Introduction
Ovarian stimulation with gonadotropins is widely used to produce multiple pre-/post-ovulatory oocytes in assisted reproduction technologies in women (Wang et al., Reference Wang, Ock and Chian2006) or in smart breeding technologies (embryo transfer) in animals, predominantly in cattle (Merton et al., Reference Merton, de Roos, Mullaart, de Ruigh, Kaal, Vos and Dieleman2003). Rarely, it might be associated with serious complications such us ovarian hyperstimulation syndrome or ovarian cancer (Brinton et al., Reference Brinton, Moghissi, Scoccia, Westhoff and Lamb2005). More importantly, it is usually accompanied with the recruitment of a relatively high number of oocytes that have not experienced a normal period of follicular maturation (Elbling & Colot, Reference Elbling and Colot1985) and show low developmental competence. This has consequential effect on the efficiency and profitability of the technologies used.
Experimental studies on mice have shown that ovarian stimulation with gonadotropins (administration of pregnant mare's serum gonadotropin followed by human chorionic gonadotropin) changed the expression of steroidogenesis-related genes and increased cholesterol content in ovaries (Wang et al., Reference Wang, Wang, Le, Li, Lou, Liu, Zheng, Qian, Chen, Jiang, Huang and Jin2015); decreased fertilization rate, altered lipid metabolism and epigenetic programming in oocytes (Huffman et al., Reference Huffman, Pak and Rivera2015; Wang et al., Reference Wang, Wang, Le, Li, Lou, Liu, Zheng, Qian, Chen, Jiang, Huang and Jin2015); delayed in vivo development of preimplantation embryos (Van der Auwera & D'Hooghe, Reference Van der Auwera and D'Hooghe2001) and decreased the proportion of morphologically normal preimplantation embryos (Ertzeid and Storeng, Reference Ertzeid and Storeng2001). It also affected the expression of several epigenetic biomarkers and ICM/TE (inner cell mass/ trophectoderm) ratio in recovered blastocysts (Wu et al., Reference Wu, Xue, Chen, Dai, Guo and Li2013; Bakhtari et al., Reference Bakhtari, Rahmani, Bonakdar, Jafarpour, Asgari, Hosseini, Hajian, Edriss and Nasr-Esfahani2014; Bonakdar et al., Reference Bonakdar, Edriss, Bakhtari, Jafarpour, Asgari, Hosseini, Sadeghi Boroujeni, Hajian, Rahmani and Nasr-Esfahani2015); increased post-implantation mortality (Ertzeid and Storeng, Reference Ertzeid and Storeng2001), retarded fetal growth and increased numbers of resorption sites after embryo transfer (Van der Auwera & D'Hooghe, Reference Van der Auwera and D'Hooghe2001). Similarly, in domestic cattle, various deviations in follicular morphology and function, abnormalities in oocyte maturation, decrease in oocyte developmental competence, decrease in embryo quality and viability and delay in conception time have been documented after superovulatory treatments (Hyttel et al., Reference Hyttel, Callesen and Greve1986; Greve et al., Reference Greve, Callesen, Hyttel, Høier and Assey1995; Blondin et al., Reference Blondin, Coenen, Guilbault and Sirard1996; Lopes da Costa et al., Reference Lopes da Costa, Silva and Silva2001). In contrast, there are reports showing no differences in pregnancy rates after the transfer of bovine embryos associated with ovarian stimulation (Hasler et al., Reference Hasler, McCauley, Lathrop and Foote1987; Merton et al., Reference Merton, de Roos, Mullaart, de Ruigh, Kaal, Vos and Dieleman2003).
Superovulatory response is characterized by a high degree of variability and unpredictability. Apparently, there are various external factors affecting the yield and quality of obtained ova/embryos (Chu et al., Reference Chu, Dufort and Sirard2012). As the metabolism of lipids is closely related to that of steroid hormones, we might hypothesize that the metabolic status of donors, especially obesity in women and overfeeding in non-lactating cows with high body condition score, could be one of the most important factors (Brewer & Balen, Reference Brewer and Balen2010; Velazquez, Reference Velazquez2011).
