It is well established that, as part of the evolutionary history of animals, stages are found in many species in which six pairs of arteries extend through the pharyngeal mesenchyme, joining the cardiac outflow tract to the descending aorta. It is the presence of six such potential symmetrical channels that underscores the so-called Rathke cartoon, which has been used to interpret the morphogenesis of several abnormalities found in human patients with congenitally malformed hearts (Fig 1). The presence of six sets of arteries encircling the trachea–oesophageal pedicle in mammalian species, however, has been controversial for well over a century. In an excellent review published in 1907, LocyReference Locy 1 described well the potential candidates for the purported fifth arch arteries, with none of the candidates described at that time extending in bilateral manner to parallel the arrangements seen in lower animals. Subsequent to this, in perhaps the most authoritative work to appear since that time, CongdonReference Congdon 2 emphasised the need for further studies before it could be presumed that such fifth arch arteries were part of normal development. The kind of investigations Congdon envisaged are still to be conducted in the setting of the developing human heart. In a recent study of the developing mouse heart,Reference Bamforth, Chaudhry and Bennett 3 nonetheless, we failed to find any evidence for the presence of fifth pharyngeal arches, containing arteries bilaterally, as part of normal development. We did find evidence for the formation of collateral channels. Seen between the distal extents of the fourth and sixth arch arteries, these pathways were found in almost half of the embryos we examined during their 11th day of development. Such collateral channels, permeable to Indian ink, had also been found by other groups investigating the developing mouse heart.Reference Lorandeau, Hakkinen and Moore 4 , Reference Geyer and Weninger 5 More significantly, however, in one of our human specimens we found evidence supporting the contention that an additional artery had initially extended through the pharyngeal mesenchyme from the aortic sac to the dorsal aorta. As we will show, the channel we found was paralleling the course of the fourth and sixth arch arteries.Reference Bamforth, Chaudhry and Bennett 3 Had this artery persisted in postnatal life, it would have run in an extrapericardial manner from the ascending to the descending components of the aorta. It is our contention that, to be considered as persistent fifth arch arteries, candidate structures should resemble the course of this vessel. As far as we are aware, this is the only channel to have been described thus far as extending from the extrapericardial systemic outflow tract to the descending aorta or the pulmonary arteries, although CongdonReference Congdon 2 showed a similar pathway in the drawings of his studied embryos.

Figure 1 The cartoon, redrawn from one used in an old edition of Gray’s Anatomy, shows the classical concept derived from the so-called Rathke diagram, in which six sets of arteries are believed to extend through the pharyngeal arches during the stages of development of the human heart.
Review of the current literature shows that a host of structures have been interpreted by paediatric cardiologists as representing persistent arteries of the fifth pharyngeal arch (Tables 1 and 2).Reference Van Praagh and Van Praagh 6 , Reference Gerlis, Ho, Anderson and Da Costa 7 , Reference Murugan, Gulati, Saxena, Juneja and Gupta 14 – Reference Boothroyd, Smith and Peart 28 , Reference Lee, Chen and Chen 38 – Reference Lee, Tsao, Wang, Lee and Chiu 94 Many of these entities produce the lesions known as the double-barrelled aortas.Reference Van Praagh and Van Praagh 6 Others produce various forms of systemic-to-pulmonary collateral channels.Reference Gerlis, Ho, Anderson and Da Costa 7 Only few of these potential candidates run a course so as to replicate the arrangement of the fifth arch artery discovered in our developing human embryo, which was itself incomplete, and hence either forming or regressing.Reference Bamforth, Chaudhry and Bennett 3 It is our opinion that many of the various structures are better interpreted on the basis of persistence of the collateral channels found in almost half of the developing mouse embryos, and also now found in the developing human heart.Reference Congdon 2 – Reference Geyer and Weninger 5 In this review, we recapitulate our account of the findings in the developing human and mouse hearts. We then compare our own interpretations with those put forward previously in favour of persistence of the arteries of the fifth aortic arch, explaining why we believe the findings are better accounted for on the basis of persistent collateral channels, or remodelling of the fourth and sixth arch arteries. We accept, nonetheless, that the channels observed in some of the cases providing systemic-to-pulmonary communications could well represent persistence of the arteries of the enigmatic fifth arch.
Table 1 List of all the cases published in the world literature implicating the fifth arch artery in the double-barrelled arch of the aorta with or without patent arterial duct or coarctation of the aorta.

* Cases that we interpret as requiring dual fifth arch arteries to explain the anatomyIn the column giving details of cases, the reference to ‘Case 2’ or ‘Case 3’ refers to the individual as identified by the original authors
Table 2 List of all the cases invoking the fifth arch arteries in the development of various forms of aortic-to-pulmonary arterial connections.

