Background
Permethrin is an insecticide that belongs to the pyrethroid family of neurotoxic agents designed to kill insects by altering the permeability of the voltage-gated sodium channel (VGSC). Pyrethroids are highly preferred to other insecticides in vector control management practices due to their low toxicity to humans, rapidity of action, and easy and fast degradability in the environment (Ray, Reference Ray, Krieger, Doull and Ecobichon2001). As such, permethrin-based insecticide treated nets and indoor residual spraying have been extensively used to control Anopheles gambiae populations in malaria endemic areas and have been effective in reducing the transmission of the parasites from infective mosquito females to humans (Enayati & Hemingway, Reference Enayati and Hemingway2006; WHO, 2007). However, over the last decade, strains of An. gambiae that are resistant to the toxic effects of permethrin and other pyrethroids have become widespread in several endemic areas in Africa (Casimiro et al., Reference Casimiro, Coleman, Hemingway and Sharp2006; Adasi & Hemingway, Reference Adasi and Hemingway2008). The most established source of permethrin resistance is represented by point-mutations within the gene encoding the VGSC that have been associated with knockdown resistance (kdr) to different insecticides (Ranson et al., Reference Ranson, Jensen, Vulule, Wang, Hemingway and Collins2000). But, enhanced metabolic detoxification mechanisms through up-regulation of cytochrome P450 monooxygenase (P450), glutathione S-transferase (GST), and non-specific esterase genes have also been reported in Anopheles mosquitoes (Vulule et al., Reference Vulule, Beach, Atieli, McAllister, Brogdon, Roberts, Mwangi and Hawley1999; Hemingway & Ranson, Reference Hemingway and Ranson2000; Djouaka et al., Reference Djouaka, Bakare, Coulibaly, Akogbeto, Ranson, Hemingway and Strode2008; Lumjuan et al., Reference Lumjuan, Rajatileka, Changsom, Wicheer, Leelapat, Prapanthadara, Somboon, Lycett and Ranson2011). Although significant progress has been made in understanding the molecular mechanisms underlying the resistance to permethrin in malaria vectors (Li et al., Reference Li, Schuler and Berenbaum2007; Soderlund, Reference Soderlund2008), there have been few reports of how alleles that confer resistance to insecticides affect other fitness characteristics of the malarial vectors in insecticide-free environments.
Life history theory applied to this problem suggests that we should expect alleles influencing resistance to insecticide to come at the cost of other fitness traits, especially in an insecticide-free environment. The life-history theory is based on the idea that physiological traits such as reproduction, storage, somatic maintenance, growth, and development are energetically costly traits. Because resources are limited, differential allocation of energy among these competing demands can produce trade-offs among traits and natural selection is thought to have shaped the way organisms partition their limiting resources to these fitness components, balancing the costs and benefits (Wiley, Reference Wiley1974). Fitness costs associated with resistance mechanisms have been reported in insecticide-free environments reviewed in (Brooke & Koekemoer, Reference Brooke and Koekemoer2010; Kliot & Ghanim, Reference Kliot and Ghanim2012). For example, work in An. gambiae and Anopheles stephensi showed that strains resistant to dieldrin had reduced fecundity compared to susceptible individuals despite similar longevity (Rowland, Reference Rowland1991). Reduced fecundity and shorter reproductive period and lifespan were also observed in carbofuran resistant aphids (Roberto & Omoto, Reference Roberto and Omoto2006). However, loss of fitness has not been observed in other studies (Okoye et al., Reference Okoye, Brooke, Hunt and Coetzee2007), suggesting that a cost of resistance may not always occur (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000; Rigby et al., Reference Rigby, Hechinger and Stevens2002). This is particularly true if different molecular mechanisms of resistance exist and/or ecological factors are involved (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000). Because evolutionary fitness costs are the cornerstones of economic optimal models of malaria vector insecticide resistance (Brown et al., Reference Brown, Dickinson and Kramer2013); more research in this area is necessary. Such research could elucidate whether new resistance management strategies for insecticide use are needed to maintain or restore its efficacy (Read et al., Reference Read, Lynch and Thomas2009).