Clinical reports on assisted reproductive technology outcomes have documented that obese women frequently require increased doses of gonadotropins (Purcell & Moley, Reference Purcell and Moley2011). Thus obesity has been associated with relative gonadotrophin resistance (Fedorcsák et al., Reference Fedorcsák, Dale, Storeng, Tanbo and Abyholm2001). After ovarian stimulation, increased incidence of follicular asynchrony and increased cancellation rates have been recorded as well (Tamer Erel & Senturk, Reference Tamer Erel and Senturk2009). Moreover, in overfed superovulated dairy heifers, enhanced in vitro embryo production was documented after short-term (6 week) reduction of body-weight gain (Freret et al., Reference Freret, Grimard, Ponter, Joly, Ponsart and Humblot2006).
The aim of the present study was to evaluate whether maternal body condition might modulate the effect of superovulation on the number, quality and developmental potential of oocytes. Experiments were performed using the mouse model.
Females with obesity-like phenotype were produced using a previously standardized breeding protocol based on over-nutrition of experimental animals during intrauterine and early postnatal development (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014). Ovarian stimulation was performed in adult mice of two body condition types: (1) Normal controls (derived from dams fed a standard diet) showing physiological body weight (around 18 g) and body fat (around 7.5%), with minimal fat deposits in the abdominal and perirenal areas; and (2) Fat mice (derived from dams fed a high-energy diet) showing significantly elevated body weight (around 23 g) and body fat (over 12%), with massive fat deposits in the abdominal and perirenal areas, displaying increased plasma glucose, insulin and leptin levels (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014; Janštová et al., Reference Janštová, Burkuš, Kubandová, Fabian, Koppel and Čikoš2017).
Furthermore, to make the data comparable with our previous findings, documenting the effect of obesity-like phenotype on reproductive parameters in spontaneously ovulating animals (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014; Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015), mice of the same strain, age and breeding line were used in the present study. The corresponding times and procedures of ova and embryo recovery were maintained as well.
Materials and Methods
Animals and experimental design
All experiments were performed on mice of the outbred ICR (CD-1 IGS) strain (Velaz, Prague, Czech Republic) and the entire experimental design was repeated two times. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. All animal experiments were approved by the Ethical Committee for Animal Experimentation at the Institute of Animal Physiology, approved by the State Veterinary and Food Administration of the Slovak Republic (Ro 2296/13–221c), and were performed in accordance with Slovak legislation based on EU Directive 2010/63/EU on the protection of animals used for experimental and other scientific purposes.
To produce females with various body conditions, a two-generation dietary model was used. Adult female mice of the parental generation (5 to 6 weeks old, n = 73) were subjected to estrous synchronization through i.p. administration of 4 IU eCG (pregnant mare's serum gonadotropin; Folligon, Intervet International, Boxmeer, Holland) and 4 IU of hCG (human chorionic gonadotropin; Pregnyl, Organon, Oss, Holland; 47 h later) and then mated with males of the same strain (12 to 24 weeks old) overnight. Mated dams were randomly divided into two groups: control (n = 24) and experimental (n = 25). Animals in both groups were fed a standard pellet diet (M1, Ricmanice, Czech Republic, 3.2 kcal/g) ad libitum. Animals in the experimental group were fed additionally with Ensure Plus high-energy nutritional product (Abbott Laboratories, IL, USA, 1.5 kcal/ml), also ad libitum. Control dams (n = 15) delivered 13.40 ± 1.14 pups on average, experimental dams (n = 16) delivered 11.38 ± 0.85 pups on average. To achieve adequate nutrition in all pups, the litter size was adjusted on the eighth day after birth up to 12 pups per nest. All animals were kept at 22 ± 1°C on a 12 h light/dark cycle (6 am to 6 pm) with free access to food and water. To minimize the effect of actual nutrition on the reproductive process, after weaning, female mice of the F1 generation delivered from both control and experimental dams were fed the standard pellet diet only (M1).
