1. Introduction
The fossil record of caprines (Caprinae, sensu Gentry, Reference Gentry1992) in the Mediterranean islands is restricted to the Balearic Islands and Sardinia. On Mallorca, five chronospecies belonging to the Myotragus phylogenetic lineage have been described, spreading from the Early–Middle Pliocene to the Holocene (Alcover, Moyà-Solà & Pons-Moyà, Reference Alcover, Moyà-Solà and Pons-Moya1981; Bover & Alcover, Reference Bover and Alcover2005): M. pepgonellae (Early/Middle Pliocene), M. antiquus (latest Middle Pliocene), M. kopperi (latest Pliocene/Early Pleistocene), M. batei (Early/Middle Pleistocene) and M. balearicus (Middle Pleistocene to Holocene). Two species are known to occur on Menorca, namely, Myotragus balearicus and a second one from an Early Pleistocene deposit described as M. binigausensis by Moyà-Solà & Pons-Moyà (Reference Moyà-Solà and Pons-Moyà1980), although it was later synonymized to M. batei by Bover & Alcover (Reference Bover and Alcover2000), a view recently questioned (Moyà-Solà et al. Reference Moyà-Solà, Köhler, Alba, Pons-Moyà, Pons and Vicens2007). M. balearicus, the terminal species of the lineage, survived on Mallorca and Menorca presumably until the first human arrival, 4300–4200 years ago (Bover & Alcover, Reference Bover and Alcover2003; Alcover, Reference Alcover2008). Evolutionary patterns within Myotragus include body size reduction (insular dwarfism), changes in skull and postcranial morphology, and reduction in number of incisors and premolars. M. balearicus displays a single ever-growing incisor in each jaw, and a single premolar in the lower dentition (p4) and two in the upper dentition (P3 and P4) (Bate, Reference Bate1909; Andrews, Reference Andrews1915). Similar changes in dentition have occurred in other island bovids. Thus, both species of the Alcelaphini genus Maremmia, M. haupti and M. lorenzi from the Late Miocene of the Tusco-Sardinian palaeoprovince (Hürzeler & Engesser, Reference Hürzeler and Engesser1976; Hürzeler, Reference Hürzeler1983; Azzaroli et al. Reference Azzaroli, Boccaletti, Delson, Moratti and Torre1986), also display one lower premolar and two upper premolars, although the Maremmian bovid has three ever-growing incisors in each jaw. Turritragus casteanensis (unassigned subfamily and tribe status, neither Neotragini nor Alcelaphini: M. R. Palombo, pers. comm.) from Casteani (Grossetto) and Fiume Santo (Sardinia) displays just one lower premolar and two upper premolars (Abbazzi et al. Reference Abbazzi, Delfino, Gallai, Trebini and Rook2008).
On the island of Eivissa (‘Ibiza’), a faunal assemblage was discovered in the beginning of the 1980s at Ses Fontanelles, a Late Miocene/Early Pliocene deposit (Moyà-Solà et al. Reference Moyà-Solà, Pons-Moyà, Alcover and Agustí1984). Two bovids were initially identified: a very hypsodont and small-sized Antilopini, and a Bovidae (Caprinae) with short metapodials (Moyà-Solà, Agustí & Pons-Moyà, Reference Moyà-Solà, Agustí and Pons-Moyà1984; Agustí & Moyà-Solà, Reference Agustí, Moyà-Solà and Azzaroli1990; Moyà-Solà et al. Reference Moyà-Solà, Quintana, Alcover, Köhler, Rössner and Heissig1999).