AP=aortopulmonary
* Cases that we interpret as requiring dual fifth arch arteries to explain the anatomyIn the column giving details of cases, the reference to ‘Case 2’ or ‘Case 3’ refers to the individual as identified by the original authors
Development of the pharyngeal arch arteries
It has become customary, subsequent to the stellar investigation of Kramer,Reference Kramer 8 to account for development of the cardiac outflow tract on the basis of formation of a “conus” and “truncus”. There remains uncertainty, however, among those who use this convention, as to the origin of the intrapericardial and extrapericardial arterial trunks. There is also, to the best of our knowledge, no consensus as to the boundaries, in the postnatal heart, of the components identified by Kramer.Reference Kramer 8 This is of particular significance with regard to the arterial valves, as there is also no consensus as to whether they, along with their supporting sinuses, belong to the “conus” or the “truncus”.
We believe that the evidence now adduced from study of the developing heart in both man and mouse using episcopic microscopy favours a redefinition of the nomenclature proposed by Kramer, providing the developing arterial valves with their own discrete part of the developing outflow tract.Reference Sizarov, Lamers, Mohun, Brown, Anderson and Moorman 9 , Reference Anderson, Chaudhry and Mohun 10 Thus, during the initial stages of development in the mouse heart, and at comparable stages of development of the human heart, the cardiac outflow tract has a decidedly serpentine configuration, permitting recognition of distal, intermediate, and proximal components (Fig 2a). The appearance of the intercalated cushions in the intermediate part of the outflow tract, described initially by Kramer in his landmark investigation as the forerunners to the arterial valves, adds further traction to the description of the intrapericardial outflow tract in terms of three, rather than two, components (Fig 2b). The intrapericardial components of the arterial trunks are formed within the distal part of the outflow tract, with the non-myocardial cells contributing to these components continuing to migrate into the arterial pole of the murine heart from the second heart field and the neural crest during the 9th and 10th days of development. The series of paired arch arteries that connect the aortic sac to the initially paired dorsal aortas are located extrapericardially within the pharyngeal mesenchyme, encircling the trachea–oesophageal pedicle (Fig 2b).Reference Congdon 2 , Reference Bamforth, Chaudhry and Bennett 3 , Reference Hiruma, Nakajima and Nakamura 11 The arterial channels form within the pharyngeal arches, a series of protuberances that develop in sequence in a cranial-to-caudal direction. Each protuberance, or arch, has an epithelial covering, comprising endoderm on the inside and ectoderm on the outside. The first four arches each contain a mesodermal core, along with mesenchyme derived from the neural crest. In addition to the arterial channels, each arch also contains a neural, bony, and muscular component.Reference Graham 12 , Reference Graham 13 The formation of the arterial channels in the mouseReference Bamforth, Chaudhry and Bennett 3 , Reference Hiruma, Nakajima and Nakamura 11 closely parallels the arrangement described for the developing human heart.Reference Congdon 2 , Reference Bamforth, Chaudhry and Bennett 3 The arches themselves, and the arterial channels developing within them, are first observed during the latter half of murine embryonic day 8, and during embryonic day 9 (Fig 3).

Figure 2 The images are from an episcopic data set prepared from a mouse embryo in the 11th day of development. Panel ( a ) shows an overview of the developing heart, with the white arrows showing the extent of the pericardial cavities. The arch arteries are within the pharyngeal mesenchyme. Panel ( b ) shows a section across the outflow tract, revealing the presence of the intercalated cushions within its intermediate component.

Figure 3 The images show the development of the pharyngeal arch arteries at embryonic (E) day 9.5 in the mouse. High-resolution episcopic microscopy data sets were manually segmented to illustrate the three-dimensional arrangement of the developing arch arteries ( a , c , and e ) and these images were overlayed onto a sagittal view of the embryo ( b , d , and f ). The numbers of somites (s) developed at a particular stage are shown in the different panels. At the stage with 19 somites ( a and b ), only the first and second arch arteries are present, connecting the as to the da. By the middle of E9.5 stage, when the embryo has 24 somites ( c and d ), the artery of the third arch has started to form and is complete by the 27 somite stage ( e and f ). By this stage, the first and second arch arteries have begun to regress. Note that the arteries are bilaterally symmetrical during these early stages of cardiovascular development. as=aortic sac; da=dorsal aorta.
The arteries of the first arch develop as loops between the bilaterally paired dorsal and ventral aortas. The second and third pairs of arch arteries sprout sequentially, extending from the extrapericardial part of the outflow tract also known as the aortic sac, and connect again with the dorsal aortas. All three paired vessels are present by the end of the 10th murine embryonic day, which is equivalent to Carnegie stage 11 in the developing human (Fig 3). The fourth pair of arteries has begun to appear at the beginning of the 11th day. By this stage, however, the first and second arch arteries have begun to regress, forming a capillary network within the developing jaw, and making no contribution to the final structure of the aortic arch (Fig 4a and b).Reference Hiruma, Nakajima and Nakamura 11 It is during this day of development in the mouse that the sixth pair of arteries become apparent (Fig 4c and d).