The employment of mechanisms of detoxification in insects can be energetically costly (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000). Thus, it is conceivable that increased metabolic detoxification in pyrethroid resistance would result in a resource allocation trade-off between the detoxification mechanisms and other energetically demanding physiological functions, such as growth or reproduction (Rivero et al., Reference Rivero, Vezilier, Weill, Read and Gandon2010). In the present study, we compared life-history traits and energy metabolism between a wild-derived permethrin resistant strain of An. gambiae, and a permethrin susceptible strain reared under laboratory conditions. The permethrin resistant strain used in our study is characterised for a kdr mutation and enhanced levels of P450 and esterase enzyme activities (Vulule et al., Reference Vulule, Beach, Atieli, McAllister, Brogdon, Roberts, Mwangi and Hawley1999) (http://www.mr4.org/). We focused on this resistant strain of An. gambiae because growing evidence suggests that pyrethroid resistance in the wild is likely due to a combination of target-site insensitivity and metabolic-based mechanisms (Brooke & Koekemoer, Reference Brooke and Koekemoer2010).
Materials and methods
Strains and colony maintenance
A strain of An. gambiae with reduced susceptibility to permethrin (RSP) and a permethrin susceptible (ASEMBO1) strain were used. The strains were obtained from the Malaria Research and Reference Reagent Resource Center (MR4) (http://www.mr4.org/).
RSP has the East African form of the kdr sodium channel mutation allele, L1014S, and increased P450 and beta-esterase activities (Vulule et al., Reference Vulule, Beach, Atieli, McAllister, Brogdon, Roberts, Mwangi and Hawley1999). RSP was originally isolated from a permethrin impregnated bed net study carried out in four adjacent villages in North West of Kisumu, Kenya (Vulule et al., Reference Vulule, Beach, Atieli, McAllister, Brogdon, Roberts, Mwangi and Hawley1999). The strain was established from a colony representative of An. gambiae selected for permethrin tolerance (Vulule et al., Reference Vulule, Beach, Atieli, McAllister, Brogdon, Roberts, Mwangi and Hawley1999) and subsequently deposited in the MR4 collection, where it has been selected for permethrin resistance for over 10 years by treating a cohort of fourth-instar larvae with 1 ppm permethrin for 24 h every three generations (http://www.mr4.org/).
The permethrin susceptible strain ASEMBO1 was originally collected 50 km west of Kisumu in a control area for the United States Agency for International Development cohort bed net project approximately 4.5 km from the study area (http://www.mr4.org/).
The stocks were obtained twice from the MRC4 (2009 and 2012) and the bioenergetics experiments described below were performed within 5 months of arrival.
Experimental conditions
Mosquitoes were maintained in insectary rooms at 25±2 °C, 80±10% relative humidity, and 16 h light/8 h dark cycle. Larvae were provided abundant food consisting of ground Tetramin (fish food). Adult mosquitoes were fed on 10% honey solution soaked in cotton sticks. Unless otherwise stated, experimental groups of each strain were 3–5-day-old adults reared in 30 cm3 cages. For egg production, young adults of the respective strains were allowed to freely mate and feed on blood from ears of a restrained rabbit. Moist filter paper was placed in cages for oviposition and the eggs collected were incubated overnight in a Petri dish and were hatched in deionised (DI) water. The larvae were reared in plastic pans (29.5×23.5×15 cm) containing approximately 1000 ml of water in environmental chambers (Thermo Scientific, Dubuque, Iowa, USA). Each pan contained groups of 50 larvae which fed ad libitum on Tetramin. Emerging adults were reared in the environmental chamber and fed ad libitum on 10% honey solution.