On Sardinia, a Plio-Pleistocene Caprinae was initially described as Antilope (Nemorhaedus) melonii by Dehaut (Reference Dehaut1911), although the genus Nesogoral was erected ulteriorly by Gliozzi & Malatesta (Reference Gliozzi and Malatesta1980) to accommodate this species from material obtained at Capo Figari (northeastern Sardinia). Remains of this caprine have been recovered from other deposits on the island, such as Capo Mannu (western central Sardinia: Van der Made, Reference Van der Made, Reumer and de Vos1999), Monte Tuttavista (eastern Sardinia: Abbazzi et al. Reference Abbazzi, Angelone, Arca, Barisone, Bedeti, Delfino, Kotsakis, Marcolini, Palombo, Pavia, Piras, Rook, Torre, Tuveri, Valli and Wilkens2004) and from an unknown locality at Campidano (southwest Sardinia: Van der Made, Reference Van der Made, Alcover and Bover2005). Two species of Nesogoral have been described thus far: N. melonii (Dehaut, Reference Dehaut1911) and N. cenisae Van der Made, Reference Van der Made, Alcover and Bover2005. In addition, four bovids were reported by Abbazzi et al. (Reference Abbazzi, Angelone, Arca, Barisone, Bedeti, Delfino, Kotsakis, Marcolini, Palombo, Pavia, Piras, Rook, Torre, Tuveri, Valli and Wilkens2004) from Monte Tuttavista: Nesogoral sp.1 aff. N. melonii (morphotype A), Nesogoral sp.2 (morphotype B), ?Caprinae gen. et sp. nov. (morphotype C) and Caprinae gen. et sp. indet. The former morphotype C was described as Asoletragus gentryi (Palombo et al. Reference Palombo, Valli, Arca and Tuveri2006b), but its definitive ascription to the Caprinae could not be established. A close phylogenetic relationship between Nesogoral and Myotragus is widely accepted (e.g. Gliozzi & Malatesta, Reference Gliozzi and Malatesta1980; Palombo et al. Reference Palombo, Bover, Valli and Alcover2006a).
We describe here the remains of a caprine recovered at Caló den Rafelino (Manacor, east coast of Mallorca). With this paper we wish to honour Miss Dorothea M. A. Bate, a pioneering fossil hunter from the beginning of the 20th century, who discovered and described the genus Myotragus after a short visit to Mallorca, exactly one hundred years ago (Shindler, Reference Shindler2005).
2. Methods
Postcranial bones and teeth of recent and fossil bovids used to establish comparisons with the new taxon are curated at three vertebrate collections: (1) ‘Museu de la Naturalesa de les Illes Balears’ (MNIB), deposited at the Institut Mediterrani d'Estudis Avançats (IMEDEA, Esporles, Mallorca, Spain) and Societat d'Història Natural de les Balears (SHNB, Palma, Mallorca, Spain); (2) the American Museum of Natural History (New York, USA); and (3) the National Museum of Natural History–Smithsonian Institution (Washington, D.C., USA). Measurements (to 0.02 mm) were taken as defined in Table 1 caption using a digital caliper.
Table 1. Measurements (in mm) of dental and postcranial remains of Myotragus palomboi n. sp.
Measurements of astragalus and phalanges following von den Driesch (Reference von den Driesch1976). Collection numbers of specimens are also provided. Teeth: L – length; W – width; Hm – mesial height of the enamel; Hd – distal height of the enamel; Hli – lingual height of the enamel; Hla – labial height of the enamel. Postcranial: GL – greatest length; TD – transverse diameter at midshaft; APD – dorso-palmar/plantar diameter at midshaft; TDp – transverse diameter proximal; APDp – dorso-palmar/plantar diameter proximal; TDd – transverse diameter distal; APDd – dorso-palmar/ plantar diameter distal; GLPe – greatest length of the peripheral (abaxial) half; BFp – proximal functional breadth; BFd – distal functional breadth; DLS – greatest diagonal length of the sole; MBS – middle breadth of the sole; GLl – lateral greatest length; Bd – distal breadth; Dm – greatest depth on medial side; Dl – greatest depth on lateral side; APFL – dorso-palmar/plantar functional diameter; ACm – medial height in dorsal view; ACm – lateral height in dorsal view; DVD – dorso-ventral diameter.
Measurements of additional recent and fossil caprines were derived from the literature: Guerin (Reference Guerin1965) for Gallogoral meneghinii and additional recent caprines; Gliozzi & Malatesta (Reference Gliozzi and Malatesta1980) for Nesogoral melonii; Van der Made (Reference Van der Made, Reumer and de Vos1999) for Nesogoral aff. melonii and Van der Made (Reference Van der Made, Alcover and Bover2005) for Nesogoral cenisae; Palombo et al. (Reference Palombo, Bover, Valli and Alcover2006a) for Nesogoral sp.; Köhler, Moyà-Solà & Morales (Reference Köhler, Moyà-Solà and Morales1995) for Norbertia hellenica; and Alcalá & Morales (Reference Alcalá and Morales1997) for Aragoral mudejar.
