INTRODUCTION
The ability to initiate and sustain purposeful finger movements while simultaneously inhibiting adjacent finger movements has been recognised as an important feature of human hand function (Jones & Lederman, Reference Jones and Lederman2006) and reflects a decided evolutionary advantage (Donald, Reference Donald1991). It is a complex motor achievement involving a delicate balance of both excitatory and inhibitory neural activity in several regions of the cortical-spinal tract. At the cortical level, the primary motor cortex (M1), the premotor cortex, the supplementary motor cortex and prefrontal cortex are involved when normally functioning individuals perform repetitive finger tapping tasks while inhibiting adjacent finger movements (Bächinger, Rea Lehner, Hanimann, Balsters, & Wenderoth, Reference Bächinger, Rea Lehner, Hanimann, Balsters and Wenderoth2019; Jones & Lederman, Reference Jones and Lederman2006). At the subcortical level, the basal ganglia has also been recognised as important for efficient starting, sustaining and stopping fast repetitive finger movements (Beck & Hallett, Reference Beck and Hallett2011).
Behaviourally, this important feature of normal hand/finger motor control has been described as “selective inhibition of movement” (Coxon, Stinear, & Byblow, Reference Coxon, Stinear and Byblow2007). It is defined as “the ability to prevent one movement while concurrently executing another” (Coxon et al., Reference Coxon, Stinear and Byblow2007, p. 2480). It has been proposed that this behavioural achievement is based on the physiological mechanism of “surround inhibition” (Beck & Hallett, Reference Beck and Hallett2011). The concept of surround inhibition is that a zone of inhibitory neural activity surrounds an area of neural activation within the cortex to permit a more controlled and precise motor activity (such as inhibiting adjacent fingers of the hand when the index finger is required to complete a task) (Sohn & Hallett, Reference Sohn and Hallett2004).
Failure to control individual finger movements when performing finger tapping tasks has been reported in a number of clinical conditions, including cerebral vascular accidents (CVA) (Birchenall et al., Reference Birchenall, Térémetz, Roca, Lamy, Oppenheim, Maier and Lindberg2019), traumatic brain injury (TBI) (Prigatano & Borgaro, Reference Prigatano and Borgaro2003), neurodevelopmental disorders (Tallet, Albaret, & Barral, Reference Tallet, Albaret and Barral2013) and focal hand dystonia (Moore, Gallea, Horovitz, & Hallett, Reference Moore, Gallea, Horovitz and Hallett2012). Interestingly, CVA patients make more frequently “unwanted extra-finger taps” than normal controls, but the pattern of findings is the same in both groups (Térémetz, Colle, Hamdoun, Maier, & Lindberg, Reference Térémetz, Colle, Hamdoun, Maier and Lindberg2015). A “neighborhood gradient” of “unwanted extra-finger taps” has been reported. “Digits anatomically far from the target (lead) digit produced fewer error taps than those close to (or immediate neighbors of) the target digit” (p. 10). If the person was attempting to tap only with the index finger, the middle finger (i.e., finger 3) more frequently moved when the person was instructed not to move any of the other fingers. The ring and little fingers (i.e., fingers 4 and 5) were less likely to move. These phenomena have been reported by others (Aoki, Shinohara, & Kinoshita, Reference Aoki, Shinohara, Kinoshita and Shinohara2009). Thus, behaviourally one might anticipate that when normal functioning adults are instructed to tap with the index finger and keep other fingers still, middle finger (finger 3) movements would occur more frequently than distal digits (fingers 4 and 5). Despite these observations, clinical neuropsychologists have not included measures of controlled individual finger movements when conducting a neuropsychological examination.
A commonly used neuropsychological test that measures fast sustained index finger movements is the Halstead Finger Oscillation Test (Halstead, Reference Halstead1947). Later referred to as the Halstead Finger Tapping Test (HFTT), it requires the person to place the index finger on a lever attached to a mechanical counter and move the lever up and down as fast as possible for a series of 10-second intervals. The individual is instructed to try and keep the adjacent fingers and the palm of the hand immobile on the tapping board (to which the counter is attached) while preforming the task. Thus it requires the individual to execute a repetitive motor sequencing task that involves inhibition and excitation of finger movements simultaneously. While the average number of recorded taps on the mechanical counter has been used in hundreds of studies to help identify the presence of underlying brain dysfunction (Lezak, Howieson, Loring, & Fischer, Reference Lezak, Howieson, Loring and Fischer2004), the potential diagnostic value of failure to inhibit adjacent finger movements while performing this task has not been studied.