On day 30 of their age, body weight was measured in all females of the F1 generation. Mice were individually scanned using MRI (Echo MRI, Whole Body Composition Analyser, Echo Medical System, Houston, Texas) and the percentage of body fat was calculated as body fat (g)/body weight (g) × 100. Females delivered from control dams (n = 89) were allocated into two groups: 1, normal controls with physiological body weight and body fat (7–8%) and 2, lean controls spontaneously displaying decreased body weight and body fat (<7%). Females delivered from experimental dams (n = 93) were classified as: 1, fat mice with significantly elevated body weight and fat (>11%) and 2, obesity-induction resistant experimental mice with physiological body weight and slightly elevated body fat (8–11%).
In the following ovarian stimulation experiments, only normal control females with physiological body weight and body fat (n = 45; 3.00 ± 0.41 per nest on average) and fat experimental females with significantly elevated body weight and fat (n = 69; 4.31 ± 0.35 per nest on average) were used.
On day 31 of their age, female mice underwent superovulation treatment through intraperitoneal (i.p.) administration of 5 IU eCG (Folligon), followed 47 h later by administration of 10 IU hCG (Pregnyl) and overnight mating with males of the same strain (12 weeks old, fed standard diet, weight 38.51 ± 0.48 g, purchased directly from Velaz). Successful copulation was confirmed by the presence of a vaginal plug. On day 1 of pregnancy, approximately half of mated dams in both groups was sacrificed by means of cervical dislocation and subjected to ova collection. Successful fertilization was confirmed by the recovery of zygotes. The remaining mated mice were sacrificed on day 4 and subjected to embryos collection (Table 1).
Table 1 Somatic parameters of normal and fat female mice and production of ova on day 1 (D1) and embryos on day 4 (D4) of pregnancy in fertilized ones
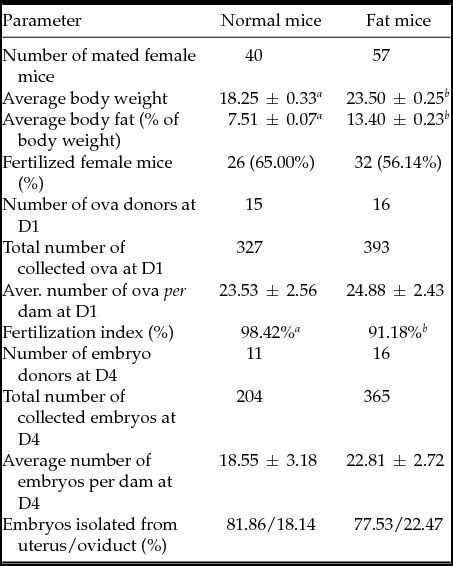
Results are expressed as mean values ± standard error of the mean (SEM) or as percentages. Different letters in superscript indicate statistical difference: body weight: Student's t-test (P < 0.001); body fat: Mann–Whitney test (P < 0.001); percentage of fertilized mice: chi-squared test; number of ova/embryos per dam: Mann–Whitney test; fertilization index (% of fertilized MII oocytes): chi-squared test (P < 0.001); transport to uterus: chi-squared test.
Ova/embryos recovery and evaluation
Ova/embryos recovery was performed by flushing oviducts (on day 1 of pregnancy) or both oviducts and uteri (on day 4 of pregnancy) of F1 dams using a flushing-holding medium (FHM) containing 1% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, USA). Oocytes were collected at 22.5 h post hCG administration (i.e. 12.5 ± 1 h after presumed ovulation). This period was chosen with regard to biological asynchrony in the natural fertilization process. Embryos (predominantly at late morula/early blastocyst stage) were collected at 90.5 h post hCG administration (i.e. 79.5 ± 1 h after presumed ovulation). This period preceding the start of implantation was chosen to avoid embryo loss.