Figure 4 The images show the stages of development during embryonic (E) day 10.5 in the mouse. As with Figure 3, episcopic data sets were manually segmented to illustrate the changes in the developing arch arteries ( a and c ), and these images were overlayed onto a sagittal view of the embryo ( b and d ). The numbers of somites (s) are again indicated. In the embryo with 32 somites, which is at the beginning of the E10.5 stage, four pairs of arteries are seen, although the first and second are now regressing, and the fourth is just forming. By late E10.5, shown in the embryo with 40 somites, there are symmetrical arteries in the third, fourth, and sixth pharyngeal arches.
The sixth arch arteries also occupy a discrete segment of pharyngeal mesenchyme. Unlike the first four arches, however, the sixth arches do not contain bony, muscular, or neural elements. By the end of the 11th day of murine development, the bilaterally symmetrical system of the arteries is fully formed (Fig 5). During the following day (E11.5), we have observed collateral channels, as shown in Figure 5, in up to half of all the embryos. They extend through the dorsal pharyngeal mesenchyme between the caudal surfaces of the fourth pair of arteries and the cranial surfaces of the sixth pair.Reference Bamforth, Chaudhry and Bennett 3 – Reference Geyer and Weninger 5 These collateral channels are not seen before the formation of the sixth pair of arch arteries.Reference Congdon 2 , Reference Bamforth, Chaudhry and Bennett 3 It is also possible to recognise additional spurs that could represent forming or regressing channels. The morphological findings suggest that the formation of the collateral channels is a normal occurrence, although their function remains unknown. It is our belief, nonetheless, that they could well be implicated in producing many of the structures previously interpreted as “fifth arch arteries”.

Figure 5 The images show comparable development as seen in Figures 3 and 4, but this time for mice during embryonic (E) day 11.5. The numbers of somites are indicated in the appropriate panels. The system begins to remodel asymmetrically during E11.5. By this stage, the intrapericardial aorta (ao) and pulmonary trunk (pt) have separated and rotated, resulting in the thinning of the right sixth arch artery, as seen in panels ( a and d ). The pulmonary arteries (pa) are forming within the pharyngeal mesenchyme. At this stage, as shown in panels ( b , c , e and f ), approximately half of mouse embryos display a collateral channel (cc) extending between the dorsal parts of the fourth and sixth arch arteries.
Having completed its formation by the end of E10.5, the initially bilaterally symmetrical system undergoes significant remodelling during E11.5 and E12.5 so as to form the mature systemic arteries. The first vessel to remodel is the right sixth arch artery, which thins and involutes during E11.5 as the outflow tract of the heart rotates (Fig 5a and d). The regression of the right sixth arch artery is accompanied by regression of the right-sided dorsal aorta (Fig 6). The left and right carotid ducts, formed from the segments of the dorsal aortas between the dorsal insertions of the third and fourth arch arteries, also reduce in size during this period, and have fully regressed by the end of the E12.5. Subsequent to the remodelling, the third arch arteries continue cranially into the remaining parts of the dorsal aortas and thus form the internal carotid arteries. On the left side, the fourth arch artery has expanded to form the aortic arch, whereas the left sixth arch artery has become the arterial duct. Further significant remodelling is needed, however, before the left subclavian artery, derived from the left cervical seventh intersegmental artery, can reach its definitive origin from the aortic arch. Similar remodelling is required on the right side so that the right seventh intersegmental artery can arise from the right horn of the aortic sac, which then becomes the brachiocephalic trunk (Fig 6).

Figure 6 The images show ongoing maturation of the arch arteries in developing mice at E12.5 and E13.5. The manually segmented data sets are shown as frontal ( a and c ) and left ( b and d ) views. By E12.5, as seen in panels ( a and b ), the left sixth aortic arch has become the arterial duct (ad), whereas the right and left pulmonary arteries (pa) have achieved their extrapericardial origin from the pulmonary trunk (pt). The arteries of the third arches have become the right and left common carotid arteries (rcc, lcc) and the right dorsal aorta (rda) has regressed, whereas the left dorsal aorta (lda) has become larger. The right and left primitive subclavian complexes (rpsc, lpsc) will form the subclavian arteries. The left and right vertebral arteries (rva, lva) are seen in panel ( b ). By E13.5, as seen in panels ( c and d ), the previously symmetrical arch arteries have fully remodelled into the mature aortic arch configuration. ao=aorta; da=dorsal aorta; lsa=left subclavian artery; rsa=right subclavian artery.
During our examination of a much smaller number of episcopic data sets obtained from the developing human embryos,Reference Bamforth, Chaudhry and Bennett 3 we encountered one specimen with remnants of an unequivocal fifth arch artery (Fig 7). Further interrogation of the episcopic data set obtained from this embryo showed that the persisting left-sided artery was contained within an additional discrete segment of pharyngeal mesenchyme, distinct from the sixth pharyngeal arch (Fig 7c and e). Had the artery of the fifth arch persisted, it would have extended from the aortic sac, which becomes the extrapericardial ascending aorta, to the descending aorta. Moreover, had it continued to feed the developing pulmonary circulation, it would have done so through the dorsal part of the sixth arch artery. We suggest that candidates to be considered as persistent fifth arch arteries should be expected to run a similar extrapericardial course. If they feed the pulmonary circulation, then this should be via an origin from the ascending aorta, and then through a dorsal serpentine continuation of the sixth arch. In this respect, it is significant that collateral channels, as found in half the mouse embryos, have also been found in the human embryos. As in the developing mice, they connect the dorsal parts of the fourth and sixth arch arteries, and take their origin from the caudal, as opposed to the cranial, parts of the extrapericardial ascending aorta. It is the similarities to the arrangements of these various developmental patterns that we have used when seeking to adjudicate on the validity of claims for the persistence of fifth arch arteries in congenitally malformed humans.