Developmental time
Groups of 16 first-instar (L1) larvae of each strain were randomly selected and transferred into 12 emergence containers (11 cm in diameter) each holding 100 ml DI water and approximately 4.8 mg of Tetramin added daily and maintained in environmental chambers. Larvae were counted daily until they reached the pupa stage when they were transferred into individual test tubes containing 3 ml of DI water.
Body weight and glycogen levels
To determine wet weight, female mosquitoes were anaesthetised with CO2, transferred into vials in groups of five, and weighed to 0.1 mg accuracy with an analytical balance (OHAUS corp. Pine Brook, NJ). Mosquitoes were then dried in a bath incubator (Fischer Scientific) at 60 °C for 1 h and the dry weight was measured as a proxy for their size (Siegel et al., Reference Siegel, Novak and Ruesink1994). Water content was calculated by subtracting the dry weight from the wet weight.
Glycogen content was assessed in 3–5-day-old females fed on 10% honey using the protocol described in (Jumbo-Lucioni et al., Reference Jumbo-Lucioni, Ayroles, Chambers, Jordan, Leips, Mackay and De Luca2010). Briefly, for each strain, ten independent replicates, each containing a group of ten mosquitoes were assayed. Groups of mosquitoes were homogenised on ice using 40 μl of homogenisation buffer (0.01 M KH2PO4 and 1 mM ethylene diamine tetraacetic acid (EDTA) pH 7.4). The homogenates were centrifuged in a microcentrifuge at 2000 rpm for 2 min at 4 °C. Aliquots of 1.67 μl of homogenate were added to 250 μl of a reagent containing 0.1 U ml−1 of amyloglucosidase. After 30-min incubation period at 37 °C, OD540 was measured. The concentration of glycogen was determined from a glycogen standard run with each replicate. An independent set of ten independent replicates was assayed for triacylglycerol content spectrophotometrically using a commercially available kit (Sigma-Triglyceride kit) following manufactures protocol. Each sample was assayed twice and the mean was used in the analyses.
Fecundity
After blood feeding, 40 mosquitoes of each strain were randomly aspirated and transferred into individual cages with moist filter paper for egg laying. The cages were provided with 10% honey solution and egg production monitored daily. The eggs produced were counted and incubated overnight in petri dishes to determine hatch rate after which the filter paper was replaced. Eggs were hatched in rearing pans in 1000 ml of DI water. Care was taken to ensure all eggs stuck on the sides of the rearing pan were in contact with DI water. Hatched eggs were counted on the second day.
Adult life span
One hundred and twenty pupae of each strain were randomly collected in pupa cups and transferred to three cages (40 per cage) in an environmental chamber. Pupa cups were removed from the cages after 24 h so that pupae that failed to emerge were excluded. The emerged adults were fed on 10% honey solution ad libitum. The cages were monitored daily and dead individuals were counted and removed until all individuals had died.
Metabolic rate measurement
Metabolic rate was determined as described in (De Luca et al., Reference De Luca, Klimentidis, Casazza, Chambers, Cho, Harbison, Jumbo-Lucioni, Zhang, Leips and Fernandez2010). Briefly, metabolic rate as CO2 production was measured using a flow-through respirometry system (Qubit System Research, Kingston, Ontario, Canada). Groups of five females of each strain were anaesthetised with CO2 and gently transferred to the respirometry chamber. CO2 was then measured for 10 min chamber−1 with a 30 s flush period between measurements at a flow rate of 30 ml min−1. The amount of CO2 produced by each group of mosquitoes was calculated using C950 Data Acquisition software (Qubit System Research, Kingston, Ontario, Canada).