Immediately after isolation, the collected material was subjected to preliminary stereomicroscopic classification (Nikon SMZ 745T, Japan) and the number of ova isolated from oviduct/the number of embryos isolated from oviduct and uterus per dam were determined. The exact classification of all isolated ova/embryos was performed after nuclear DNA staining (Hoechst 33342, Sigma-Aldrich; 10 μg/ml in phosphate-buffered saline (PBS) for 5 min at room temperature).
Ova with one polar body and an acentric mitotic spindle (metaphase chromosomes) were classified as unfertilized MII oocytes. Ova with one or two polar bodies displaying two pronuclei or equatorially localized mitotic spindles were classified as zygotes. The developmental advance of zygotes [pronuclear stages 1 to 5 (PN1–PN5)] was evaluated according to the distance and the size of maternal and paternal pronuclei: PN1, small pronuclei located at the periphery of the embryo; PN2, pronuclei increased in size and migrating towards the centre of the embryo; PN3, large pronuclei migrating towards the centre; PN4, large pronuclei close to each other in the centre of the embryo; PN5, large apposed central pronuclei (Adenot et al., Reference Adenot, Mercier, Renard and Thompson1997; Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015). Ova showing non-typical morphology (e.g. absent polar body, highly translucent cytoplasm with non-homogenous structure, cytoplasmic autolysis, cytoplasmic fragmentation) were classified as immature or degenerated oocytes. The fertilization index (%) was calculated as the number of zygotes/total number of mature oocytes × 100.
Embryos with a blastocoele cavity were classified as blastocysts, embryos with up to 32 blastomeres were classified as morulas, and embryos with 2-cells to 16-cells were classified as cleavage stage embryos. Zona pellucida-enclosed embryos containing an undefined number of cells with massive cytoplasmic fragmentation or remnants of degraded biological material only were classified as degenerated embryos.
In the recovered blastocysts, morphological fluorescence staining was used for the assessment of the following qualitative parameters: mean number of cells per embryo and dead-cell incidence. Blastocysts were first stained with propidium iodide (PI, 10 μg/ml; Sigma-Aldrich; stains dead cells only), then washed in PBS containing BSA, Sigma-Aldrich), fixed in 4% paraformaldehyde (Merck, Darmstadt, Germany) in PBS at room temperature for 1 h and optionally stored in 1% paraformaldehyde in PBS at 4°C. Fixed embryos were washed, permeabilized for 1 h in PBS with 0.5% Triton X-100 (Sigma-Aldrich) and again washed in PBS. Then they were incubated in TUNEL assay reagents (DeadEndFluorometric TUNEL System, Promega, WI, USA) for 1 h at 37°C in the dark. After the TUNEL reaction all embryos were counterstained with Hoechst 33342 (10 μg/ml in PBS; stains all nuclei, shows nuclear morphology) for 5 min at room temperature, washed, mounted on slides with Vectashield (Vector Laboratories, Burlingname, CA, USA) and observed under a fluorescence microscope at ×400 magnification (BX50 Olympus, Japan).
According to the nuclear morphology, the presence of specific DNA fragmentation in the nucleoplasm and PI positivity/negativity, embryonic cells were classified as: normal (without morphological changes in nuclei, without TUNEL labelling, and without PI inclusion in the nucleoplasm) or dead (showing at least one of the following features: fragmented or condensed nucleus, positive TUNEL labelling or positive PI staining). Dead cells showing nuclear fragmentation or condensation and TUNEL positive labelling were classified as apoptotic. The dead-cell index (%) was calculated as the number of dead cells/total number of cells in the blastocyst × 100. Its average value was separately determined for all blastocysts in the experimental group and for blastocysts containing at least one dead cell in the experimental group.
Statistical analysis
Statistical analysis was performed using GraphPad Prism Software 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). The results are expressed as the mean values ± standard error of the mean (SEM).