Figure 7 The reconstruction of the developing pharyngeal arch arteries from a human embryo at Carnegie stage (CS) 14 reveals a fifth arch artery. This stage is equivalent to E10.5 in the mouse. The episcopic data set was manually segmented to illustrate the developing arch arteries, and the pharyngeal endoderm has been labelled in green in panels ( a and b ). Panel ( a ) shows that the third, fourth, and sixth arch arteries are bilaterally symmetrical. In addition, evidence of a fifth arch artery is seen on the left side in panel ( b ). The course of the arterial channel is such that it indicates it took origin from the aortic sac (as). It terminates in the dorsal portion of the left sixth arch artery, which then continues to join the left-sided descending aorta. Panel ( c ) shows a coronal section, and panels ( d and e ) show sagittal sections of this embryo, the latter viewed from the right ( d ) and the left ( e ). They demonstrate that the left-sided fifth arch artery is contained within a discrete segment of the pharyngeal mesenchyme bounded by endodermal and ectodermal epithelial surfaces. da=dorsal aorta; pa=pulmonary arteries.
The candidates for persistent fifth aortic arch arteries
Although Gerlis et alReference Gerlis, Ho, Anderson and Da Costa 7 have drawn attention to a description provided by Hudson in 1965, it is Van Praagh and Van Praagh,Reference Van Praagh and Van Praagh 6 in 1969, who are generally credited with making the suggestion that the so-called double-barrelled aorta could be interpreted on the basis of persistence of the artery of the fifth aortic arch. They claimed that “from the developmental standpoint, this anomaly appears clearly to indicate that a fifth arterial arch can occur in man, thereby resolving a long-standing embryologic dispute”. Furthermore, they suggested that “the double lumen extends from the level of the innominate artery proximally to the level of the left subclavian artery and ductus arteriosus distally”. Such has been the impact of this simplified, or arguably oversimplified, interpretation that many subsequent investigators have labelled any vessel arising from the distal part of the ascending aorta, but proximal to the level of the brachiocephalic artery, as representing the persisting artery of the fifth arch. Advances in the field of cardiac imaging have now resulted in many cases being reported in the world literature not only to explain double-barrelled aorta, but also to account for systemic-to-pulmonary arterial channels, and for abnormal branching of the brachiocephalic arterial structures. We have reassessed these reports for the purposes of this review, so as to assess their validity in terms of representing persistence of the fifth arch arteries as opposed to collateral channels, or remodelling of the fourth and sixth arch arteries.
Double-barrelled aorta
The emphasis placed by Van Praagh and Van PraaghReference Van Praagh and Van Praagh 6 on the relatively proximal origin of the arterial channel running parallel to the aortic arch as evidence in favour of its origin as the fifth arch artery found support from Gerlis et al.Reference Gerlis, Ho, Anderson and Da Costa 7 The latter investigators, furthermore, claimed that, rather than being non-existent, the fifth arch may prove to be underdiagnosed. It is certainly the case that such channels are now interpreted with frequency on this basis, as 40 cases of double-barrelled aorta have now been reported as the representing fifth arch arteries (Table 1), with more and more cases being labelled in this manner simply because of the presence of the double lumen aorta. Many of the investigators making this diagnosis, however, do not even establish whether the origin of the second channel is proximal to that of the brachiocephalic artery. The determination of the precise proximal nature of the channel, however, does not prove conclusive at times. In one of the cases reported from one of our own Institutions,Reference Murugan, Gulati, Saxena, Juneja and Gupta 14 for example, assessment using angiography was not directly comparable to that obtained using CT (Fig 8). In another of our cases with double-barrelled aorta, which we examined echocardiographically, the proximal origin of the location of the arterial duct is highly suggestive that the findings are better explained on the basis of persistence of collateral channels combined with remodelling of the lesser curvature of the aortic arch (Fig 9). Those arguing in favour of persistence of the fifth arch artery would find the proximal location of arterial duct extremely difficult to explain in this setting. To date, furthermore, to the best of our knowledge, there has been no acknowledgement that the collateral channels formed between the dorsal insertions of the fourth and sixth arch arteries could provide an alternative explanation of the findings. Ignorance of this alternative explanation is more significant; as we have explained, we found such collateral channels in up to half of all our developing mice,Reference Bamforth, Chaudhry and Bennett 3 – Reference Geyer and Weninger 5 and also in developing human embryos.Reference Bamforth, Chaudhry and Bennett 3

Figure 8 The images show the channel interpreted by Murugan et alReference Murugan, Gulati, Saxena, Juneja and Gupta 14 as representing persistence of the artery of the fifth arch. Their published images might suggest that the origin of the second channel is distal to the origin of the brachiocephalic artery. Analysis of the angiographic ( a ) and the echocardiographic ( b ) images from this patient show that the origin is opposite to that of the brachiocephalic artery. These discrepancies illustrate the problems in determining whether the second channel truly represents a fifth arch artery, or is better interpreted on the basis of remodelling of the arterial duct. If we invoked Occam’s razor, then the rarity of the existence of the fifth arch artery, compared with the frequency of the arterial duct, favours the latter option.

