Introduction
Agricultural practices, particularly pesticide applications, have a strong impact on biodiversity in agroecosystems (Geiger et al., Reference Geiger, Bengtsson, Berendse, Weisser, Emmerson, Morales, Ceryngier, Liira, Tscharntke, Winqvist, Eggers, Bommarco, Pärt, Bretagnolle, Plantegenest, Clement, Dennis, Palmer, Oñate, Guerrero, Hawro, Aavik, Thies, Flohre, Hänke, Fischer, Goedhart and Inchausti2010). Efforts to preserve the environment and establish sustainable agriculture practices have resulted in interest in alternative pest control strategies. In pome fruit (mainly apple and pear) orchards, pest control strategies can be roughly classified into three categories: (i) conventional pest management that mainly relies on chemical pesticide applications following a systematic calendar-based approach; (ii) organic pest management that discards agrichemicals and favours the activity of pest predators and parasites to make the most of ecological processes; (iii) an intermediate approach known as Integrated Pest Management (IPM) that integrates both non-chemical and chemical means of pest control, in which chemical control is used only when necessary.
Over past decades, numerous studies have explored the overall effect of alternative agricultural practices on biodiversity in agroecosystems (e.g., Paoletti et al., Reference Paoletti, Schweigl and Favretto1995; Feber et al., Reference Feber, Firbank, Johnson and Macdonald1997; Doles et al., Reference De Lillo2001; Letourneau & Goldstein, Reference Letourneau and Goldstein2001; Mäder et al., Reference Mäder, Fliebbach, Dubois, Gunst, Fried and Niggli2002; Weibull et al., Reference Weibull, Bengtsson and Nohlgren2003; Fuller et al., Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005, Geiger et al., Reference Geiger, Bengtsson, Berendse, Weisser, Emmerson, Morales, Ceryngier, Liira, Tscharntke, Winqvist, Eggers, Bommarco, Pärt, Bretagnolle, Plantegenest, Clement, Dennis, Palmer, Oñate, Guerrero, Hawro, Aavik, Thies, Flohre, Hänke, Fischer, Goedhart and Inchausti2010, Flohre et al., Reference Flohre, Fischer, Aavik, Bengtsson, Berendse, Bommarco, Ceryngier, Clement, Dennis, Eggers, Emmerson, Geiger, Guerrero, Hawro, Inchausti, Liira, Morales, Oñate, Pärt, Weisser, Winqvist, Thies and Tscharntke2011). The meta-analysis by Bengtsson et al. (Reference Bengtsson, Ahnström and Weibull2005) has shown that species richness and/or the abundance of living organisms are reduced overall in conventional farming systems compared with organic ones. However, the effect size depends on the taxa under consideration: a significantly positive effect size of organic farming was shown on both abundance and species richness for plants and a slightly less significant effect size was shown for birds. Interestingly, Bengtsson et al. (Reference Bengtsson, Ahnström and Weibull2005), as well as specific studies by Fuller et al. (Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005) and Flohre et al. (Reference Flohre, Fischer, Aavik, Bengtsson, Berendse, Bommarco, Ceryngier, Clement, Dennis, Eggers, Emmerson, Geiger, Guerrero, Hawro, Inchausti, Liira, Morales, Oñate, Pärt, Weisser, Winqvist, Thies and Tscharntke2011), revealed a reduced response in arthropods compared with plants or birds.
The reduced response of arthropods may come as a surprise considering that arthropods are the target organisms of many agrichemicals used in conventional and IPM systems. As such, these organisms could be expected to respond more specifically than non-target, distantly related vertebrate organisms such as birds and to respond as much as plants that are targets of widely used herbicides.