The anatomical terminology used in the description follows Köhler (Reference Köhler1993) for metatarsal and phalanges, Mead & Taylor (Reference Mead and Taylor2005) for cubonavicular (except ‘internal’ and ‘medial’ processes of these authors have been changed to ‘medial’ and ‘central’ processes, respectively, in this paper), and Schaller (Reference Schaller1992) and Bover, Fornós & Alcover (Reference Bover, Fornós, Alcover, Alcover and Bover2005) for the rest of postcranial bones. Dental terminology is according to Gentry & Hooker (Reference Gentry, Hooker and Benton1988) and Gentry (Reference Gentry1992).
3. Deposit
The deposit is located on the eastern coast of Mallorca, close to Caló den Rafelino, in the municipality of Manacor (Fig. 1). It is very close to the seashore, and consists of hardened sediment belonging to one of the relatively common palaeo-collapses (e.g. Fornós, Reference Fornós1998; Robledo & Pomar, Reference Robledo and Pomar2000) present in the Upper Miocene limestones of the so-called Reef Complex (Pomar et al. Reference Pomar, Rodríguez-Perea, Sabat and Fornós1990; Gómez-Pujol, Balaguer & Fornós, Reference Gómez-Pujol, Balaguer, Fornós, Fornós, Ginés and Gómez-Pujol2007), Late Tortonian–Messinian in age (Bizon et al. Reference Bizon, Bizon, Bourrouilh and Massa1973).

Figure 1. Location map (left) and topographic survey (right) of the palaeontological deposit from Caló den Rafelino. Arrows and grey coloured zone indicate the exact location of the fossiliferous breccia. Translation: ‘secció’ – cross-section, ‘planta’ – plan, ‘secció longitudinal’ – longitudinal section, ‘topografia’ – topographic survey and ‘abril’ – April.
The fossiliferous deposit (Fig. 1) occupies a very small portion of the red silt sediment of the fossil cave (roughly 1 m2), and contains a faunal assemblage not recorded before in Mallorca. In addition to the caprine studied herein, remains of four mammals (a new species of Hypolagus, a large-sized new genus of cricetid, a glirid and a soricid), several reptiles (thus far, a large viperid, a colubrid, a tortoise, a lacertid and an anguid, and more may be identified), a bird and some fish teeth have been recovered (Bover et al. Reference Bover, Quintana, Agustí, Bailon and Alcover2007; Quintana et al. in press).
4. Description and comparisons
Following the criteria of Gentry (Reference Gentry1992) and Gentry, Rössner & Heizmann (Reference Gentry, Rössner, Heizmann, Rössner and Heissig1999), the combination of characters displayed by the Caló den Rafelino bovid, such as short metapodials, weak flanges distally on metatarsals, dorso-plantar compression of metatarsal shaft, simple outline of central fossettes on upper molars, and reduced p2 (see description of material for further details) clearly favour its inclusion in the Caprinae. The lack of remarkable differences in size, the morphological consistency of the complete caprine bone sample obtained, together with the insular character of the fauna (as derived from absence of mammal predators, presence of endemic species, and characteristic changes in body size) suggest that all these remains belong to the same species.
The tentative Early Pliocene chronology of the deposit, the lack of Miocene bovids in the Balearic Islands, and the known Mallorcan faunal assemblage during the Pliocene and Pleistocene, all suggest that the recovered material should be compared with Late Miocene–Early Pliocene western European and Pliocene Balearic caprines in order to establish its phylogenetic relationships. Thus, comparisons between the caprine from Caló den Rafelino (CdR caprine) and Myotragus, Nesogoral and Gallogoral meneghinii (a mainland caprine from the Villafranchian of Europe (Guerin, Reference Guerin1965) that has been related to Myotragus (Alcover, Reference Alcover1976)) will be established. Although two species of Nesogoral have been described (N. melonii and N. cenisae), the main difference between them lies in the degree of metapodial robustness (those of N. melonii being more robust than N. cenisae) (Van der Made, Reference Van der Made, Alcover and Bover2005). Consequently, Nesogoral s.l. will be used in the comparisons except for the metapodials. We also compared the CdR caprine with a few remains from the caprine of Ses Fontanelles (Eivissa). Only the material of this caprine curated in Mallorca (Spain) has been available for comparative analysis.
