian Schistosoma mansoni cycled through its host snail Biomphalaria glabrata; (B) Egyptian S. mansoni cycled through its host snail B. alexandrina; (C) Egyptian S. mansoni cycled experimentally through B. glabrata (5 cycles). *P<0·05 vs control (t-test).

Fig. 2. Miracidial responses (%) to snail conditioned water from two Biomphalaria species and the non-host Lymnaea stagnalis. (A) Pure-bred Brazilian Schistosoma mansoni strain; (B) pure-bred Egyptian S. mansoni strain; (C) F1 hybrid of male Brazilian and female Egyptian S. mansoni strain; (D) F1 hybrid of male Egyptian and female Brazilian S. mansoni strain. *P<0·05 vs control (t-test).

Fig. 3. Miracidial responses (%) to snail conditioned water from the non-host Lymnaea stagnalis in decreasing dilutions and from Biomphalaria alexandrina. (A) Pure-bred Brazilian Schistosoma mansoni strain; (B) pure-bred Egyptian S. mansoni strain; (C) F1 hybrid of male Brazilian and female Egyptian S. mansoni strain; (D) F1 hybrid of male Egyptian and female Brazilian S. mansoni strain.
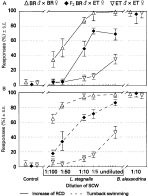
Fig. 4. Miracidial responses (%) of a F2 hybrid of Brazilian and Egyptian Schistosoma mansoni to snail conditioned water from the non-host Lymnaea stagnalis in decreasing dilutions and from B. alexandrina as compared with the responses of the pure-bred parental strains. For clarity reasons, the behavioural patterns ‘Increase of RCD’ (A) and ‘Turnback swimming’ (B) are shown in separate graphs. Pure-bred BR strain, 89–696 observations (6–15 replicates); F2 hybrid BR[male ]×ET[female], 67–393 observations (4–9 replicates); pure-bred ET strain, 114–401 observations (6–15 replicates).

Table 1. Infection rates in Biomphalaria glabrata and B. alexandrina after exposure to single miracidia of a Brazilian and an Egyptian Schistosoma mansoni strain and their F1 hybrids in both parental combinations