A major challenge in analysing the potential diagnostic significance of failure to inhibit adjacent finger movements has been a lack of data that addresses the question: “How frequent are failures at selective motor inhibition in normally functioning adults when performing the HFTT?” Motor physiology studies have suggested that young adults (i.e., age 40 and younger) often have no difficulty inhibiting adjacent finger movements when performing a finger tapping task using the index finger, but older adults demonstrate a decline in selective motor inhibition (Levin, Fujiyama, Boisgontier, Swinnen, & Summers, Reference Levin, Fujiyama, Boisgontier, Swinnen and Summers2014; Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl, & Seidler, Reference Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl and Seidler2019; Seidler et al., Reference Seidler, Bernard, Burutolu, Fling, Gordon, Gwin and Lipps2010). The cause of that decline remains unclear, but appears linked to a decline in neuronal integrity involving the somatosensory cortex (Ruitenberg et al., Reference Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl and Seidler2019) as well as a decline in levels of gamma-aminobutyric acid (GABA) (Pauwels, Maes, Hermans, & Swinnen, Reference Pauwels, Maes, Hermans and Swinnen2019). We recently used kinematic recordings to assess how age, educational level and the sex of healthy adults correlated with the speed of finger tapping while performing a modified version of the HFTT (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019). Males often had faster start and stop times compared to females. More educated individuals also had faster start, but not stop times. Age, however, appeared related only to the actual number of valid counts (or taps) achieved per 10-second trial.
In the present report, we extend our kinematic analysis to the study of adjacent finger movements in normally functioning adults. Kinematic recordings of adjacent finger movements were obtained while subjects performed a modified version of the HFTT. Based on earlier clinical observations (Prigatano & Hoffman, Reference Prigatano and Hoffman1997), an attempt was made to classify finger movements into four basic patterns. Pattern 1 involved executing the tapping task as instructed. The index finger repeatedly tapped the lever on the mechanical counter as fast as possible for 10 seconds while the other fingers did not move and the palm of the hand remained immobile on the tapping board. Pattern 2 exhibited an inability to suppress movement of the adjacent ipsilateral middle finger while the other fingers and the palm of the hand remained immobile on the board during the task. This middle finger lifted off the board when the index finger was tapping. This was considered one manifestation of failure to maintain selective motor inhibition. Pattern 3 consisted of the inability to suppress movement of two or more adjacent fingers while the index finger completed the task. This typically involved the middle finger and the fourth finger lifting off the board and at times included the fifth digit as well. This was considered a greater failure at selective motor inhibition. Pattern 4 involved lifting the entire hand off the board when attempting to perform the task. While the index finger tried to move the lever up and down as fast as possible, the entire hand also moved up and down. All fingers and the palm of the hand rose over the tapping board. Pattern 4 appeared to represent the most severe form of failure to inhibit adjacent finger movements. It resembled an apraxic difficulty. No specific hypotheses were made, however, regarding which patterns would be most frequent given the limited data available using the HFTT.
The frequency of Patterns 1, 2, 3 and 4 were studied as a function of the individuals’ age group (young, middle-aged and older adults), educational level (high school education vs. college degree) and sex. Given that these variables had small-to-medium effects on the average number of counted taps while performing both the HFTT (Leckliter & Matarazzo, Reference Leckliter and Matarazzo1989) and the modified HFTT (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019), our exploratory hypothesis was that all three variables would correlate at a similar level with failures to maintain selective finger inhibition. Our major goal, however, was to determine the frequency of Patterns 1 to 4 in normally functioning adults.
METHODOLOGY
Participants
In total, 107 healthy volunteers between the ages of 20 and 80 years participated in this study. The mean age was 48.9 years (SD = 16.3). Participants were placed into three age groups: 20–39 years old (32.7% of the sample); 40–59 years old (40.2% of the sample) and 60–80 years old (27.1% of the sample). Fifty-eight participants (54.2%) were college graduates; 49 (45.8%) had a high school diploma. Fifty-five were women (51.4% of the sample) and 52 were men (48.6%). All subjects were right hand dominant, by their subjective report. Forty-three of the subjects were recruited from a convenience sample of the staff of the SARAH Network of Rehabilitation Hospitals in Brasilia and had successfully completed their annual physical and psychological screening tests. The remaining 64 subjects were caregivers of patients treated in the hospital network and deemed by hospital staff to be physically and psychologically capable of caring for the patients they accompanied to the hospital at discharge. Each subject was informed of the purpose of the study and what would be required of them if they chose to participate. They signed an informed consent to participate. The study was approved by the Ethics Committee of the SARAH Network of Rehabilitation Hospitals under Regulation #94830518.50000.0022.