Although several species of presumed Caprinae have been described from the Late Miocene of the Mediterranean–European region (e.g. Köhler, Reference Köhler1987; Alcalá, Morales & Moyà-Solà, Reference Alcalá, Morales and Moyà-Solà1989–1990; Köhler, Moyà-Solà & Morales, Reference Köhler, Moyà-Solà and Morales1995; Gentry, Rössner & Heizmann, Reference Gentry, Rössner, Heizmann, Rössner and Heissig1999; Vislobokova, Reference Vislobokova2006), just one species has been recorded in the western European mainland: Aragoral mudejar (Late Vallesian) from Aragón (Spain). It displays a robust metacarpal and a hypsodont dentition with a reduced premolar row (Alcalá & Morales, Reference Alcalá and Morales1997). This species has been related to the more modern Norbertia hellenica from the Turolian/Ruscinian transition of Maramena (Greece) (Köhler, Moyà-Solà & Morales, Reference Köhler, Moyà-Solà and Morales1995). Although the latter species has not been recorded in western Europe, it will be considered when establishing comparisons because it has been phylogenetically related with Aragoral (Alcalá & Morales, Reference Alcalá and Morales1997).
In addition to the fossil caprines already listed, almost all recent caprines (Ammotragus, Budorcas, Capra, Capricornis, Hemitragus, Nemorhaedus, Oreamnos, Ovibos, Ovis, Pseudois and Rupicapra) have been included in the analysis, with special attention paid to Ovis, the closest relative to Myotragus according to molecular data (Lalueza-Fox et al. Reference Lalueza-Fox, Castresana, Sampietro, Marqués-Bonet, Alcover and Bertranpetit2005; Ramírez et al. Reference Ramírez, Gigli, Bover, Alcover, Bertranpetit, Castresana and Lalueza-Fox2009).
4.a. Upper dentition
4.a.1. Upper second premolar (P2)
Material. IMEDEA 90142, left (Fig. 2a).

Figure 2. Dental material of Myotragus palomboi, n. sp. (a) left P2 IMEDEA 90142: 1 – lingual view; 2 – mesial view; 3 – labial view; 4 – distal view; 5 – occlusal view; (b) left P3 IMEDEA 90275: 1 – lingual view; 2 – mesial view; 3 – labial view; 4 – distal view; 5 – occlusal view; (c) left i3 IMEDEA 90145: 1 – occlusal view; 2 – mesial view; 3 – labial view; (d) right dc IMEDEA 90146: occlusal view; (e) left p2 IMEDEA 90143: 1 – labial view; 2 – lingual view; 3 – mesial view; 4 – distal view; 5 – occlusal view; (f) right p2 IMEDEA 90144: 1 – labial view; 2 – lingual view; 3 – mesial view; 4 – distal view; 5 – occlusal view. Scale bar 2 cm.
A very hypsodont tooth with subequal length and width in occlusal view. The tooth is slightly wider than long (L = 6.5 mm, W = 6.7 mm; see Table 1), and the labial wall is slightly concave, almost flat, with styles poorly developed or absent. No ribs on the labial wall can be observed. The parastyle is almost absent because it is extremely reduced, appearing as a small rib on the mesial side of the tooth. This weak parastyle is placed very close to the mesial extreme of the central cavity, which is mesio-distally elongated and placed on the mesial half of the tooth. This tooth is poorly developed mesially to the protocone, while distally it is large and expands lingually. The enamel is more extended on the mesial and labial walls than on the distal and lingual walls. The occlusal surface is worn and almost flat. The distal edge on the occlusal surface is sharp and divided into two lobes.
As remarked previously, the tooth of the CdR caprine is wider than long (ratio L/W < 1), as in specimens of M. pepgonellae, Ovis vignei and Capra pyrenaica (P. Bover, unpub. data). The remaining fossil and extant caprines display a P2 which is longer than wide.