Mitochondrial respiration rate assay
Mosquitoes were anaesthetised with CO2 and thoraces dissected from 30 females per replicate. All mitochondrial isolation steps in the six replicates were performed on ice. Mosquitoes were chilled briefly on ice and thoraces were separated from the heads and abdomens. Dissected thoraces were placed into 200 μl of ice-cold isolation buffer [(250 mM sucrose, 5 mM Tris-HCl, 2 mM EDTA, 1% (w/v) bovine serum albumin (BSA), pH 7.4 at 4 °C (Miwa et al., Reference Miwa, St-Pierre, Partridge and Brand2003) supplemented with protease inhibitors (leupeptin 1 mg ml−1, aprotinin 1 mg ml−1, and pepstatin 1 mg ml−1)] in a 1.5 ml Eppendorf tube. The samples were pounded gently 126 times over a 2 min period, using a custom-built, motorised micromortar. Mashed mosquitoes were filtered through a 5 μm nylon mesh, and the volume was raised to 400 μl by washing the nylon membrane with additional isolation buffer. A cycle of low-speed centrifugation (1 min centrifugation at 1000g) was followed by centrifugation of the filtered solution for 10 min at 3000g at 4 °C, and the pellet re-suspended in 100 μl of isolation buffer. Protein concentrations in the mitochondrial fractions were determined using a Lowry assay.
Using freshly isolated mitochondria, mitochondrial respiration assays were performed using a polarographic oxygen sensor (Oroboros oxygraph, OROBOROS® INSTRUMENTS, Innsbruck, Austria) with 0.2 mg ml−1 of freshly isolated mitochondria incubated in respiration medium (120 mM KCl, 5 mM KH2PO4, 3 mM Hepes, 1 mM EDTA, 1 mM MgCl2, and 0.2% BSA, pH 7.2; (Ferguson et al., Reference Ferguson, Mockett, Shen, Orr and Sohal2005). Oxygen consumption rates were measured at 25 °C (Sacktor & Sanborn, Reference Sacktor and Sanborn1956). As implemented by Miwa et al. (Reference Miwa, St-Pierre, Partridge and Brand2003), we measured state 3 and state 4 respiration rates using the nicotinamide adenine dinucleotide (NAD+)-linked substrates pyruvate 5 mM proline−1 5 mM to deliver electrons into mitochondrial complex I predominately. NAD+-linked substrates were added to the chamber and allowed to equilibrate for 1 min, followed by the addition of adenosine diphosphate (ADP) at a concentration of 400 μM to elicit ADP-dependent state 3. This was followed by the determination of the state 4 respiration rate, once all the added ADP had been exhausted and a steady state is reached (Affourtit et al., Reference Affourtit, Quinlan and Brand2012). Mitochondrial coupling efficiency (P:O ratio), e.g. the relationship between adenosine triphosphate (ATP) synthesis and oxygen consumption, was calculated as the amount of ADP consumed per oxygen being reduced during state 3 (Jumbo-Lucioni et al., Reference Jumbo-Lucioni, Bu, Harbison, Slaughter, Mackay, Moellering and De Luca2012). All assays were performed within 3 h of mitochondrial isolation. Data were analyzed using the software DatLab Version 4.1.0.8.
Detection of reactive oxygen species (ROS)
Mitochondria for ROS analysis were stored in ice cold conditions and used within 5 h after isolation. ROS levels were measured using 10-acetyl-3, 7-dihydroxyphenoazine (Amplex Red, AR; Molecular Probes, Eugene, OR), to detect hydrogen peroxide (H2O2) in the presence of horseradish peroxidase, producing the red-fluorescent oxidation product (excitation/emission=571/585 nm), resorufin. The ROS production of isolated mitochondria was measured as a basal rate (state 2 respiration) initiated by the reaction of saturated levels of substrates (pyruvate and proline), which evaluates ROS produced in a leak-like state without ADP and no oxidative phosphorylation. ROS production was also measured in the presence of rotenone and antimycin A, known inhibitors of complex I and complex III of the electron transport chain (ETC), respectively. Complexes I and III have been reported as the primary sites of mitochondrial ROS production (Hinkle et al., Reference Hinkle, Butow, Racker and Chance1967; Boveris et al., Reference Boveris, Oshino and Chance1972; Cadenas et al., Reference Cadenas, Boveris, Ragan and Stoppani1977; Raha et al., Reference Raha, McEachern, Myint and Robinson2000) and these inhibitors would assess that ROS production capacity of the ETC at these complexes. All these reactions were performed in triplicate for both strains at the same time in the same plate. In addition, a vehicular control was included to provide values for non-mitochondrial ROS. The data in arbitrary units are mean activity of the reaction for 30 min measured in a Synergy 2 plate reader at 600 nm.