Arthropods' responses to practices and their value as indicators may depend on their life histories. Most studies focused on either large and mobile predatory arachnids or insects (mainly Araneae, Carabidae and Staphylinidae: e.g., Mäder et al., Reference Mäder, Fliebbach, Dubois, Gunst, Fried and Niggli2002; Weibull et al., Reference Weibull, Bengtsson and Nohlgren2003; Fuller et al., Reference Fuller, Norton, Feber, Johnson, Chamberlain, Joys, Mathews, Stuart, Townsend, Manley, Wolfe, Macdonald and Firbank2005; Geiger et al., Reference Geiger, Bengtsson, Berendse, Weisser, Emmerson, Morales, Ceryngier, Liira, Tscharntke, Winqvist, Eggers, Bommarco, Pärt, Bretagnolle, Plantegenest, Clement, Dennis, Palmer, Oñate, Guerrero, Hawro, Aavik, Thies, Flohre, Hänke, Fischer, Goedhart and Inchausti2010; Flohre et al., Reference Flohre, Fischer, Aavik, Bengtsson, Berendse, Bommarco, Ceryngier, Clement, Dennis, Eggers, Emmerson, Geiger, Guerrero, Hawro, Inchausti, Liira, Morales, Oñate, Pärt, Weisser, Winqvist, Thies and Tscharntke2011), large butterflies (Weibull et al., Reference Weibull, Bengtsson and Nohlgren2003), strictly herbivorous communities (e.g., Feber et al., Reference Feber, Firbank, Johnson and Macdonald1997; Letourneau & Goldstein, Reference Letourneau and Goldstein2001) or soil arthropods (Paoletti et al., Reference Paoletti, Schweigl and Favretto1995; Doles et al., Reference De Lillo2001). These organisms may be good indicators for global impact studies of agricultural practices, but are not optimal for the assessment of direct toxic impact of pesticide use. For example, mobile arthropods (ambulatory or flying) may provide relevant information on the large-scale impact of agricultural practices, but may be considered poor indicators regarding the impact of agricultural practices on local biodiversity because they can escape in space. Choosing mobile species to assess the impact of pest control strategies may thus confuse the issue because the dynamics of these species also largely depend on landscape characteristics. The choice of mobile organisms may also be partly responsible for the large effect of landscape features on biodiversity, a crucial conclusion of the meta-analysis by Bengtsson et al. (Reference Bengtsson, Ahnström and Weibull2005), and on the confounding effects of landscapes and pest control strategies (Winqvist et al., Reference Winqvist, Bengtsson, Aavik, Berendse, Clement, Eggers, Fischer, Flohre, Geiger, Liira, Pärt, Thies, Tscharntke, Weisser and Bommarco2011). More so than other species, phytophagous species (pest or not) that feed on any cultivated plant are prone to have undergone stronger and repeated selective pressure typical of crop agroecosystems. Therefore, these communities are expected to encompass resistant populations and may be unable to reflect the baseline toxicity of the different control methods. Soil arthropods can be affected not only by the type of pest control management but also by the accumulation of organic matter in soils (Doles et al., Reference De Lillo2001), soil compaction and/or surface accumulation of vegetal debris (Paoletti et al., Reference Paoletti, Schweigl and Favretto1995).
In the present study, we conducted a pilot study aiming at assessing the impact of pest control strategies on the arthropodofauna of tit nests built within nestboxes installed in orchards. Bird nests constitute islands par excellence in which particular arthropod community structures develop because of the presence of a vertebrate. Indeed, the bird inhabitant brings skin scales, droppings and/or wastes and parasites into this isolated habitat, which promotes: (i) a guild of saprophagous/microbivorous arthropods that feed on bird-generated detritus or on fungi and other micro-organisms; (ii) a guild of bird ectoparasites that directly feed on bird chicks and/or adults; (iii) a guild of predators that may feed on members of the two former guilds (Lesna et al., Reference Lesna, Wolfs, Faraji, Roy, Komdeur and Sabelis2009). This effect usually results in rich and balanced microecosystems, and inventories of insects and arachnids in these microhabitats in natura have revealed noteworthy diversity (e.g., Nosek & Lichard, Reference Nosek and Lichard1962; Zeman & Jurík, Reference Zeman and Jurík1981; Burtt et al., Reference Burtt, Chow, Babbitt, Loye and Zuk1991; Fain & Galloway, Reference Fain and Galloway1993; Fend'a & Schniererová, Reference Fend'a and Schniererová2004; Merkl et al., Reference Merkl, Bagyura and Rózsa2004; Majka et al., Reference Majka, Klimaszewski and Lauff2006; Lesna et al., Reference Lesna, Wolfs, Faraji, Roy, Komdeur and Sabelis2009). The unusual isolation of such microhabitats results in more or less nest-specialized arthropods in each of the three above guilds, with some exclusively nidicolous species and some more opportunistic generalist species (Lesna et al., Reference Lesna, Wolfs, Faraji, Roy, Komdeur and Sabelis2009). We expected these communities to respond to pesticide management because the species are sedentary (and are thus unable to take refuge in any non-crop biotope), unlinked to plants and not living in the soil.