Procedures and Materials
Modified Halstead Finger Tapping Test
Each participant was asked to perform the HFTT with two modifications. First, the tapping key was placed on the left side of the Veeder-Root counter, which was used in the original HFTT (model #0727215 –001), for tapping with the left index finger. The tapping key was on the right side of the counter for tapping with the right index finger, as was traditionally performed. Second, the participant was instructed to tap as fast as possible, moving only their right index finger, for three consecutive 10-second trials. Next, the participant was asked to tap as fast as possible, moving only their left index finger, for three consecutive 10-second trials. The participant was then told to repeat tapping with the right hand for three consecutive 10-second trials followed by the left index finger for three consecutive 10-second trials. Finally, there was one more trial with the right hand followed by a single trial with the left hand. This procedure resulted in seven trials for each hand.
The participant was expressly instructed to keep the palm of the hand and wrist on the board while tapping as fast as they could for each 10-second trial. They were also asked to not move any of the adjacent fingers when performing the task, which included the thumb and the middle, fourth and fifth fingers. This was demonstrated to each participant during the practice phase before the recordings were obtained. A single reminder was given: the first time the neuropsychologist administering the test noticed movement of the adjacent fingers. The reminder was not based on kinematic recordings.
Kinematic measurements of finger movements and selective motor inhibition on the modified Halstead Finger Tapping Test
Kinematic recordings were obtained using a motion capturing system that was developed to measure movements of the fingers 5 seconds before, during, and 3 seconds after completing the modified version of the HFTT. Movement detectors (reflective markers) were placed on the mechanical counter as well as each participant’s hands, fingers and arms. Methodological details, a photo of the placement of the markers and the actual motion capturing system can be found in Prigatano et al. (Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019). We used a VICON movement analysis system with 12 MXF40 cameras and the Nexus program, version 1.7.1. Kinematic recordings were processed offline in Matlab (version R 2014.a). This methodology permitted tracking of adjacent finger movements while the task was being performed, and also counted the incidence of adjacent finger movements on each trial in normally functioning adults.
The motion capturing system is capable of tracking the reflective markers down to millimeters. Thus, an automatic classification algorithm was developed to filter the marker motion and reliably detect finger lift as described by Prigatano and Hoffman (Reference Prigatano and Hoffman1997). The automatic classification algorithm determines the minimum detectable elevation by an adjacent finger that yielded a recorded vertical movement (dH), as well as an accumulated time threshold to capture reliable adjacent finger movement (dT). This automatic classifier algorithm was calculated based on a small convenience sample of 12 individuals (42 trials for each hand) selected to train and validate the classifier. The authors manually classified each subject trial according to the Prigatano and Hoffman (Reference Prigatano and Hoffman1997) description of adjacent finger movement patterns. A trial was classified as Pattern 1 when no adjacent finger movement was detected on middle, fourth and fifth fingers; Pattern 2 when movement was detected only on the middle finger; Pattern 3 when movement was detected on the middle and fourth fingers; and Pattern 4 when the entire hand lifted off the board (i.e., movement was detected on the thumb and middle, fourth and fifth fingers and the back of the hand). In the Prigatano and Hoffman (Reference Prigatano and Hoffman1997) classification system, thumb movements were not included, except for Pattern 4.
Two subjects were selected to train the classifier (i.e., training set) and the remaining 10 subjects (i.e., testing set) were used for testing the classifier. Both training and testing samples included all four finger movement patterns. The optimum values for dH and dT were obtained when all possible combinations were tested in each training interval. The optimum parameters (dH = 8.95 mm; dT = 0.17 seconds) classified the training set with 100% accuracy. Those values were tested with the testing set, also resulting in 100% accuracy.
Each finger was analysed by the algorithm and labelled as “still” or “moved”. If the middle, fourth and fifth fingers were recorded as “still”, the trial was classified as Pattern 1. If fingers were labelled as “moved”, then the trial was identified as Patterns 2, 3, or 4. As these recordings were obtained, we noted that a participant could exhibit different performance patterns throughout the seven 10-second trials. Thus, in this study a trial was operationally defined as Pattern 1 only if there were no adjacent finger movements (measured by the dH and dT criteria) at any time during that trial. If a participant showed Pattern 1 for most of the trial but at some point the kinematic recordings detected Pattern 2 activity, then the trial was labelled Pattern 2. The same method of classification was used to identify trial Patterns 3 and 4. Pattern classification was therefore determined by the most deviant movements recorded during that trial.
This study comprised 107 participants who completed seven 10-second trials each, yielding 749 trials for each hand. The finger and hand movements in each trial were labelled according to the aforementioned classification algorithm.
STATISTICAL ANALYSIS
The descriptive analysis included demographic variables (three age groups, two educational levels and sex) and the qualitative classification pattern (i.e., pattern type) for each trial applied the kinematic measures described above. Participants were classified as “failure to inhibit the adjacent finger” when Patterns 2, 3 or 4 were observed on three or more of the seven trials.