Quantitative (q) PCR
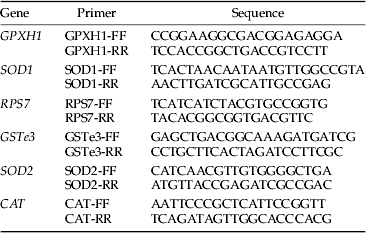
Groups of ten permethrin susceptible and RSP females each in six replicates were homogenised using TRI REAGENT (Promega) as described in the manufacturer's instruction. First strand cDNA was synthesised from 500 ng μl−1 of total RNA using SuperScript III, RNaseOUT, reverse transcriptase buffer, MgCl2, and an Oligo(dT)20 (Invitrogen) as described in the manufacturer's protocol. Thirty five cycles of amplification were performed in PCR machine. The amplification cycle was as follows: 95 °C for 5 min, 95 °C for 30 s, 53 °C for 30 s and 72 °C for 45 s and 72 °C for 7 min. We performed quantitative qPCR using a SYBR Green Master mix and 50 ng total of cDNA per reaction and run in a Stratagene Mx3000P® qPCR machine. The primers used for qPCR on the same total RNA are listed in table 1.
Table 1. Reproductive characteristics for the permethrin susceptible and RSP strains of Anopheles gambiae.

Mean±SD (range).
Data analysis
A χ2 test was used to compare mean longevity between strains. A log-rank test was used to compare median longevity between strains and a Kaplan–Meier curve was provided to illustrate the difference between strains. The data analysis for metabolic rate and glycogen levels was performed using analysis of covariance, with body weight used as a covariate. A two-sample t-test was used to compare differences between means for the other phenotypes. Statistical significance was set at α<0.05 for each test.
Results
Developmental time
We found that the median developmental time from L1 to pupa was significantly longer (4%) in the RSP strain compared to the permethrin susceptible strain, (log-rank test χ2 1=8.9, P=0.003) (fig. 1). Significantly longer (6%) was also the median developmental time from L1 to adult emergence (log-rank test χ2 1=13.6, P=0.0002) (fig. 1).

Fig. 1. Developmental time from first-instar larva to pupa (Panel A; n=465) and to adult emergence (Panel B, n=337) in permethrin susceptible and RSP strains.
Body weight, energy storage, and metabolic rate
Evidence exists of a strong trade-off between larval developmental time and adult weight in insects (Santos et al., Reference Santos, Fowler and Partridge1994). Therefore, we measured both wet body weight and dry weight of 3–5-day-old females. RSP females had significantly higher wet weight than permethrin susceptible females (P=0.044) (fig. 2A). On the other hand, there were no significant differences in dry body weight between the strains (P=0.250) (fig. 2B). As expected considering the higher wet weight, the RSP females had on average 5% more water content (P=0.026) than permethrin susceptible female (fig. 2C).

Fig. 2. Adult wet weight (Panel A; n=30), dry weight (Panel B; n=30), water content (Panel C; n=30), glycogen levels (Panel D; n=20), triacylgycerol storage (Panel E; n=20) and metabolic rate (Panel F; n=10) in permethrin susceptible and RSP females. Glycogen and triacylgycerol levels are normalised for body weight. Values are expressed as mean±SE. *P<0.05.