In this pilot study, we investigated the impact of pest control strategies against arthropod pests on the number and diversity of arthropod taxa developing in nests built by Parus major L., 1758 (great tits) in nestboxes located in commercial apple and pear orchards. We estimated the taxonomic and ecological diversity of arthropodofauna in nests and assessed the extent to which the whole and nest-specific arthropodofauna were affected by pest control strategies.
Material and methods
Sampled orchard nests
The study area
The study area was located in south-eastern France in the Avignon region (43°96′27″N to 43°51′23″N, 4°51′12″E to 4°57′34″E). The orchards studied were commercial apple and pear orchards located on privately owned farms. For each tree species, the orchards were distributed as follows: five were managed under organic, five under conventional and five under IPM strategies. In the organic orchards, the use of synthetic chemicals (both fertilizers and pesticides) was excluded according to the commission regulation (European Community 473/2002) that amended the council regulation (European Economic Community 2092/91). The conventional orchards were managed using synthetic chemical pesticides following advice from technical institutes. The IPM orchards were managed according to the International Organisation for Biological and Integrated Control of Noxious Animals and Plants (IOBC) guidelines (Cross, Reference Cross2002), and growers used mating disruption against Cydia pomonella (L., 1758), the main insect pest (see online supplementary material S1). All orchards consisted of a cultivated area of approximately 1 ha inside a larger orchard unit, and they were bordered by single-rowed hedgerows used for protection against the prevailing north wind. All but two studied apple and pear orchards were surrounded only by orchards managed under a similar pest control strategy. Orchards were also chosen for their similar pattern in terms of local and landscape features that might influence bird communities (see Bouvier et al., Reference Bouvier, Ricci, Agerberg and Lavigne2011 for apple orchards, personal observation for pear orchards). In 2008, the analysis was conducted in 30 orchards (15 orchards of each fruit tree species). In 2007, a reduced sampling campaign was carried out with only apple orchards tested (15 orchards in all).
Nestboxes and birds
Nest material for analysis of arthropod communities was collected from nestboxes. The nestboxes were of the Schwegler 1B type (woodcrete material, a blend of wood, concrete and clay). Each orchard had five nestboxes per 1 ha at least two years before our study started. Nestboxes were located on a tree, 2.5 m above ground level, 30 m away from its nearest neighbour and 20 m from surrounding hedgerows. The entrances to all nestboxes faced southeast to avoid both the prevailing north wind and the prevailing south rain.
Birds occupying nestboxes were in most cases P. major. In a few nestboxes, nestlings were found of blue tits Cyanistes caeruleus (L., 1758) (1%) or Eurasian tree sparrows Passer montanus (L., 1758) (9%). To consistently perform comparisons, the nests of the last two species were excluded from analyses, and only nests of P. major were analysed. Each nest in occupied nestboxes was collected on average 75 days after the young had fledged (mean ± 1s.d.: 76.7 ± 22.4 in 2007; 75.0 ± 20.4 in 2008). Complete nests were removed from the nestbox and stored in a closed plastic bag.
Nest analysis
Arthropods were isolated from nests following the immersion and sieving method described in de Lillo (2001), with slight modifications (see Roy et al., Reference Roy, Dowling, Chauve, Lesna, Sabelis and Buronfosse2009). Compared with the Berlese funnel method used in Burtt et al. (Reference Burtt, Chow, Babbitt, Loye and Zuk1991) where only live arthropods are caught, de Lillo's method allows the detection of both living and dead Arthropoda.
The arthropodofauna was explored following two different approaches: (i) the notation of simple presence or absence of arthropods in the nests being studied and (ii) the assignment of isolated arthropods to any of the four guilds defined below based on ecological habits and linkage to the nest habitat.
Assignment to ecological groups
A rough identification at a high taxonomic level (see Primary Taxonomic Groups (PTGs) as defined in the Results section) was first performed using a stereoscopic magnifying glass (2007 and 2008 samples). Species-level identification was then performed on individuals of recurrent taxa and on taxa with possibly ambiguous guild assignments (e.g., beetles). Finally, individuals were assigned to one of the four ecological guilds described below.