Figure 9 The echocardiographic images obtained from this patient with the double-barrelled aorta show that the origin of the lower channel is opposite to, or distal to, the take-off of the persistently patent arterial duct. This makes an interpretation on the basis of persistence of a collateral channel just as likely, if not more likely, than the alternative explanation of persistence of the fifth arch artery (see also Fig 8).
Aortic-to-pulmonary arterial communications (Table 2)
Aortic-to-pulmonary arterial confluences
Congdon,Reference Congdon 2 when commenting on the alleged findings of fifth arch arteries in the developing human embryos, stated that “if it is established that these vessels are true fifth arches, their usual termination would indicate strongly that the upper end of the pulmonary arch is the homologue of the distal portion of the fifth”. We take this to mean that, if a fifth arch artery is the structure feeding the pulmonary arteries, it would do so via the dorsal portion of the sixth arch arteries. It was this concept, combined with origin of the channel proximal to the brachiocephalic artery, that received endorsement from Macartney et alReference Macartney, Scott and Deverall 15 when describing such a candidate channel. The cartoon provided by Gerlis et alReference Gerlis, Ho, Anderson and Da Costa 7 to illustrate one of their cases (Fig 10), however, shows the purported fifth arch artery as arising from the aortic sac, but terminating directly in the pulmonary arteries, and not involving the sixth arch arteries. We have, nonetheless, encountered a channel arising from the lateral aspect of the ascending aorta in one of our patients with ventricular septal defect and pulmonary atresia in the setting of discordant ventriculoarterial connections (Fig 11). This channel, similar to the one described by Lee et alReference Lee, Chiu, Dai, Lin, Wu and Wu 16 and Subramanyan et al,Reference Subramanyan, Sahayaraj, Sekar and Cherian 17 is directly comparable to the cartoon drawn by Gerlis et al, albeit with no interruption of the aorta.

Figure 10 The cartoon drawn by Gerlis et al,Reference Gerlis, Ho, Anderson and Da Costa 7 which we have redrawn to produce this figure, illustrates the presumed explanation of the fifth arch arteries arising from the aortic sac proximal to the origin of the brachiocephalic artery, with the right-sided artery feeding the right pulmonary artery. The fifth arch arteries as illustrated, however, are not using the dorsal component of the aortic arch to feed the pulmonary arteries, which would be the situation if they were truly arteries of the fifth arch (see Fig 7). The star shows the missing portion of the true right-sided fifth arch artery. Gerlis et al used their cartoon to interpret a heart having aortic atresia with interruption of the aortic arch, as will be shown in our Figure 16. In our opinion, the artery they have interpreted to be the right fifth arch artery is better considered as being the right-sided arterial duct.

Figure 11 The image shows the arterial pathways in the heart from a patient with discordant ventriculoarterial connections, ventricular septal defect, and pulmonary atresia. The channel feeding the pulmonary arteries arises from the ascending aorta proximal to the origin of the brachiocephalic artery. Its distal extent, however, feeds the confluence of the pulmonary arteries in retrograde manner, in other words through the termination of the left sixth arch artery. This favours strongly its origin as a fifth arch artery. This channel is similar to the one described by Lee et alReference Lee, Chiu, Dai, Lin, Wu and Wu 16 and Subramanyan et al.Reference Subramanyan, Sahayaraj, Sekar and Cherian 17 Lcc=left common carotid; lsc=left subclavian.
Fifth arch arteries versus distal aortopulmonary windows
As with the double-barrelled aorta, subsequent investigators have identified any vessel arising from the distal ascending aorta proximal to the brachiocephalic artery, and joining the superior aspect of bifurcation of the pulmonary trunk, or supplying either the right or left pulmonary artery, as representing a fifth arch artery. A good example of a channel that can justifiably be interpreted as a fifth arch artery is seen in the autopsy specimen housed in the museum of Children’s Hospital of Pittsburgh (Fig 12). This type of connection, when seen in alternative settings, however, has been interpreted by some investigators as representing a distal aortopulmonary window.Reference Gerlis, Ho, Anderson and Da Costa 7 , Reference Freedom, Silver and Miyamura 18 – Reference Lee, Chiu, Fang, Chen, Wang and Chaou 23 The distal aortopulmonary window is, however, intrapericardial. This would not be the case for either a collateral channel or a fifth arch artery, both of which would run extrapericardial courses. Most of the cases reported as representing the fifth arch arteries have been associated with intracardiac anomalies, producing reduced pulmonary blood flow, although some were found in isolation, presenting as left-to-right shunts.Reference Wu, Chiu, Lin and Huang 19 – Reference Chiu, Wu, Chen and Lin 21

Figure 12 The image shows the arterial pathways in the heart from a patient with tetralogy of Fallot and pulmonary atresia. The channel feeding the pulmonary arteries arises from the ascending aorta proximal to the origin of the brachiocephalic artery. When traced distally, it feeds the confluence of the pulmonary arteries in retrograde manner, in other words through the termination of the left sixth arch artery. This again favours its origin as a fifth arch artery.
The abnormal connections cannot be explained on the basis of conventional understanding of the development of the pharyngeal arch arteries without invoking the presence of a fifth arch artery, or alternatively a collateral channel. In either event, the purported channels must incorporate part of the original sixth arch arteries to feed the pulmonary confluence, and hence, as shown in the case illustrated in Figure 13, or in the third case reported by Gerlis et al,Reference Gerlis, Ho, Anderson and Da Costa 7 must take a serpentine course. The channels must also take their proximal origin from the ascending aorta, again as shown in the case illustrated in Figure 13, rather than the transverse segment of the aortic arch. In the latter setting, which is the case for the majority of reported cases, the channels could just as well represent persistence of the arterial duct (see the following section).