A partial maxilla of M. pepgonellae (MNIB 59199) bearing P2–M3 shows that the P2 displays the same morphology as in the CdR caprine. According to the description of the P2 of Norbertia hellenica, styles are absent and the tooth is longer than broad (Köhler, Moyà-Solà & Morales, Reference Köhler, Moyà-Solà and Morales1995). This morphology is similar to that displayed by the CdR caprine, M. pepgonellae and Gallogoral, although it might be related to the fact that these remains share the same level of wear. Like Norbertia, Aragoral mudejar has a longer than broad P2 (Alcalá & Morales, Reference Alcalá and Morales1997) and, as observed in the CdR caprine, its mesial side is reduced (area placed mesially to protocone poorly developed). No P2 of Nesogoral appears described or figured in the bibliography, and no P2 from Ses Fontanelles is available for study.
Although the morphology of this premolar is conditioned by wear, the shape in occlusal view of the Caló den Rafelino P2 is more similar to Ammotragus, Ovis and Capra than to other caprines. Differences among these taxa rely on the lesser development of the area placed mesially to the protocone in the CdR caprine and Ammotragus, and the presence of a labial projection of a feeble metastyle in Capra and Ovis.
4.a.2. Upper third premolar (P3)
Material. IMEDEA 90275, left (Fig. 2b).
Although the tooth is highly worn, it is clearly hypsodont. As in P2, the P3 is wider than long (L = 8.3 mm; W = 10.8 mm; see Table 1). In occlusal view, it has a square-trapezoidal shape. The parastyle is very reduced and placed on the mesio-labial corner, and is expanded labially. The labial wall is also relatively flat and only the paracone prolongation to the root through the wall can be observed. The area placed mesially to the protocone is well developed and expanded lingually. The area placed distally to the protocone is poorly developed. The enamel is more expanded on the labial and medial walls. The central cavity is elongated and U-shaped. The medial extreme of this cavity is placed close to the parastyle, between the parastyle and the paracone. As in P2, the distal crest present on the occlusal surface is well marked and sharp. The occlusal surface is almost flat with a broad groove running in the lingual–labial direction.
A wider than long P3 (ratio L/W < 1) is also displayed by M. pepgonellae (Moyà-Solà & Pons-Moyà, Reference Moyà-Solà and Pons-Moyà1982), Gallogoral (Guerin, Reference Guerin1965) and some specimens of Capra and Ovis (P. Bover, unpub. data). Although this tooth is more hypsodont in M. pepgonellae, its morphology is very similar to the CdR caprine. Norbertia hellenica and Aragoral mudejar have a weak metastyle (like the CdR caprine), but they display a flat lingual surface, while in the CdR caprine this surface is strongly convex. Gallogoral and Nesogoral have a well-developed metastyle, but Nesogoral has a weaker parastyle than Gallogoral, although it is more developed than in the CdR caprine.
Although the shape of the occlusal surface also seems to be influenced by wear, similarities in development of the protocone and parastyle between the CdR caprine and specimens of Ovis, Capra and Pseudois with similar tooth wear can be clearly noticed.
4.b. Lower dentition
4.b.1. Third incisor (i3)
Material. IMEDEA 90145, left (Fig. 2c).
This tooth has a typical chisel shape and displays a clear distinction between root and crown. It is not a very hypsodont tooth (crown length 9.5 mm; crown maximum width 4.6 mm; root length 8.2 mm). The root is compressed mesio-distally and displays a shallow groove along the mesial side. The crown is labially curved, especially in ventral view. The wear surface has a subrectangular-oval shape. It is divided into two concavities by a medial rib and has a distinctive wear facet on the tip (labial extreme). The enamel has no longitudinal grooves and covers the entire crown region (except the wear surface).
In bovids, incisors and canines (incisiforms) usually fall from the mandible shortly after death. It is rare to get jaws retaining incisiforms in fossil specimens. When more than one species is present in the same deposit, it is difficult to assign isolated incisors and canines to one of them. Thus, for comparative purposes, only incisors and canines from recent caprines have been used, together with some teeth of Myotragus pepgonellae.
Although it depends on the wear conditions, the shape of this tooth in the CdR caprine is similar to the i3 of all caprine species studied except Myotragus. The general shape of the tooth is even more similar to Capricornis, Nemorhaedus and Rupicapra.