Specific identifications were performed by specialists: Mites: astigmatic and prostigmatic Acariformes by P. Klimov and B.M. O'Connor (University of Michigan, Ann Arbor, USA) (2007 samples) and by F. Faraji (MITOX Consultants, Amsterdam, The Netherlands) (2008 samples); hematophagous mesostigmatic mites were identified by L.R. Insects; Psocoptera were identified by Z. Kucerova (Crop Research Institute, Prague, Czech Republic); Coleoptera by R. Allemand (Université Lyon 1, Villeurbanne, France).
Approximately 2% of isolated arthropod individuals were undetermined because of poor condition or to a life stage inappropriate for identification, such as the larval/nymphal stages of some holometabolous insects or some mites. As for Psocoptera, only a sample of isolated individuals has been identified at the species level (27 individuals, distributed in six organic, five integrated and four conventional orchard nests).
To characterize arthropod communities of bird nests, different guilds were distinguished according to the assumed ecological link to bird nests (for details see online supplementary material S2). Note that as Guild 1's link to its nest habitat is by far the weakest, the first guild was not considered in some of the analyses (see section ‘Prevalence estimate’). Guilds were differentiated as follows:
Guild 1: Accidental visitors
In addition to the typical nest guests described in the introduction, some individual arthropods may end up in nests via plant material that birds use for nest construction or directly originating from the tree environment (plant-dwelling species) (Lesna et al., Reference Lesna, Wolfs, Faraji, Roy, Komdeur and Sabelis2009). The presence of these arthropods is fortuitous; they do not develop within nests. All fortuitous visitors were included in this guild, without any distinction according to feeding habits (e.g., phytophagous, predatory, etc.).
Guild 2: Saprophagous/microbivorous guests
These arthropods can be generalists having found a convenient habitat in the nests. They take advantage of detritus produced/brought by the bird inhabitant and adapt to life within nests. However, these arthropods can also be nest specialists.
Guild 3: Predatory guests
These arthropods feed on either Guild 2, Guild 4 arthropods, or both, and may be more or less specialized in nest environments. Parasitoid insects are also included in this guild.
Guild 4: Bird ectoparasites
These arthropods feed on blood or external products (feathers, scales) of the bird host (chicks and/or adults).
Prevalence estimate
Two different approaches were adopted to assess the presence/absence status.
The first was a simple listing of PTGs per nest based on the assignment to PTGs, as defined in the Results section. The following classification of nests was thus proposed:
Arthropod-free nest: a nest from which no arthropod has been isolated.
Arthropod-poor nest: a nest from which only one or two individuals belonging to a single PTG (as defined below, in the Results section) have been isolated.
Arthropod-rich nest: a nest from which more than one PTG or more than two individuals of a single PTG have been isolated. Note that the term ‘rich’ is used as relative to other categories; nest communities in general had low richness.
The second approach consists of assigning arthropods to ecological guilds depending on their degree of association with their nest environment. Considering that the core guilds (i.e., the guilds that are expected to be the most common within nests) are Guilds 2, 3 and 4 and that Guild 1 is by definition unlinked to the nest environment, we classified nests as follows:
Nidicole-free nest: a nest from which no individuals assigned to Guilds 2, 3, or 4 were isolated, individuals of Guild 1 being present or not.
Nidicole-occupied nest: a nest from which individuals assigned to Guilds 2 and/or 3 and/or 4 were isolated, individuals of Guild 1 being present or not.
Statistical tests
We tested whether the proportion of arthropod-free nests, as well as of nidicole-free nests, differed according to crop fruit (‘species’, qualitative, two levels), years (qualitative, two levels), pest control strategy (qualitative, three levels: organic, integrated and conventional) or interval between date of fledging and nest collection (‘date’, quantitative, Julian days) using a logistic regression (proc GENMOD, SAS 9.01, SAS Institute, Cary, NC, USA) on the binary variable describing whether nests were free of arthropods and a logit link function. As nests within orchards were not statistically independent, we introduced a random orchard level into the model. The same models were used to analyse the presence of the three most represented PTGs: Psocoptera, detritivorous Astigmata and hematophagous Mesostigmata, and the presence of Guilds 1, 2 and 4 (Guild 3 being represented by too few individuals).