Pearson’s Chi-squared and Fisher’s exact tests were used to evaluate the association between a subject’s demographic variables and the four patterns classifying finger performance. The effect size was evaluated by Cramer’s V and interpreted as small, medium or large when V was .10, .30 and .50, respectively. When a 3 × 2 contingency table (DF = 2) was analysed, the thresholds for small, medium or large were .07, .21 and .35 (Cohen, Reference Cohen1988, p. 222).
McNemar’s test was used to assess the incidence of failure to inhibit the adjacent fingers between dominant and non-dominant hand. In this case, the odds ratio (OR) was calculated only for effect size purpose.
We employed a mixed logistic regression approach to determine which demographic variables significantly correlated with the failure to inhibit adjacent finger movements on all trials (N = 749 per hand).
A linear regression model assessed the association between the failure to inhibit adjacent finger movements and the mean of taps adjusted by the demographic variables.
All analyses were performed with R Package (version 3.5.2) using core and external libraries.
The significance level was set at p ≤ .05, two-tailed. For the statistical inferences with the data stratified by demographic variables (Tables 3, 4 and 6) the significance level (p ≤ .05) was divided by the number of categories in each variable (Bonferroni correction).
RESULTS
Age Effects
Normally functioning young adults (age range 20–39) had no difficulty inhibiting adjacent finger movements when performing the modified version of the HFTT with their right, dominant hand. Only one individual out of the 35 participants in the 20–39 age range (2.9%) had difficulty inhibiting adjacent finger movements (Table 1). When adjacent finger movements occurred, it was primarily the middle finger that moved (i.e., Pattern 2); the other fingers rarely moved (Pattern 3). No individual in this age range lifted the entire hand when performing the task (i.e., Pattern 4) (Table 2).
Table 1. Failure to inhibit adjacent finger movements in normal adults as a function of age, education and sex

DF = degree of freedom.
Failure to inhibit movements as recorded when Patterns 2, 3 or 4 were observed on three or more of the seven trials.
a Fisher’s Exact Test: Sex (p = .519), Age Group (p = .587), Educational Level (p = .181).
Table 2. Most observed deviant failure to inhibit movement by sex, age group and educational level – Dominant hand (sample size = 749 trials, 107 subjects)

a Logistic regression with random effects using failure as dichotomous (failure vs. no failure). Variance of subject (intercept): 7.07 (sex), 6.00 (age group) and 6.38 (educational level).
In the middle adult years (ages 40–59), 23.3% (i.e., 10 out of 43 participants) exhibited a failure to inhibit adjacent finger movements with the dominant hand. Pattern 2 was most common, while Patterns 3 and 4 rarely occurred (Tables 1 and 2).
In older adults (60–80 years of age), 31.0% (i.e., 9 out of 29 individuals) had difficulty inhibiting adjacent finger movements with their dominant hand. Again, Pattern 2 was the most frequent failure to inhibit movement classification, followed by rare occurrences of Patterns 3 and 4, respectively (Tables 1 and 2).
Age was significantly associated with the difficulties inhibiting adjacent finger movements in normal healthy adults, X 2(2, N = 107) = 9.271, p = .010, Cramer’s V = .29). The effect size was medium. As Figure 1 illustrates, the incidence of failure to inhibit adjacent finger movements in this study was very low prior to the age of 50. However, after 50 the incidence increased and remained relatively stable for the other older age ranges.

Fig. 1. The percentage of adult individuals who demonstrate any failure to inhibit adjacent finger movements on three or more of the seven trials as a function of decades of life, beginning in the 20’s.
It is important to note that failure to inhibit adjacent finger movements also occurred in the non-dominant left hand (9.3%), but these movements were relatively less frequent than what was observed in the right dominant hand (18.7%), McNemar test, X 2(1, N = 107) = 5.063, p = .024, OR = 4.3) (Table 1).
Education Effects
Educational level (high school vs. college education) was also related to presence or absence of adjacent finger movements when performing the modified version of the HFTT (Table 1). Only 10.3% of individuals with a college education were unable to inhibit adjacent finger movements with the dominant right hand compared to 28.6% of those with only a high school education (X 2(1, N = 107) = 4.669, p = .031, Cramer’s V = .23, a medium size effect). A strong interaction effect was observed between educational level and participants in the 40–59 age range (see Table 3). In total, 45% of middle-aged adults who had only a high school education showed failures to inhibit adjacent finger movements in the dominant hand, compared to only 4.3% of college educated middle-aged adults (Fisher’s exact test, N = 43, p = .003).
Table 3. Failure to inhibit adjacent finger movements in normal adults as a function of Age × Education.

a Failure to inhibit movements as recorded when Patterns 2, 3 or 4 were observed on three or more of the seven trials.
b Fisher’s Exact Test.