The capacity of glycogen to bind water is 3–5 times its own weight (Schimdt-Nielsen, Reference Schimdt-Nielsen and Schimdt-Nielsen1997). To explore whether the higher water content of RSP females was accompanied by an increase in glycogen level, we measured the glycogen content of whole-body sugar-fed females. Compared to permethrin susceptible females, RSP females had on average 9.5% higher levels of body weight-adjusted glycogen in (P=0.014) (fig. 2D). No statistical difference in triacylglycerol levels (P=0.27) was, however, observed between the two strains (fig. 2E). The mean triacylglycerol content for the RSP and permethrin-susceptible females was 11.36 μl mg−1±1.71 and 14.51 μl mg−1±2.17, respectively.
Previous studies of desiccation resistance and water balance in natural populations of Drosophila (Gibbs & Matzkin, Reference Gibbs and Matzkin2001) have shown a positive correlation between metabolic rates and water-loss rates (Gibbs & Matzkin, Reference Gibbs and Matzkin2001). The relationship between metabolic rate and water loss has been observed in a variety of other insects as reviewed by (Chown & Gaston, Reference Chown and Gaston1999) and is explained by the fact that reducing metabolic rates can help the insect to conserve water by reducing the need for gas exchange. Thus, we measured CO2 production in the two strains. There was a statistical difference in metabolic rate between the strains (P=0.034). As predicted, the mean VCO2 in RSP was lower than in permethrin susceptible females, 20.48 μl h−1±1.27 and 25.07 μl h−1±1.48, respectively (fig. 2F).
Fecundity
The mean number of eggs produced in one gonotrophic cycle between the two strains was not significantly different (table 2). No differences were also observed in the number of eggs hatched and the time from blood feeding to laying eggs between the two strains (table 2).
Table 2. Nucleotide sequence of primers used for qPCRs.
Longevity and mitochondrial bioenergetics
We observed a significantly reduced adult life span of RSP females compared to permethrin susceptible females (log-rank test χ2 1=10.49, P=0.0012) (fig. 3).

Fig. 3. Adult female survivorship curves for the permethrin susceptible and RSP strains of Anopheles gambiae. Survival assays were carried out using population cages with initial population sizes of 46–50 individuals.
Previous work in Drosophila reported a positive correlation between inter-individual variability in survival and mitochondrial bioenergetics (Melvin & Ballard, Reference Melvin and Ballard2006). Thus, we next measured mitochondrial coupling efficiency (P:O ratio) and H2O2 production rate using NADH-linked substrates. Notably, mitochondria isolated from thoraces of RSP females showed a significantly lower coupling efficiency than those isolated from thoraces of permethrin susceptible females (P=0.050) (fig. 4A). Furthermore, the RSP females’ mitochondria produced significantly more ROS with only substrates present and no ADP (no oxidative phosphorylation or ATP production occurring), reflective of the leakiness of the system to proton re-entry (state 2; P=0.010), and also produced significantly more ROS from complex I (rotenone; P=0.017) than those from susceptible females (fig. 4B). There was no statistically significant difference in ROS production from complex III between the strains (antimycin A; P=0.096) (fig. 4B).

Fig. 4. Coupling efficiency (or P:O ratio) (Panel A) and ROS production (Panel B) of mitochondria isolated from thoraces of permethrin susceptible and RSP females using NAD+-linked substrates (pyruvate and proline). Values are given as means±SE of six independent replicates. *P<0.05.