The i3 of M. pepgonellae displays a very different shape. It has a wear surface subtriangular in outline and is relatively hypsodont (Hypsodonty Index, HI: 2.12–2.22, calculated as crown total length/maximum crown width), but it is less hypsodont than the i3 of M. antiquus (HI: 3.1) (Moyà-Solà & Pons-Moyà, Reference Moyà-Solà and Pons-Moyà1982). The HI for the i3 of the CdR caprine is 2.06, and its shape is completely different (see above).
4.b.2. Deciduous canine or third incisor? (dc or di3?)
Material. IMEDEA 90146, right (Fig. 2d).
Crown of a small incisiform, probably a deciduous canine. Crown length 5.5 mm; crown maximum width 2.7 mm. The outline of the crown is oval.
4.b.3. Lower second premolar (p2)
Material. IMEDEA 90143, left, 90144, right (Fig. 2e, f; Table 1).
This is not a very hypsodont tooth (Table 1). Entoconid and entostylid are fused (entoconid–entostylid complex; fusion observed even in an unworn tooth, IMEDEA 90143). Paraconid and parastylid are also fused (paraconid–parastylid complex). The labial wall is almost completely flat, while there are two slightly marked ribs on the lingual wall. They correspond to the ventral expansion of the paraconid–parastylid complex and the metaconid. The paraconid–parastylid complex is small and reduced to a crest on the mesial margin of the tooth. The protoconid is just in front or placed slightly mesial to the metaconid. In the worn p2 (IMEDEA 90144) the metaconid and protoconid are the highest points of the tooth and are placed on the mesial margin of the occlusal surface. Thus, the occlusal surface is flat and obliquely inclined towards the distal part. In one of the specimens (IMEDEA 90143), two large roots can be observed.
In M. pepgonellae the presence of a second lower premolar remains controversial. In the description of the species by Moyà-Solà & Pons-Moyà (Reference Moyà-Solà and Pons-Moyà1982), the presumed presence of an alveolus for a reduced p2 was reported, but Bover & Alcover (Reference Bover and Alcover2005) mentioned that it was not observable. Thanks to a recent exhaustive cleaning of the type of M. pepgonellae (MNIB 57321) and of a second mandible (MNIB 59196) of this species, both completely covered by an opaque layer of consolidant, the root of a small tooth was revealed mesially to the p3. It is unclear if it corresponds to a p2 or to a relict dp2. The size of this premolar should be extremely reduced in relation to a ‘normal’-sized premolar (alveolar length: 1.9 mm, 2.5 mm; alveolar width: 2 mm, 2.9 mm, respectively, in the two mandibles), and its shape should have looked like a small column (like the dp3 of M. balearicus; see Bover & Alcover, Reference Bover and Alcover1999). The morphology of this tooth in the CdR caprine is more similar to the p2 of other fossil and recent caprines studied. Unfortunately, the unavailability for study of other premolars of the CdR caprine precludes the establishment of comparisons between the size of the p2 and the size of the p3 and p4, and thus of evolutionary changes or size reduction in this tooth. Nevertheless, the p2 is proportionally wider in the CdR caprine (W/L: 0.75 for IMEDEA 90143 and 0.95 for IMEDEA 90144; see Table 1) than in other caprines studied (W/L ranging between 0.55 and 0.67 except in very worn specimens, in which this ratio is higher). In other caprines studied (except Aragoral; p2 not figured in Alcalá & Morales, Reference Alcalá and Morales1997) the protoconid and metaconid are also the highest points of the tooth, but they are placed in the middle of the occlusal surface.
4.c. Postcranial skeleton
4.c.1. Magnum
Material. IMEDEA 90272, right, and 90273, right (Fig. 3a, b).

Figure 3. Postcranial material of Myotragus palomboi, n. sp. (a) Right magnum IMEDEA 90272: 1 – proximal view; 2 – distal view; 3 – palmar view; (b) right magnum IMEDEA 90273: 1 – proximal view; 2 – dorsal view; (c) left pisiform IMEDEA 90138: 1 – medial view; 2 – dorsal view; (d) left astragalus IMEDEA 90131: 1 – dorsal view; 2 – plantar view; 3 – proximal view; 4 – distal view; (e) left cubonavicular IMEDEA 90141: 1 – proximal view; 2 – distal view; 3 – dorsal view; (f) right metatarsal (holotype) IMEDEA 90140: 1 – dorsal view; 2 – medial view; (g) proximal phalanx IMEDEA 90132: 1 – dorsal view; 2 – axial view; (h) proximal phalanx IMEDEA 90133: 1 – dorsal view; 2 – abaxial view; (i) proximal phalanx IMEDEA 90274: 1 – dorsal view; 2 – axial view; (j) medial phalanx IMEDEA 90134: 1 – dorsal view; 2 – abaxial view; (k) medial phalanx IMEDEA 90135: 1 – dorsal view; 2 – abaxial view; (l) distal phalanx IMEDEA 90136: 1 – proximal view; 2 – solar view; 3 – axial view; 4 – distal view; (m) distal phalanx IMEDEA 90137: axial view. Scale bar 2 cm.