While individuals with a high school education versus college education differed in the frequency of failures to inhibit adjacent finger movements in the dominant hand, the same relative frequency of pattern performance was observed. Pattern 1 was most common, followed by Pattern 2. Patterns 3 and 4 rarely occurred in both the dominant (Table 2) and non-dominant hand (not illustrated in Table 2)
Sex Effects
Males and females did not differ in their ability to inhibit adjacent finger movements in the dominant hand (19.2% and 18.2%, respectively) or the non-dominant hand (11.5% and 7.3%, respectively) while performing the finger tapping test (Table 1). There were no age versus sex interaction effects with the dominant hand (Table 4). There was a slight interaction in the non-dominant hand (Table 4). Four out of 20 males (20%) in the 40–59 age range had difficulty inhibiting adjacent finger movements in the non-dominant hand (Fisher’s exact test, N = 43, p = .039). The pattern of adjacent finger movements was also the same for males and females in the dominant hand (Table 2) and the non-dominant hand (not illustrated in Table 2).
Table 4. Failure to inhibit adjacent finger movements in normal adults as a function of Age × Sex
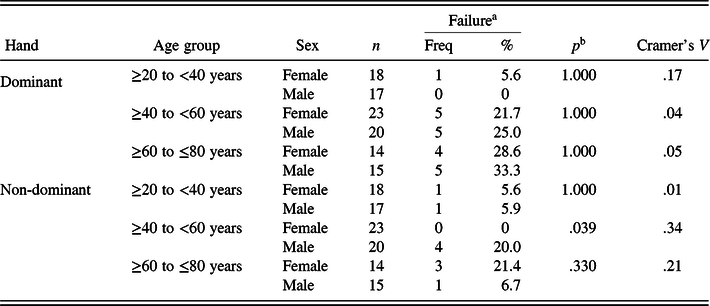
a Failure to inhibit movements as recorded when Patterns 2, 3 or 4 were observed on three or more of the seven trials.
b Fisher’s Exact Test.
Frequency of Patterns 1, 2, 3, and 4 as a Function of the Seven Trials
As Figure 2 illustrates, the frequency of Patterns 1, 2, 3 and 4 was fairly consistent across each of the seven trials. Learning and fatigue effects for the different patterns were not observed.

Fig. 2. The percentage of Patterns 1, 2, 3, and 4 observed on Trials 1, 2, 3, 4, 5, 6, and 7 in a normal sample of adults.
Linear Regression Analysis for Predicting the Mean Number of Taps in the Dominant hand
The demographic variables of the individual’s age group, educational level and sex are known to correlate with mean number of taps in the dominant hand during the modified version of the HFTT (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019). In addition to including these measures as predictive variables, we included the failure to inhibit adjacent finger movements as a predictor variable. While males tapped faster (B = 6.5) and older adults tapped slower (B = −10.0), failure to inhibit adjacent finger movements in college-educated individuals resulted in lower number of taps per 10-second trials (β = −.20, p = .043) (Table 5). Table 6 lists actual mean finger tapping scores for the dominant and non-dominant hands as a function of failure to inhibit adjacent finger movements, and the individual’s age group, educational levels and sex.
Table 5. Linear regression for mean of taps, dominant hand (sample size = 107 subjects)

Table 6. Effect of failure to inhibit adjacent finger movements in number of taps by sex, age and education (sample size = 107 subjects)

DISCUSSION
The findings of the present study provide the first estimates of failure to inhibit adjacent finger movements in normally functioning adults who perform the modified version of the HFTT. The method of classifying the different patterns of failure to inhibit adjacent finger movements was based on clinical observations and not predetermined by any previous behavioural or neural hypothesis. By and large, effective selective finger inhibition is quite common in young adults (ages 20–39). This is in keeping with other reports on selective motor inhibition using other finger tapping tasks (e.g., (Ruitenberg et al., Reference Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl and Seidler2019). While the incidence of failures to inhibit adjacent finger movements increases over three broad age ranges (i.e., 20–39 vs. 40–59 vs. 60–80), our findings suggest that after age 50 a clear increase occurs in normally functioning individuals and remains fairly stable after that age period (Figure 1). It should be emphasised, however, that there is primarily only one type of finger inhibition failure that is observed in normally functioning adults. In about 20% of the trials for middle-aged and older adults, Pattern 2 occurred. This finding is compatible with finger tapping data from other studies using different methods of measuring finger tapping movements (Aoki et al., Reference Aoki, Shinohara, Kinoshita and Shinohara2009; Dupan et al., Reference Dupan, Stegeman and Maas2018; Térémetz et al., Reference Térémetz, Colle, Hamdoun, Maier and Lindberg2015). It potentially may be explained by the fact that the thumb and index finger show the highest degree of independent movement control. The middle finger and fourth finger can move independently, but with slightly less individual control (Häger-Ross & Schieber, Reference Häger-Ross and Schieber2000). Earlier research on patterns of impairment in digit independence have also noted that in both controls and persons with subcortical stroke, it is most difficult to keep the middle (third) finger still or stationary while other fingers attempt to move independently (Raghavan, Petra, Krakauer, & Gordon, Reference Raghavan, Petra, Krakauer and Gordon2006). Middle-aged and older adults who cannot inhibit adjacent index finger movements on the majority of trials (e.g., 50% or more) may show a decline in selective motor inhibition that goes beyond the normal aging process. Future research will have to investigate this possibility and its potential clinical relevance.