Antioxidant gene expression
To control for the oxidative stress induced by the enhanced production of ROS in mitochondria, cells produce different enzymes that scavenge ROS, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and GST (Hayes & McLellan, Reference Hayes and McLellan1999). Previous work in An. gambiae showed that the expression of GSTe3 is significantly enhanced following H2O2 exposure (Kumar et al., Reference Kumar, Christophides, Cantera, Charles, Han, Meister, Dimopoulos, Kafatos and Barillas-Mury2003; Ding et al., Reference Ding, Hawkes, Meredith, Eggleston, Hemingway and Ranson2005). Additionally, it has been reported that CAT is the primary antioxidant enzyme involved in oocyte protection by ROS damage in An. gambiae (DeJong et al., Reference DeJong, Miller, Molina-Cruz, Gupta, Kumar and Barillas-Mury2007). To assess whether the increased mitochondrial ROS production in the RSP strain is associated with the induction of components of the antioxidant system, we measured the expression of GSTe3, CAT, GPXH1, SOD1, and SOD2 genes using mRNA extracted from whole-body of females of each strain. We found a statistically significant difference in GSTe3 (P=0.011) and CAT (P=0.0068) expression levels between the two strains, with RSP females showing on average 80% GSTe3 (fig. 5A) and 92% CAT (fig. 5B) respectively higher transcript levels than permethrin susceptible females. No significant difference was observed in the expression levels of either GPXH1 (P=0.650) (fig. 5C), SOD1 (P=0.430) (fig. 5D) or SOD2 (P=0.480) (fig. 5E).

Fig. 5. Relative mRNA expression of antioxidant enzymes in whole body extracts of permethrin susceptible and RSP females. (Panel A) GSTe3: Glutathione S Transferase e3; (Panel B) CAT: Catalase; (Panel C) GPXH1: Glutathione Peroxidase; (Panel D) SOD1: Superoxide Dismutase 1; and (Panel E) SOD2: Superoxide Dismutase 2. Transcript levels of the five genes were normalised to RSP7 Ribosomal Protein S7. Values are given as means±SE of six independent replicates. *P<0.05.
Discussion
Here, we compared several life history and energy metabolism traits of a permethrin susceptible strain of An. gambiae and a strain harbouring kdr and metabolic-based mechanisms conferring permethrin resistance (RSP strain). We observed that the RSP strain had lower metabolic rate, slower developmental time, and shorter adult life span than the permethrin susceptible strain. Additionally, consistent with previous evidence in different organisms of a relationship between enhanced levels of ROS and age-associated decline (reviewed in Marchi et al., Reference Marchi, Giorgi, Suski, Agnoletto, Bononi, Bonora, De Marchi, Missiroli, Patergnani, Poletti, Rimessi, Duszynski, Wieckowski and Pinton2012), mitochondria isolated from the thoraces of the RSP females were found to have lower coupling efficiency and higher ROS production rates. Collectively, these results suggest that permethrin resistance alleles could affect energy metabolism, oxidative stress, and adult survival of An. gambiae and therefore impose strong fitness costs to the malaria vector. However, one major limitation of this study is that the RSP and permethrin susceptible strains have different genetic background and thus we cannot exclude the possibility that different loci may be responsible for the phenotypic differences observed between the strains. Also, although our strains of An. gambiae were originally isolated from the wild in the same geographic area in Kenya, they were subsequently reared under laboratory conditions for over 10 years and may suffer from inbreeding depression. Therefore, our results need to be interpreted with caution and replicated in independent studies in order to have significant implications for malaria transmission and vector control.
In our study, we found that both developmental times from L1 to pupation and from L1 to adult emergence were longer in RSP when compared to the permethrin susceptible strain. However, despite the longer developmental time, we did not observe a difference in dry body weight between females of the two An. gambiae strains. This result is interesting considering the strong relationship between molecular and physiological mechanisms that regulate the duration of developmental time and rate of growth and final body size (Shingleton, Reference Shingleton, Flat and Heyland2011). Compared to females of the permethrin susceptible strain, adult females of the RSP strain also showed higher water and glycogen levels, which is in agreement with the high energetic investment that is required to support the body's detoxifying apparatus (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000).