The differences between groups of data showing normal Gaussian distribution (body weight of female mice and the mean number of cells per blastocyst) were assessed using Student's parametric test. The differences between groups of data that did not pass the normality tests (amount of body fat in female mice, the number of collected embryos per dam, cell death incidence per blastocyst) were assessed using the Mann–Whitney non-parametric test.
For the assessment of differences between score-type data [collected ova/embryos classified according to: fertilization status, reached developmental stage, progress of decay, localization of recovery (oviduct versus uterus), and presence of cell death], standard chi-squared tests with one or more degrees of freedom (depending on the number of specified categories) were used.
Differences in P-values < 0.05 were considered to be statistically significant.
Results
As shown in Table 1, fat female mice (delivered from dams fed the high-energy diet) showed significantly elevated body weight and body fat amounts compared with the normal controls (delivered from dams fed the standard diet). Overnight mating with males resulted in successful fertilization in more than 55% of superovulated females of both body condition types.
There were no significant differences between the average numbers of ova or embryos collected from normal and fat mice on day 1 and on day 4 of pregnancy respectively (Table 1). However, the recovered ova and embryos differed in several qualitative or developmental parameters:
On day 1, higher numbers of immature oocytes were recovered from fat females than from control dams (Fig. 1). Furthermore, in superovulated fat mice, lower proportions of fertilized oocytes (zygotes) were recorded (Fig. 1). Such zygotes also showed slightly retarded development: lower numbers of advanced stage (PN4 and PN5) zygotes were collected from fat dams (Fig. 2).
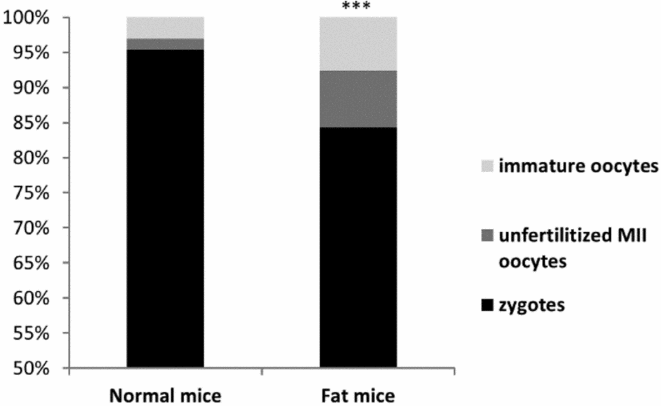
Figure 1 Proportion of zygotes, unfertilized MII oocytes and immature oocytes recovered from normal and fat mice on day 1 of pregnancy.
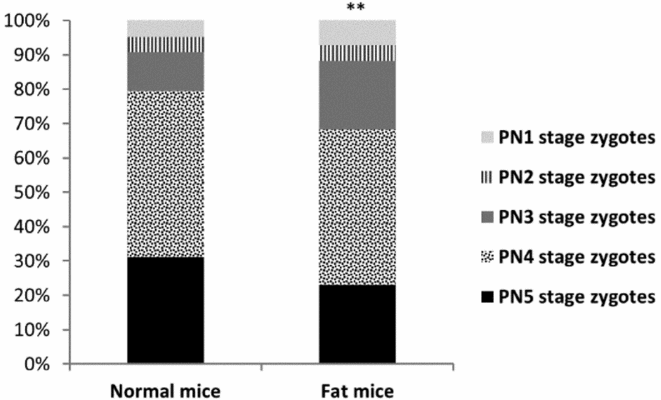
Figure 2 Stage-distribution of zygotes recovered from normal and fat mice on day 1 of pregnancy. PN1–PN5, pronuclear stages 1 to 5.
On day 4, most embryos developed in the reproductive tract of both normal and fat females were able to reach the morula or the blastocyst stage (Fig. 3). However, in the fat mice group the proportion of recovered blastocysts was significantly lower (47.95% vs. 57.35%, P < 0.05). Although the numbers of degenerates collected from fat dams also tended to be higher, statistical analysis did not confirm any relevant difference between the groups (22.20% vs. 17.64%, P > 0.05). However, they apparently differed in morphology: while almost half of the degenerates from normal mice showed advanced autolysis (45%), the majority of degenerates from fat mice were just at the stage of gradual cytoplasmic fragmentation (88%) (P < 0.001).