The other important finding is that failure to inhibit more than the middle finger (i.e., Pattern 3) and inability to keep the palm of the hand on the tapping board while performing the modified HFTT (i.e., Pattern 4) is a very rare occurrence in normally functioning individuals at all age ranges. These observations are also compatible with the findings of Térémetz et al. (Reference Térémetz, Colle, Hamdoun, Maier and Lindberg2015). When Patterns 3 and 4 are observed, it may suggests more serious selective motor inhibition failures.
Age, Education and Sex Effects
The three age groups and two educational levels showed a medium effect on failure to inhibit adjacent finger movements, as anticipated. Older individuals have more difficulty controlling adjacent finger movements while the index finger is moving rapidly. This basic finding of reduced motor inhibition in the elderly has been reported in several studies (King et al., Reference King, Van Ruitenbeek, Leunissen, Cuypers, Heise, Santos Monteiro and Swinnen2017; Pauwels et al., Reference Pauwels, Maes, Hermans and Swinnen2019). Quite interestingly, a substantial interaction was observed in individuals with a high school education who were in the middle age range (i.e., 40–59 years). They exhibited the greatest incidence of failure to inhibit adjacent finger movements when tapping with the dominant right index finger. This interaction effect was not observed in the younger (20–39 years) or older (60–60 years) age groups. It may be that during this middle adult age range, the effects of education on selective, individual finger motor inhibition is actually stronger than age effects. The opposite appears to be true for younger and older groups of individuals.
During the mid-50s the actual level of performance (i.e., raw scores) on many neuropsychological tests often falls within the “impaired” range if educational level is not considered in the interpretation of the data (Prigatano & Grant, Reference Prigatano, Grant, McSweeny and Grant1988). It is also well known that white matter tracts within the cerebral hemispheres rapidly develop in the late 20s and early 30s and then decline in the late 60s and middle 70s (Westlye et al., Reference Westlye, Walhovd, Dale, Bjørnerud, Due-Tønnessen, Engvig and Fjell2009). During these time periods, changes in brain functioning connectivity may override or diminish educational effects as they relate to selective finger/motor inhibition/control.
It is possible that educational experiences impact white matter tracts. Research is this area, however, has reported mixed findings specifically as they relate to frontal white matter tracts (Arenaza-Urquijo et al., Reference Arenaza-Urquijo, Landeau, La Joie, Mevel, Mézenge, Perrotin and Chételat2013; Kim, Chey, Kim, & Kim, Reference Kim, Chey, Kim and Kim2015; Rojkova et al., Reference Rojkova, Volle, Urbanski, Humbert, Dell’Acqua and De Schotten2016). Recent neuroimaging correlates of literacy acquisition in middle-aged adults from Brazil suggests that increased functional connectivity does occur in many brain regions, including the left fronto-parietal white matter tracts (López-Barroso et al., Reference López-Barroso, de Schotten, Morais, Kolinsky, Braga, Guerreiro-Tauil and Cohen2020). Lower levels of education may result in less robust connectivity between fronto-parietal regions and this may contribute to failures to inhibit adjacent finger movements when performing a modified version of the HFTT. While this remains clearly a speculation, several years ago O’Boyle, Gill, Benbow, and Alexander (Reference O’Boyle, Gill, Benbow and Alexander1994) noted that boys gifted in mathematics demonstrated faster finger tapping scores than boys with average mathematical skills. A later neuroimaging study showed that children who perform high on standardised math tests have greater fronto-parietal connectivity than children not gifted in math and the difference could not be explained purely on the basis of IQ level (Emerson & Cantlon, Reference Emerson and Cantlon2012).