Figure 13 Volume-rendered reformatted CT images, looking from the back ( a ) and front ( b ), show the supply to the pulmonary arterial tree in a patient with tetralogy of Fallot with pulmonary atresia. The right lung is supplied by the systemic-to-pulmonary collateral arteries arising from the descending aorta ( a ). The supply to the left lung, however, is through a serpentine vessel that arises from the ascending aorta ( b ) and terminates in the left pulmonary artery. As in the heart shown in Figure 12, the course strongly favours its origin as a fifth arch artery (Reproduced with permission of Dr Gurpreet S. Gulati, All India Institute of Medical Sciences, New Delhi).
Anomalous origin of a pulmonary artery from the ascending aorta
The fifth arch artery has been implicated by some investigators to explain the morphogenesis of anomalous origin of the right or left pulmonary artery directly from the ascending aorta. In our opinion, such interpretations do not reflect current knowledge concerning the development of the intrapericardial components of the arterial trunks. Recent data from developing mouse embryos have shown that the right and left pulmonary arteries, which develop within the pharyngeal mesenchyme, take their origins from the sixth arch arteries as the exit from the floor of the aortic sac. They would have no direct connection with a fifth arch artery, should such an artery be present (Fig 7). It is the unequal separation of the aortic sac that results in either the right or left pulmonary artery remaining anomalously attached to the ascending aorta, rather than retaining its connection with the pulmonary trunk.
In reality, relatively few investigators have chosen to invoke the fifth arch arteries in these situations. In one such case, nonetheless, the left pulmonary artery was described as being constricted at the presumed site of junction of the fifth arch artery with the left pulmonary artery.Reference Koch, Ludwig, Zink and Singer 24 In another reported example, a double lumen was observed in the left-sided aortic component of a patient with the common arterial trunk, along with the absence of the right pulmonary artery, whereas the left pulmonary artery arose from the dominant aortic component of the common trunk.Reference Parmar, Pillai, Kulkarni and Sivaraman 25 The explanation offered by these authors, however, would require the presence of two fifth arch arteries on the same (left) side, as would be the case for the patient found to have double-barrelled aorta and anomalous origin of the left pulmonary artery from the ascending aorta,Reference Wang, Wu and Yang 26 although this latter patient must have had the bilateral fifth arch artery to be able to explain the anatomy described. In all these instances, we are dubious that the evidence substantiates the claim for persistence of the arteries of the fifth arch.
Large aortopulmonary connections from the lateral aspect of the ascending aorta
Several investigators have suggested that, in the setting of patients having ventricular septal defect with pulmonary atresia, the finding of systemic-to-pulmonary collateral arteries arising from the distal part of the ascending aorta, but proximal to the origin of the brachiocephalic artery, can best be explained as representing the persistent fifth arch arteries. Such major systemic-to-pulmonary arteries are the rule rather than the exception in patients with tetralogy of Fallot and pulmonary atresia. The collateral arteries typically arise from the descending rather than the ascending aorta, or from its brachiocephalic branches. It is also well recognised, nonetheless, that the collateral arteries can arise from the coronary arteries, and may rarely be sufficiently large to account for all the pulmonary blood flow. In the setting of tetralogy of Fallot with pulmonary atresia, it is the reduced pulmonary blood flow that promotes the development of the large aortopulmonary collateral arteries. Simply on the basis of probabilities, therefore, invoking the formation of systemic-to-pulmonary collateral arteries to explain origin from the ascending aorta seems more plausible than implicating the fifth arch artery. The course of the channel arising from the ascending aorta and supplying the confluence of the pulmonary arteries (Fig 11), or the left pulmonary artery in the patient illustrated from one of our own Institutions (Fig 13), nonetheless, is highly suggestive of its origin as an artery of the fifth arch.
Aortic-to-pulmonary connections from the undersurface of the transverse aortic arch
It is not uncommon in patients with tetralogy of Fallot and pulmonary atresia to encounter a channel that takes its origin from the undersurface of the transverse component of the aortic arch and feeds one or both the pulmonary arteries. On occasion, nonetheless, the channel may arise proximally, from the ascending part of the arch, as shown in the heart illustrated in Figure 12. This channel is comparable to the persistently patent arterial duct, but has a more proximal origin that is usually the case for the duct in these patients. This proximal origin has been cited as evidence to support the structure as representing persistence of the fifth arch artery. It is known, nonetheless, that marked remodelling of the persistently patent duct supplying the pulmonary circulation can occur as a result of intrauterine flow patterns, as in the setting of pulmonary atresia with intact ventricular septum. Significant remodelling might be expected, therefore, in such situations. The histology of the purported fifth arch artery is also said to resemble that of the arterial duct. This suggests that, rather than representing a fifth arch artery, it may simply be the remodelled arterial duct.Reference Freedom, Silver and Miyamura 18 Our own bias, therefore, is to explain the channels, such as the one shown in Figure 8, on the basis of remodelling of the sixth arch arteries, rather than incorporation of the elusive fifth arch artery, unless the serpentine course of the channel, or its markedly proximal origin, favours the alternative explanation.
Systemic-to-pulmonary arterial connections
Another subset of patients needs special mention at this point. In these reported cases,Reference Lim, Kim, Kim, Lee, Kim and Kim 27 we interpret the anatomy is being that of the common arterial trunk with pulmonary dominance and interruption of the aortic arch (Fig 14). In this setting, it was the absence of a connection of the pulmonary arteries with the channel that continued as the descending thoracic aorta that led the authors to argue that it was the fifth, rather than the sixth, arch artery that had persisted. In another of the cases described by Gerlis et al in 1989, the specimen exhibited aortic atresia, interruption of the aorta beyond both the carotid arteries, and aberrant retroesophageal origin of the right subclavian artery (Fig 15). This heart, in addition, showed a channel connecting the right pulmonary artery to the diminutive ascending aorta. The channel was interpreted by them as an artery of the fifth arch, which was providing blood to flow from the pulmonary trunk to the ascending aorta and, in turn, to the carotid arteries. To us, the putative fifth arch artery seems more akin to the right-sided sixth arch derivative, as it takes origin from a right-sided brachiocephalic artery and connects directly to the right pulmonary artery. We interpret the channel, therefore, as more likely representing the right-sided arterial duct. In still other instances, nonetheless, such as in the case reported by Boothroyd et al,Reference Boothroyd, Smith and Peart 28 the anatomy is bizarre so as to call into question any interpretation made on the basis of our current knowledge of cardiac development.