Figure 3 Stage-distribution of embryos recovered from normal and fat mice on day 4 of pregnancy. Graph shows the proportion (%) of blastocysts, morulas, cleavage stage (2-cell to 16-cell) embryos and degenerated embryos.
Finally, in fat females a higher percentage of blastocysts contained dead cells and also displayed increased incidence of cell death per blastocyst (Table 2). Morphological analysis of the dead cells showed that the majority of these were of apoptotic origin. Conversely, there was no difference in mean number of cells between blastocysts collected from control and fat dams (Table 2) or in the speed of embryo transition from oviduct to uterus (Table 1).
Table 2 Qualitative parameters of blastocysts collected on Day 4 from normal and fat mice
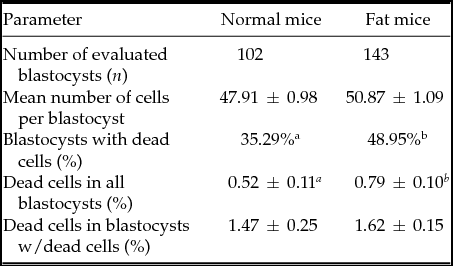
Results are expressed as mean values ± standard error of the mean (SEM) or as percentages. Different letters in superscript indicate statistical difference: mean number of cells: Student's t-test (P = 0.05); blastocysts with dead cells: chi-squared test (P < 0.05); dead cells in blastocysts: Mann–Whitney test (P < 0.01).
Discussion
Normal and fat female mice showed similar sensitivity to hormonal treatment: in both groups the average number of collected ova on day 1 of pregnancy ranged around 24, i.e. it was double compared with their spontaneously ovulating counterparts evaluated in our previous experimental study (around 11.4 per dam) (Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015). An equivalent situation was observed on day 4 of pregnancy. We recovered 18.55 and 22.81 embryos from normal and fat superovulated mice respectively, and 10.00 and 11.59 from spontaneously ovulating mice (normal ‘CN’ and fat ‘EXF’ females respectively) (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014). Our results do not confirm any increased gonadotrophin dose requirement in obese females (Fedorcsák et al., Reference Fedorcsák, Dale, Storeng, Tanbo and Abyholm2001), and they are more in accordance with observations that demonstrated no difference in the ovarian response to gonadotropin stimulation between obese and non-obese women (Lashen et al., Reference Lashen, Ledger, Bernal and Barlow1999; Dechaud et al., Reference Dechaud, Anahory, Reyftmann, Loup, Hamamah and Hedon2006; Martinuzzi et al., Reference Martinuzzi, Ryan, Luna and Copperman2008). However, as multiple ovulation is a physiological process in rodents, species-specificity cannot be excluded.
Although not statistically significant, the ability of fat females to derive a slightly higher number of embryos on day 4 (compared with normal controls), observed in both our present and previous studies (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014), appears to be important. Our results suggest that embryo loss occurred between day 1 and day 4 in both groups of mice, but was lower in fat females. This result might be explained by reduced ability of fat mice to eliminate low quality (degenerated) oocytes completely during their passage through the oviduct. This explanation is supported by the lower numbers of degenerates at more advanced stages of lysis [translucent bodies with remnants of degraded biological material) recovered from fat dams (12%)] compared with normal dams (45% from total number of degenerates).