Sex effects were not observed to impact an individual’s ability to inhibit adjacent finger movements in the dominant and non-dominant hands while performing the modified version of the HFTT. Males and females also showed a very similar pattern of the types of adjacent finger inhibition failures. While sex does relate to the number of taps achieved per 10-second intervals (Leckliter & Matarazzo, Reference Leckliter and Matarazzo1989) and the frequency of invalid taps during 10-second trials (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019), it does not appear to be related to the capacity to inhibit adjacent finger movements. The absence of a sex effect is perhaps not surprising given that sex effects have not been reported in other studies dealing with selective motor inhibition (Pauwels et al., Reference Pauwels, Maes, Hermans and Swinnen2019; Ruitenberg et al., Reference Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl and Seidler2019). Even developmental studies on selective response inhibition have failed to find sex differences, although age effects are often quite robust (Bedard et al., Reference Bedard, Nichols, Barbosa, Schachar, Logan and Tannock2002; Booth et al., Reference Booth, Burman, Meyer, Lei, Trommer, Davenport and Mesulam2003). Motor inhibitory control is a basic neuropsychological function crucial for adaptation, learning and finger dexterity. Therefore, differences between the sexes would seem improbable from an evolutionary perspective and is compatible with behavioural data that shows males and females perform very similarly on tests involving finger-hand motor dexterity (Oxford Grice et al., Reference Oxford Grice, Vogel, Le, Mitchel, Muniz and Vollmer2003).
Dominant Hand and Failures to Inhibit Adjacent Finger Movements
One of the most striking findings of this study was that failures to inhibit adjacent finger movements while performing the modified version of the HFTT occurred primarily in the dominant hand with much fewer failures in the non-dominant hand. This is especially interesting given that independence of finger movements does not appear to be different in the dominant versus non-dominant hands (Häger-Ross & Schieber, Reference Häger-Ross and Schieber2000). Yet, as Hausmann, Kirk, and Corballis (Reference Hausmann, Kirk and Corballis2004) have pointed out, a dominant hand advantage is often observed when the individual performs a “simple” finger tapping task compared to more complicated finger tapping tasks.
All individuals in this study were right-hand dominant. Thus, a special role of the left cerebral hemisphere in motor inhibition of finger movements seems likely. Shin, Sohn, and Hallett (Reference Shin, Sohn and Hallett2009) observed a hemispheric asymmetry of surround inhibition in the human motor system. They noted that transcranial magnetic stimulation (TMS) applied to M1 suppressed average motor evoked potential (MEP) amplitudes when performing an index finger movement in the right hand, but not the left hand. They interpreted their findings as suggesting that selective or surround inhibition was typically more efficient in the dominant hand and perhaps contributed to greater dexterity in the dominant hand. In light of these observations, excessive failure of adjacent finger movement inhibition while performing the modified version of the HFTT with the dominant hand may signal early decline of left hemisphere functioning. This finding might also help explain the age versus education interaction effect noted earlier. Assuming that education greatly influences language functions typically mediated by the left cerebral hemisphere in right-handed individuals (Dehaene et al., Reference Dehaene, Pegado, Braga, Ventura, Nunes Filho, Jobert and Cohen2010; Thiebaut de Schotten, Cohen, Amemiya, Braga, & Dehaene, Reference Thiebaut de Schotten, Cohen, Amemiya, Braga and Dehaene2014), less education may result in less developed left cerebral hemisphere neural networks, which in turn degrade level of finger motor inhibition.
Absence of Fatigue or Learning Effects on Failure to Inhibit Adjacent Finger Movements
While fatigue has been related to failures of inhibitory motor control (Bächinger et al., Reference Bächinger, Rea Lehner, Hanimann, Balsters and Wenderoth2019), the present findings did not reveal a fatigue effect when performing a modified version of the HFTT. The frequency of Patterns 1, 2, 3 and 4 did not change over trials. Patterns 2,3 and 4 were not worse on trial 6 compared to trial 4 (which were used to measure fatigue effects in a previous study (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019)). In contrast, fatigue effects have been observed in relation to the number of taps performed during the modified HFTT (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019). Keeping a finger still is an isometric activity that does require force/energy particularly in the ring finger (Dupan et al., Reference Dupan, Stegeman and Maas2018). Fatigue effects associated with finger force while performing an isometric activity have been reported when the task is a minute or longer (Singh, SKM, Zatsiorsky, & Latash, Reference Singh, SKM, Zatsiorsky and Latash2010). The time interval for each trial using the modified HFTT was only 10 seconds and this shorter time may have been responsible for failure to observe a fatigue effect. Also, fatigue effects associated with isometric activities have been related to spinal and supraspinal motor circuits, while fatigue associated with finger tapping have been related to the primary motor cortex (Arias et al., Reference Arias, Robles-García, Corral-Bergantiños, Madrid, Espinosa, Valls-Solé and Cudeiro2015). Differences in underlying neural mechanisms associated with fatigue in these two different motor activities may also contribute to the observed findings.