We then compared the average number of primary groups in each nest containing Arthropoda (arthropod-rich or arthropod-poor) according to crop fruit (‘species’, qualitative, two levels), years (qualitative, two levels), pest control strategy (qualitative, three levels: organic, integrated and conventional) and interval between date of fledging and nest collection (‘date’, quantitative, Julian days) using a generalized linear model assuming a Poisson distribution of the number of taxa and a log link function (proc GENMOD, SAS 9.01). As mentioned above, we introduced a random orchard level in the model to account for dependence among nests within orchards.
Results
Composition and structure of the communities
PTG
PTG represent a consensus between ecological and taxonomical knowledge related to first-glance available morphological characteristics (first stage of identification). By far, the PTGs best represented in orchard nests (⩾10% occurrence in both years 2007 and 2008, sometimes recovered in high number) were Psocoptera (Insecta, detritivorous/saprophagous), detritivorous/saprophagous Astigmata (Acari) and hematophagous Mesostigmata (Acari) (fig. 1). Other arachnid and insect PTGs were represented by very few occurrences. With respect to taxa, the only significant difference between the two years concerned the percentage of spider occurrences in orchard nests (31% in 2007, <3% in 2008).

Fig. 1. PTGs and guilds detected in orchard nests of P. major. Numbers above indicate: Guild 1, accidental visitors; Guild 2, saprophagous/microbivorous nest guests; Guild 3, predatory nest guests; Guild 4, bird ectoparasites.
Composition of ecological guilds
Species-level identification was performed for the above dominant PTGs, as well as for Coleoptera (bird nest beetles are known to belong to various guilds and to be more or less nest specific; Šustek & Kriŝtofik, Reference Šustek and Kriŝtofik2002; Merkl et al., Reference Merkl, Bagyura and Rózsa2004). Some Coleopteran larvae could not be identified at the species level because identification requires characteristics exclusive to the adults. In most cases, a single larva was isolated per nest, and they all matched the morphology of dermestid larvae.
Seven species were recurrently collected (see table 1). For detailed information on taxa recorded in nests, see online supplementary material S3. The occurrences of PTGs were then calculated from these identifications and classified according to guild (fig. 1).
Table 1. Species detected in the four focused primary groups (% of nests containing individuals of the species under consideration). Coleopteran larvae were excluded from the table because no specific identification was performed on them.
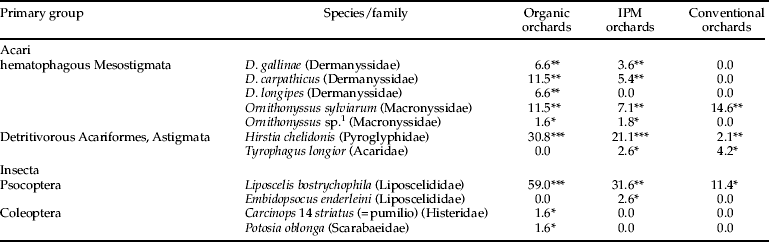
*Rare, **common, yet never abundant, ***common and sometimes abundant.
1 Non-sylviarum individuals of Ornithonyssus were very similar to, but slightly different from, O. bacoti (Hirst, 1913), according to Micherdzi'ski (Reference Micherdziński1980) and compared with individuals of O. bacoti from a lab strain in MNHN.
Comparisons between the three pest control strategies
Prevalence of arthropods and guilds
Arthropod-free nests and nests with arthropods (arthropod-poor + arthropod-rich) were found in similar proportions in apple and pear orchards for both years. However, the proportions varied depending on the pest control strategy (fig. 2, table 2). A greater proportion of arthropod-free nests was found in conventional orchards than in organic orchards (P < 0.0001, organic vs. conventional). Furthermore, the probability of observing an arthropod-free nest decreased when the interval between fledging and nest collection also decreased.

Fig. 2. Prevalence of arthropods within nests. (a) Percentage of nests containing arthropod based on richness classification: see Material and methods section); (b) Percentage of nests containing nest arthropods (Guilds 2, 3 and 4: see Material and methods section).
Table 2. Effect of tree species, pest control strategy, year and interval between sampling and date of fledging on the proportion of arthropod-free nests, the proportion of nidicole-occupied nests and the total number of PTGs.