P450 monooxygenase-mediated detoxification is a major mechanism of resistance to insecticides due to the wide variety of substrates that P450s can metabolise (Félix & Silveira, Reference Félix, Silveira and Perveen2012). The P450 monooxygenase system consists of two main components: the cytochrome P450, which acts as the substrate binding protein (and terminal oxidase), and the NADPH-cytochrome P450 reductase (P450 reductase), which transfers electrons from NADPH to cytochrome P450 (Kawano et al., Reference Kawano, Kamataki, Yasumori, Yamazoe and Kato1987; Wheelock & Scott Reference Wheelock and Scott1990; Guzov et al., Reference Guzov, Houston, Murataliev, Walker and Feyereisen1996). The overproduction of detoxifying enzymes in insects can be energetically costly (Coustau et al., Reference Coustau, Chevillon and ffrench-Constant2000), and investment in resistance can be expected to produce reduced investment in competing organismal functions. Notably, our study did not show evidence of a reproductive fitness cost associated with the presence of both target-site insensitivity and metabolic-based mechanisms of permethrin resistance in An. gambiae. However, the lifespan of RSP females was significantly reduced compared to the permethrin susceptible females. A potential explanation for these findings is that the resistant strain might invest more energy in enhancing the antioxidative and detoxification mechanisms and maintaining reproductive functions at the cost of somatic maintenance and repair, allowing faster rates of aging and decreased longevity (Rose & Charlesworth, Reference Rose and Charlesworth1981a , Reference Rose and Charlesworth b ). Consistent with this idea, we found lower mitochondrial coupling efficiency and higher ROS production rates in the mitochondria isolated from the thoraces of the RSP females. ROS, including superoxide and its dismutation product H2O2, are essential as signalling molecules in defence against infection and in reproduction (Sanz & Stefanatos, Reference Sanz and Stefanatos2008). But, if produced in excess, they can oxidise and damage various cellular components, including mitochondrial proteins, membranes, lipids, and nuclear and mitochondrial genomes, and thus have been implicated in the aging process of a variety of species (Sanz & Stefanatos, Reference Sanz and Stefanatos2008), including Anopheles (Monaghan et al., Reference Monaghan, Metcalfe and Torres2009). In insects, increased levels of P450 activity has been associated with high levels of ROS production as byproducts of the detoxification processes (Murataliev et al., Reference Murataliev, Guzov, Walker and Feyereisen2008). As such, it is tempting to speculate that the RSP strain may exhibit higher levels of ROS produced by P450 than the permethrin-susceptible strain. These ROS may in turn oxidise and damage mitochondrial DNA, protein, and lipids, possible leading to mitochondrial dysfunction and excessive production of ROS by redox-coupled reactions within the ETC, as suggested by our mitochondrial bioenergetic results.
Antioxidant enzymes are produced by the cell in response to the oxidative stress induced by the enhanced production of ROS in mitochondria. We observed an 80 and 90% increase in expression of GSTe3 and CAT, respectively, in young RSP females compared to females of the susceptible strain. As mentioned above, previous work reported that the antioxidant enzyme CAT is responsible for oocyte protection by ROS damage in An. gambiae (DeJong et al., Reference DeJong, Miller, Molina-Cruz, Gupta, Kumar and Barillas-Mury2007). Based on this observation, our gene expression data not only suggest that RSP mosquitoes might activate a strong antioxidant response to counterbalance an increased oxidative environment, but also support our hypothesis of an investment of energy resources to maintain fecundity.
Acknowledgements
We thank Michelle Moses Chambers and Gin Chuang for helping with metabolic rate and mitochondrial ROS production measurements, respectively. We also thank Jeff Leips for providing insightful comments on the manuscript. We are grateful to Edward W. Hook III and Sally Fried of the Division of Infectious Diseases, William C. Gorgas Center for Geographic Medicine, for granting permission to use the mosquito facility. The RSP strain was obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: Anopheles gambiae RSP, MRA-334, deposited by J Vulule, MQ Benedict. The ASEMBO1 strain was obtained through the MR4 as part of the BEI Resources Repository, NIAID, NIH: Anopheles gambiae ASEMBO1 [AB], MRA-186, deposited by MQ Benedict. This study was supported by the Department of Biology at the University of Alabama at Birmingham, the Bioanalytical Redox Biology Core (DRTC P60 DK079626), and NIH grant R01 DK084219 to MD.