Comparing frequency of Patterns 2, 3 and 4 on trials 4 versus 1, as done in a previous study (Prigatano et al., Reference Prigatano, Goncalves, de Oliveira, Denucci, Pereira and Braga2019), also did not reveal a learning effect. This may be a task specific finding given the relatively short periods of time in which motor inhibitory activity was required. Carrying out other motor tasks with prolonged practice may result in a learning effect (Hiraoka, Ito, Lutton, Nakano, & Yonei, Reference Hiraoka, Ito, Lutton, Nakano and Yonei2020).
Potential Implications of these Findings for Neuropsychological Assessment
The frequency of failures of inhibitory adjacent finger movements while finger tapping may help identify persons with an underlying brain disorder when controlling for age and education effects. Young adults would not be expected to demonstrate failures of inhibitory control, but patients with a history of moderate-to-severe TBI have demonstrated such difficulties (Prigatano & Borgaro, Reference Prigatano and Borgaro2003). Improvement of motor inhibitory control may also reflect recovery after various brain disorders (Birchenall et al., Reference Birchenall, Térémetz, Roca, Lamy, Oppenheim, Maier and Lindberg2019) as well as reflecting normal developmental improvements in school-aged children (Prigatano, Gray, & Legacy, Reference Prigatano, Gray and Legacy2008).
Abnormalities of finger tapping performance in older individuals may be a neuropsychological marker of the preclinical phases of Alzheimer Disease (AD) (Albers et al., Reference Albers, Gilmore, Kaye, Murphy, Wingfield, Bennett and Devanand2015; Buchman & Bennett, Reference Buchman and Bennett2011; Mollica et al., Reference Mollica, Tort-Merino, Navarra, Fernández-Prieto, Valech, Olives and Sánchez-Valle2019). Roalf et al. (Reference Roalf, Rupert, Mechanic-Hamilton, Brennan, Duda, Weintraub and Moberg2018) recently brought attention to the fact that many neurodegenerative disorders, including AD, present with mild disorders of motor functioning. While speed and variability of finger tapping separates groups of patients with mild cognitive impairments (MCI) and AD patients from normal healthy controls (Roalf et al., Reference Roalf, Rupert, Mechanic-Hamilton, Brennan, Duda, Weintraub and Moberg2018), the present findings raise the possibility that persistent failure to inhibit adjacent finger movements may also have further diagnostic value in separating MCI from AD patients. Finger tapping speed and variability has been associated with amyloid-B positivity in cognitively normal individuals (Mollica et al., Reference Mollica, Tort-Merino, Navarra, Fernández-Prieto, Valech, Olives and Sánchez-Valle2019).
LIMITATIONS OF THE PRESENT STUDY
The findings of the present study do not provide normative data on failures of selective motor inhibition when performing the HFTT. Sample size for the age ranges was moderate and was dictated by the cost of conducting kinematic recordings. In addition, the study sample was from Brazil and may not represent selective motor inhibition failures in an American or other populations. Nevertheless, the findings provide guidelines for what might be expected when assessing adjacent finger movements using the modified version on HFTT.
The findings are compatible with a large literature on motor inhibition and aging. Young adults typically do not exhibit failures at selective motor control, but older individuals do. Reduction of inhibitory control has been reported around 40 years of age (Ruitenberg et al., Reference Ruitenberg, Cassady, Reuter-Lorenz, Tommerdahl and Seidler2019). We observed the same phenomena, but for the task employed failure to inhibit adjacent finger movements was most frequent after the age of 50.
Another potential limitation of this study was that the effects of education were limited to either a high school or college education. Having a broader range of educational levels (e.g., from eighth grade to the doctorate level) may reveal a more profound effect of education on finger motor inhibitory control.
It should be noted that this study does not test hypotheses regarding potential neural mechanisms underlying selective motor inhibition, but rather describes the frequency of inhibitory failures while performing a modified version of the HFTT. The four patterns of finger movements studied here were based on clinical observations and not driven by any a prior behavioural or neural hypotheses.
CONCLUSIONS
Using a convenience sample of 107 adults representing three broad age ranges (young, middle-aged and older adults), we found that age appeared to have a medium effect on the individual’s ability to control adjacent finger movements when performing a modified version of the HFTT with the dominant hand. Young adults are very good at controlling adjacent finger movements, but increased difficulties appear at age 50 and older. Education also has a medium effect. Sex of the individual was not related to the frequency of selective motor inhibition failures. The most common failure was the inability to control the adjacent middle finger when tapping (i.e., Pattern 2). The potential diagnostic significance of frequent failures at selective motor inhibition using a modified version of the HFTT will have to be determined in future studies. Future neuroimaging studies will also be needed to determine if there are different underlying structural or functional correlates of the different patterns of motor inhibitory failures observed in this study.
ACKNOWLEDGEMENTS
The SARAH Network of Rehabilitation Hospitals provided funding to conduct this study.
CONFLICT OF INTEREST
The authors have nothing to disclose.