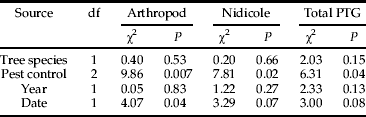
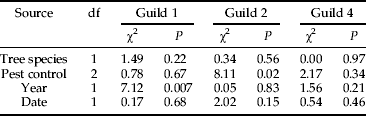
When using the guild classification, a similar pattern emerged. There was no effect of tree species or of sampling year on the proportion of nidicole-occupied nests, but a significant effect of the pest control strategy was recorded, with increasing proportions of nidicole-occupied nests from conventional to organic orchards (fig. 2, table 2). The pairwise comparison between organic and conventional crop management was highly significant (P = 0.0016), while a less significant effect was detected by comparing conventional to IPM systems (P = 0.0113) and no difference between organic and IPM systems were observed (P = 0.1448). Similarly, the pest control strategy affected the probability that a nest hosted individuals of Guild 2 (saprophagous/microbivorous guests), but the same was not observed for Guild 1 or Guild 4. For Guild 2, the probability of occurrence increased from conventional orchards to organic orchards, IPM orchards being intermediate (P < 0.0001, organic vs. conventional; table 3).
Table 3. Effect of tree species, pest control strategy, year and interval between sampling and date of fledging on the proportion of nests hosting guild 1 (accidental visitors), guild 2 (Saprophagous/microbivorous guests) and guild 4 (bird ectoparasites).
Arthropod diversity
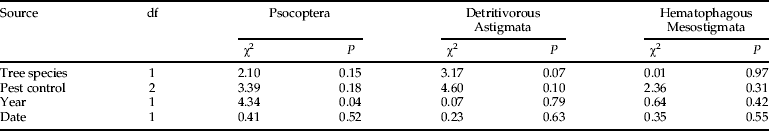
Considering all nests that were surveyed, the total number of PTGs did not depend on tree species, year of study or interval between sampling and date of fledging, but did depend on the pest control strategy (fig. 1 and table 2). However, when only nests with nidicolous species were considered, this last effect was not significant and the only significant difference was between years, the number of PTGs being higher in 2007. Examining the composition of the communities in these nests did not provide a clearer picture. The proportion of nests occupied by each of the main PTGs was not sensitive to crop protection strategy, suggesting that the presence of arthropods, rather than the composition of the community, was impacted by phytosanitary treatments (table 4).
Table 4. Effect of tree species, pest control strategy, year and interval between sampling and date of fledging on the proportion of nests hosting each of the most frequent PTGs. Only nidicole-present nests were considered.
Discussion
Composition of arthropod nest communities
Overall, guilds and higher taxa found in nests were in accordance with some previous records of arthropods from bird nests (e.g., Burtt et al., Reference Burtt, Chow, Babbitt, Loye and Zuk1991, hole-breeding passeriforms from North America: Tachycineta bicolor, Troglodytes aedon, Sialia sialis, Krištofík et al., Reference Krištofík, Šustek and Mašán2002, Reference Krištofík, Mašán and Šustek2007, non-hole-breeding passeriforms from Europe: Remiz pendulinus, Lanius collurio, L. minor and Panurus biarmicus). Interestingly, taxonomic and ecological compositions of the communities were not closer to those of arthropodofauna found in specific nests of P. major than in nests of other hole-breeding birds (see online supplementary material S3B). No general pattern can be drawn from these comparisons. Indeed, records from nests of P. major sampled from different geographic areas (from Slovakia, Ambros et al., Reference Ambros, Krištofík and Šustek1992; Šustek & Krištofík, Reference Šustek and Kriŝtofik2002; from UK, Goodenough & Hart, Reference Goodenough and Hart2012) diverge from the present results and from each other. Differences may be partly because of the cultivated environment we sampled but may also result from biogeographic and/or climatic factors (see online supplementary material S3B).
The occurrence of main taxa in the present study was consistent between years, except for spiders, a group of accidental visitors (see online supplementary material S2).
Effect of pest control strategies
The present study was designed to allow the testing of toxic impact of phytosanitary products on non-target arthropods. First, the arthropodofauna under scrutiny were isolated and sedentary and thus unable to escape pesticide treatment applications. As a result, detected effects of pest management strategies may not result from the repellent action of phytosanitary products. Second, it was not linked to soil features and mostly (apart from Guild 1) unlinked to any cultivated plant.