Figure 14 We have redrawn the cartoon of Lim et al, showing our preferred explanation of their case as representing the common arterial trunk with pulmonary dominance and interruption of the aortic arch.
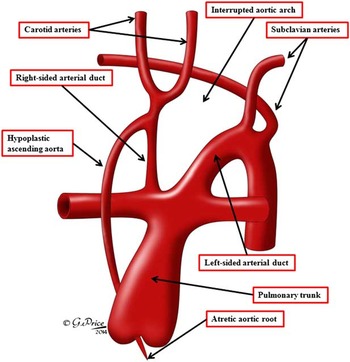
Figure 15 We have redrawn the cartoon of Gerlis et alReference Gerlis, Ho, Anderson and Da Costa 7 that illustrated the heart from a patient with aortic atresia and interruption of the aortic arch. As we show, we interpret the right-sided channel arising from the brachiocephalic artery, and feeding the pulmonary arteries, as a persistently patent arterial duct, thus respresenting a sixth, rather than a fifth, arch artery (See also Fig 10).
Abnormal origin of the brachiocephalic arteries
There are several abnormal arrangements of the brachiocephalic arteries from the ascending aorta or the aortic arch that have been interpreted on the basis of persistence of the fifth arch arteries, again with limited developmental evidence provided to support the inference made (Table 3). As we have already shown, the arteries contained within the pharyngeal arches undergo extensive remoulding during the embryonic period before attaining their final configuration (Figs 3–6). It is also well acknowledged that variations in the pattern of branching of the extrapericardial arteries arising from the aortic arch are common. The normal pattern is represented by the first branch being the right-sided brachiocephalic artery, which branches to become the right common carotid and subclavian arteries. This normal pattern, however, is seen in only two-thirds to four-fifths of patients, with the other patients showing the multiple variant patterns.Reference Rahme, Abruzzo and Ringer 29 The most frequent variant is for the brachiocephalic trunk and the left common carotid artery to take a common origin from the aortic arch, an arrangement said to occur in up to one-quarter of individuals, and known as the bovine pattern. Other common variants are direct origin of the left vertebral artery from the aortic arch, reported to occur in up to one-twentieth of patients, and aberrant retrooesophageal origin of the right subclavian artery from the descending aorta, found in around 1% of otherwise normal patients.Reference Natsis, Tsitouridis and Didagelos 30
Table 3 List of all the cases published in the world literature having abnormalities of the brachiocephalic arteries that have been explained based on the fifth arch artery.