The quality of ova recovered on day 1 of pregnancy significantly differed between the two groups of donors: higher numbers of immature oocytes, non-fertilized mature oocytes and lower-stage zygotes were collected from fat females. As the production of higher numbers of immature oocytes and retarded zygote development in overweight mice were also observed in our previous non-superovulatory study (Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015), this result is probably not related to hormonal ovarian stimulation. Conversely, the fertilization capacity of ovulated MII oocytes was influenced differently in spontaneously ovulating and hormonally stimulated fat females: While spontaneously ovulating fat mice displayed no difference in the percentage of fertilized matured oocytes (or even increased fertilization index at earlier time of collection) compared with normal dams (Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015), superovulated fat mice showed significantly decreased percentages of fertilized ova. Decreased fertilization index was also documented in superovulated non-lactating obese dairy cows compared with lactating lean cows (Velazquez et al., Reference Velazquez, Hadeler, Herrmann, Kues, Ulbrich, Meyer, Remy, Beckers, Sauerwein, Niemann and Niemann2011).
The higher percentage of immature oocytes recovered from hormonally treated normal mice (3.06%; present study) than from unstimulated normal mice (1.61%; Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015) is in accordance with the usual observations in mice (Elbling & Colot, Reference Elbling and Colot1985) and other species (Chrenek et al., Reference Chrenek, Makarevich, Vasícek, Laurincík, Bulla, Gajarská and Rafay1998).
The exact reason for lower quality of ova collected from fat female mice has not been elucidated. Based on our recent in vitro results we might speculate that the higher incidence of immature oocytes and lower developmental competence of zygotes observed in both non-superovulated and superovulated fat females might be connected with different secretory activity of ovarian cells as ovaries isolated from fat mice released less IGF-I and more progesterone and testosterone than ovaries of normal control mice (Sirotkin et al., Reference Sirotkin, Fabian, Babeľová-Kubandová, Vlčková, Alwasel and Abdel2017). It is known that the insulin-like growth factor system is crucial for ovarian function and that bioactivity of IGF-I can be disrupted by hyperinsulinemia (Velazquez et al., Reference Velazquez, Zaraza, Oropeza, Webb and Niemann2009) regularly present in obese females (Janštová et al., Reference Janštová, Burkuš, Kubandová, Fabian, Koppel and Čikoš2017; Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014). Moreover, the sensitivity of secretory ovarian cells to hormonal stimulation can also be influenced by maternal metabolic status. Whereas ovaries isolated from normal control females exposed in vitro to FSH showed increased testosterone release only, ovaries isolated from fat females showed significant increase in both testosterone and progesterone secretion (Sirotkin et al., Reference Sirotkin, Fabian, Babeľová-Kubandová, Vlčková, Alwasel and Abdel2017). It has been shown that the presence of high concentrations of progesterone in the oocyte environment during specific periods of its development can decrease oocyte fertilization ability (Hunter, Reference Hunter1968), and this might be the explanation for the decreased fertilization index recorded in superovulated fat mice females in the present study.
The quality of embryos recovered on day 4 of pregnancy also significantly differed between the two groups of donors: retardation of preimplantation development and increased incidence of apoptotic cell death in blastocysts were observed in fat mice. As the same results were observed in our previous non-superovulatory study (Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014), these effects probably do not represent consequences of ovarian stimulation. Conversely, the comparison with our previous data shows that superovulation treatment significantly slowed down the overall in vivo development of mice preimplantation embryos. This retardation was observed in both normal and fat females: although the time of embryo recovery was the same in both non-superovulation and superovulation studies (day 4, 79.5 ± 1 h after supposed ovulation), the overall proportion of recovered morulas and blastocysts was lower in hormonally treated females (77.94% in normal and 75.07% in fat mice) than in spontaneously ovulating ones [89.68% in normal (P < 0.001) and 85.17% in fat mice (P < 0.001); Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014]. The mean numbers of cells in blastocysts collected from superovulated females (47.91 ± 0.98 in normal and 50.87 ± 1.09 in fat mice) were also lower than those in blastocysts collected from non-superovulated ones [56.34 ± 0.83 in normal (P < 0.001) and 55.29 ± 0.75 in fat mice (P < 0.001); Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014]. Conversely, the lower dead-cell indices found in blastocysts in the present study are probably not related to superovulation treatment. More likely, they are the consequence of the physiologically lower ability of embryos at the lower stages of development to eliminate damaged cells by apoptosis or to respond to external induction of apoptosis (Fabian et al., Reference Fabian, Bystriansky, Makarevich, Chrenek and Koppel2009, Reference Fabian, Makarevich, Chrenek, Bukovská and Koppel2007).