The significant increase in the ratio of arthropod-free, arthropod-poor or arthropod-rich nests as well as of nidicole-free or nidicole-occupied nests across the three pest control strategies cannot be due to landscape characteristics because all orchards under study were in similar landscape environments (Bouvier et al., Reference Bouvier, Ricci, Agerberg and Lavigne2011 for apple orchards, personal observation for pear orchards). It is known that chemical pest control can have a strong impact on orchard avifauna diversity and reproductive success of birds (Bouvier et al., Reference Bouvier, Toubon, Boivin and Sauphanor2005, Reference Bouvier, Ricci, Agerberg and Lavigne2011). The present results show that chemical pest control also has a strong impact on many of the non-target arthropods usually found in bird nests. Given the configuration of nestboxes (single entrance hole ca. 32 mm diameter, located >15 cm above the tit nest) and their position (2.5 m above ground level), it is unlikely that nests were directly sprayed. Chemicals may be introduced into nestboxes through aerosols as well as by the bird itself, whose feathers may be contaminated by sprayed products during foraging. While further work is needed, this descriptive preliminary study showed that environmental impregnation by agrichemicals dramatically affects non-target arthropods in orchards, which was highlighted by the gradually decreasing gradient of prevalence from organic to IPM to conventional systems. However, the limited abundance data prevented a detailed assessment of the effects on nest ecosystem functioning since rare species usually contribute less than abundant species to ecosystem function.
Diversity of arthropoda
The biodiversity of arthropodofauna in orchard nests was not as informative as were the approaches based on nest classification (arthropod-free, arthropod-poor or arthropod-rich nests or nidicole-free or nidicole-occupied nests) when the three pest control strategies were assessed. This result was at least partly because different guilds were very weakly diversified: recurrent detritivorous arthropods were restricted to two mite species and one insect species, predators were almost absent and parasites were mainly represented by five hematophagous mesostigmatic species.
Conclusions and perspectives
The prevalence of arthropods in bird nests contained in nestboxes was affected by pest control strategies in apple and pear orchards. A marked contrast between the conventional and organic modalities and an intermediate status of nests sampled in IPM orchards was observed. Our results provide evidence of environmental insecticide impregnation in orchards, which may also affect species visiting the orchard such as mobile pest enemies, beneficials or pollinators. In contrast, the taxonomic diversity within arthropod communities did not provide as clear-cut information as was observed for simple presence or absence notation.
This study is thus a first step towards demonstrating that crop protection strategies affect nest-dwelling arthropod communities. Understanding consequences for the nest ecosystem requires further investigation. First, bearing in mind that even in organic orchards substances are applied that specifically target arthropods (see online supplementary material S1), an interesting perspective would be to compare nest communities between treated, including organic systems, and un-treated abandoned or family orchards. Second, considering not only the prevalence but also the abundance of nest arthropods would allow understanding better how pest control strategies affect nest ecosystem functions.
The supplementary material for this article can be found at http://www.journals.cambridge.org/BER.
Acknowledgments
The authors want to warmly thank Z. Kucerova (Crop Research Institute, Prague, Czech Republic), R. Allemand (Université Lyon 1, Villeurbanne, France), F. Faraji (MITOX Consultants, Amsterdam, The Netherlands) as well as P. Klimov and B.M.O'Connor (University of Michigan, Ann Arbor, USA) for insect or mite identifications, M.W. Sabelis and I. Lesna (IBED, University of Amsterdam, Amsterdam, The Netherlands) for valuable comments, B. Mullens (University of California, Riverside, USA), F. Salmon (Centre de Recherche sur la Biologie et les Populations d'Oiseaux, Paris, France), A. Marchal and M. Serre (Association Ornithologique Rhodanienne, Lyon, France), S. Calabro (Association Ornithologique Becs Crochus Centre Est, Relevant, France), I. Rubinoff (Hy-Line International, Dallas Center, USA), K. McCoy (Institut de recherche pour le développement, Montpellier, France), T. Boulinier (Centre d'Ecologie Fonctionnelle et Evolutive, Montpellier, France) for useful samples and information and M.A. Arnaud for English proofreading.
We also thank two anonymous reviewers for valuable comments, which greatly improved the manuscript. Finally, L.R. would like to offer her most sincere thanks to G. Lallemand (LEGTA des Mandailles, Chateauneuf-de-Galaure, France) for his technical participation.