The cases with the single brachiocephalic trunk containing the bilateral carotid and subclavian arteries are labelled as “True bovine arch”. The cases with the two arch vessels with the left common carotid artery arising either from the brachiocephalic artery or as a common trunk from the aorta with the subclavian artery arising separately are labelled as “pseudobovine arch”, as it is different from the “bovine arch” but is being labelled inappropriately in the literatureIn the column giving details of cases, the reference to ‘Case 2’ or ‘Case 3’ refers to the individual as identified by the original authors
The generic label of the “bovine arch” includes both common origin of the brachiocephalic trunk and the left common carotid artery, as well as origin of the left common carotid artery from the brachiocephalic trunk, with reported occurrences of 13 and 9%, respectively. Both these variants are seen more commonly in black when compared with white populations. This so-called “bovine arch”, however, bears no resemblance to the pattern of branching of the aortic arch as seen in cattle, and is possibly one of the most egregious misnomers in the medical literature.Reference Layton, Kallmes, Cloft, Lindell and Cox 31 The “bovine arch”, as seen in cattle and other animals with a deep chest, is represented by a single brachiocephalic trunk originating from the aortic arch, which then divides into the bilateral carotid and subclavian arteries. This arrangement of the single trunk coming off the aortic arch is arguably to compensate for the long distance between the thoracic inlet and the neck. The true bovine arch, like the other reported variations, is thought to remain asymptomatic and therefore may remain undetected. The arrangement, nonetheless, has rarely, if ever, been reported in analyses of large numbers of patients, indicating that it is an exceedingly rare variation. Perhaps the most extensive analysis of the potential patterns that might occur subsequent to remoulding of developing the arch arteries is that reported on the basis of study on 453 human cadavers by Nizankowski et al.Reference Nizankowski, Rajchel and Ziolkowski 32 Although the authors discovered abnormal patterns in only 15 specimens, they illustrated 25 possible variations in branching. Some of these patterns, however, have been interpreted on the basis of persistence of the arteries of the purported fifth arch. For example, Moes et alReference Moes, Benson, Burrows, Freedom, Williams and Duckworth 33 implicated the artery of the fifth arch when describing four patients in whom the left subclavian artery arose as the first branch of the left-sided aortic arch, with Oppido and DaviesReference Oppido and Davies 34 supporting this interpretation. The left subclavian artery itself, nonetheless, migrates over a significant length of the aortic arch as the seventh segmental intersegmental artery is converted into the definitive vessel (Fig 6). We would suggest that exuberant continuing migration is just as likely to produce the abnormal pattern of branching as is persistence of the extremely rare artery of the fifth arch.Reference Boechat, Gilsanz and Fellows 35 , Reference Hernandez, Shepard, Bamforth and Anderson 36 As already discussed, aberrant retroesophageal origin of the subclavian artery is not uncommon, even in the otherwise normal population. This lesion is well explained on the basis of the hypothetical double aortic arch, as propounded by Edwards.Reference Edwards 37 When reporting two additional cases, Lee et alReference Lee, Chen and Chen 38 implicated the artery of the fifth arch as explaining proximal origin of the carotid artery. This again seems most unlikely, as the remoulding of the initially bilaterally symmetrical system of the arches provides a much more realistic explanation (Figs 3–6).
Conclusions
The arteries of the fifth pharyngeal arches, if they exist, are exceedingly rare entities. In his monumental review of 1922, CongdonReference Congdon 2 reviewed some previously described potential candidates, but was dubious to their validity. We have studied large numbers of the developing mouse embryos and are yet to observe bilaterally symmetrical channels that parallel the formation of the arteries of the first through fourth pharyngeal arches, or the subsequent sixth arch arteries, with the latter set of arteries lacking any specific pharyngeal arches. We have, however, discovered collateral channels extending between the distal insertions of the fourth and sixth arch arteries in up to half of all developing mice.Reference Bamforth, Chaudhry and Bennett 3 Such channels have been described as “fifth arch arteries” by other investigators.Reference Lorandeau, Hakkinen and Moore 4 , Reference Geyer and Weninger 5 They do not, however, originate from the aortic sac, and do not run in parallel with the previously existing bilateral channels. We have also observed such collateral channels in the developing human embryos.Reference Bamforth, Chaudhry and Bennett 3 We have, nonetheless, now discovered a channel in a developing human embryo that has an appearance appreciably more in keeping of what might be expected from a fifth arch artery (Fig 6). The channel that we found is in potential communication with the aortic sac and does terminate in the dorsal aorta. Furthermore, it occupies a discrete segment of the pharyngeal mesenchyme. Our embryo, however, does not possess a similar channel on its right side. Therefore, much depends on the interpretations made by the clinical investigators of the previous descriptions of embryonic development, if we are to adjudicate the validity of the multiple channels that have now been described within the world literature as persistence of the arteries of the fifth arches (Tables 1 and 2).
Having taken all these features into consideration, and examined the findings as described in the literature, we are dubious regarding the majority of claims made concerning the persistence of arteries of the fifth arch. The examples of the double-barrelled aorta rarely extend from an origin proximal to the take-off of the brachiocephalic artery, although they often originate opposite to the brachiocephalic artery. As such, we consider it just as likely, if not more likely, that they represent persistence of the dorsal collateral channels found in up to half of all the developing mouse embryos, and seen in two of our human embryos. In the case of the systemic-to-pulmonary channels, we do believe that persistence of the fifth arch provides a reasonable explanation for a minority of cases, such as the example we found in the archive of Pittsburgh Children’s Hospital, and the channel seen in some of our patients. Many of the cases, nonetheless, are equally well explained on the basis of persistence and abnormal remoulding of the arterial duct, or persistence of the collateral channels. In the setting of tetralogy with pulmonary atresia, it is also possible that some candidates are no more than the systemic-to-pulmonary collateral arteries, although it is difficult in some instances to deny that the channels could represent the arteries of the fifth arch. In the case of the manifold variations in patterns of branching of the arteries arising from the aortic arch, our own preference is to opt for excessive remoulding of the known arch arteries, rather than to consider persistence of a fifth arch artery. In the final analysis, nonetheless, we acknowledge that our own interpretations are just as speculative as those depending on persistence of the arteries of the fifth arch. We have, therefore, summarised our suggestion in Table 4, hoping to stress the potential clinical implications of correct identification of the elusive fifth arch arteries.
Table 4 The conclusions regarding the reviewed cases described in the world literature as involving the persistent fifth arch arteries.

Acknowledgements
The authors are grateful to Dr S.S. Kothari, Dr Anita Saxena, Dr Rajnish Juneja, Dr S. Ramakrishnan, Dr Sanjiv Sharma, and Dr Gurpreet S. Gulati from the All India Institute of Medical Sciences, New Delhi, in helping with the identification of cases with possible fifth arch artery.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.






