Data documenting the delay in preimplantation development of embryos after ovarian stimulation match that of the previously published report by Van Der Auwera & D'Hooghe (Reference Van der Auwera and D'Hooghe2001) showing delayed morula formation, blastocoele formation and zona pellucida lysis in mice of another strain (C57Bl mated with CBA) superovulated with 5 IU eCG and 5 hCG. This retardation might be explained by the long-term presence of non-physiological levels of gonadotropins in the environment of developing embryos. While the half-lives of FSH and LH are about 3 h, the half-life of eCG is 40–120 h and hCG 24–36 h. This theory is supported by the fact that the delay in development diminishes (Van der Auwera & D'Hooghe, Reference Van der Auwera and D'Hooghe2001) or completely disappears (Wang et al., Reference Wang, Wang, Le, Li, Lou, Liu, Zheng, Qian, Chen, Jiang, Huang and Jin2015) in ova from stimulated mice transferred to in vitro conditions.
Results documenting the negative effect of maternal obesity on the quality and in vivo development of ova and embryos were discussed in our previous studies (Fabian et al., Reference Fabian, Kubandová, Čikoš, Burkuš, Fabianová, Račeková, Czikková and Koppel2015; Kubandová et al., Reference Kubandová, Čikoš, Burkuš, Czikková, Koppel and Fabian2014). The present study proves that humoral change in the maternal environment of fat female mice increases ovulation of immature oocytes, slows down development of zygotes and preimplantation embryos and increases the incidence of apoptotic cell death in produced blastocysts, even after the involvement of ovarian stimulation.
The question of whether overweight itself might affect the ovulatory capacity of mice remains open. Our observations on both superovulated and non-superovulated mice suggest that the presence of high amounts of body fat in the maternal body do not alter the average number of recovered ova. Similarly, no differences in the numbers of collected oocytes or zygotes between obese and non-obese donors were found in the majority of other experimental studies (Brannian et al., Reference Brannian, Furman and Diggins2005; Igosheva et al., Reference Igosheva, Abramov, Poston, Eckert, Fleming, Duchen and McConnell2010; Wakefield et al., Reference Wakefield, Lane, Schulz, Hebart, Thompson and Mitchell2008). However, there are reports that document fewer mature oocytes in mice with diet-induced obesity and hormonally induced ovulation (Jungheim et al., Reference Jungheim, Schoeller, Marquard, Louden, Schaffer and Moley2010; Tortoriello et al., Reference Tortoriello, McMinn and Chua2004), and, in contrast, also reports showing that a diet containing high fatty acid content might increase the total number of oocytes collected from non-superovulated female mice (Minge et al., Reference Minge, Bennett, Norman and Robker2008).
In conclusion, our results show that the response of normal control female mice (delivered from dams fed the standard diet) and fat mouse females (delivered from dams fed the high-energy diet) to hormonal ovarian stimulation is similar in that they produced almost the same numbers of ova on average. In both groups, more than 90% of recovered ova were classified as mature (at the MII stage). Conversely, in mice with elevated amounts of body fat, the presence of externally administered gonadotropins negatively affected the fertilization index. In both groups, most fertilized oocytes were able to cleave and reach the higher stages of development. However, compared with non-stimulated female mice (assessed in our previous studies), the overall rate of their development was significantly delayed. Furthermore, in fat mice lower numbers of blastocysts of lower quality (showing increased apoptotic index) were recovered on day 4 of pregnancy.
Acknowledgements
We thank Anna Olšavská and Dana Čigašová for their technical assistance, and Andrew Billingham for his English proofreading.
Financial support
This work was supported by the Slovak Academy of Sciences (www.vega.sav.sk) under contract VEGA 2/0001/14.