Like other postcranial remains, this bone is robust. It is slightly proximo-distally compressed, and the insertion areas of ligaments and tendons are poorly preserved, as occurs with the crests present on the articular facets. The separation between the semilunar and scaphoid articular facets is relatively low in dorsal view. The distal surface is completely flat and, while in specimen IMEDEA 90272 the articular facet for the metacarpal is expanded over all the surface, in IMEDEA 90273 there is a non-articular facet on the latero-palmar part of this surface.
One of the specimens (IMEDEA 90273) displays gnawing marks on the proximo-medial and proximo-dorsal margin of the bone, probably produced by a rodent (a cricetid and a glirid have been recorded in the deposit).
The only remarkable morphological feature of the magnum of the CdR caprine is the relatively low height difference between the semilunar and scaphoid articular facets. The ratio medial height/lateral height (ACM/ACm; see Table 1) is low in the CdR caprine (mean = 1.16; n = 2; range = 1.11–1.21). Only M. kopperi (mean = 1.22; n = 2; range = 1.17–1.27) (no magnum of M. pepgonellae or M. antiquus is available), Budorcas (mean = 1.24; n = 3; range = 1.15–1.28) and Ovibos (mean = 1.18; n = 5; range = 1.12–1.21) show similar values. This ratio is markedly higher in the other species studied (e.g. Oreamnos; mean = 1.5; n = 5; range = 1.41–1.59) and Nesogoral (1.35; n = 1, obtained from figured specimen in Van der Made, Reference Van der Made, Alcover and Bover2005).
4.c.2. Pisiform
Material. IMEDEA 90138, left (Fig. 3c).
The pisiform of the CdR caprine is robust and of globular shape. The articular facet for the unciforn and the ulna are separated by a ridge. For comparison only pisiforms of M. balearicus, Gallogoral, Oreamnos, Capra, Ovis, Rupicapra, Capricornis, Budorcas and Ovibos have been used. Although some features of the pisiform are very variable (Bover, Fornós & Alcover, Reference Bover, Fornós, Alcover, Alcover and Bover2005), the general globular shape of this bone in the CdR caprine is very similar to M. balearicus, Oreamnos and Ovibos.
4.c.3. Astragalus
Material. IMEDEA 90131, left (Fig. 3d).
The astragalus is incomplete and partially eroded, but some of its features can be described. It is short and broad. The plantar articular facet for the calcaneus is concave but not very rounded and is extended distally. The groove placed between the two proximal trochleae is broad and deep, and in dorsal view it is closed U-shaped. In this same view, and although it is slightly eroded, the lateral proximal trochlea is placed above the level of the medial one, which is expanded plantarly.
Astragali from recent caprines Budorcas, Oreamnos, Capricornis, Ovibos, Ovis and Capra and fossil Myotragus, Nesogoral and Gallogoral have been compared with the astragalus of the CdR caprine. The caprine astragali are generally short, but this bone is not significantly more robust in the CdR caprine. Values for the ratio Distal Breadth/Lateral Greatest Length (Bd/GLl × 100; measurements following von den Driesch, Reference von den Driesch1976) reach 58.5 in the CdR caprine (Table 1) and 61.8 in M. pepgonellae (n = 1) (in M. antiquus even more slender; mean = 57; n = 3; range = 55.4–58.1). In the rest of the caprines studied, this value is larger than 65 except in Nemorhaedus goral (mean = 61.2; n = 3; range = 59.2–62.4), Nesogoral (63.6) and Gallogoral (56.2).
Additionally, resemblances between the CdR caprine and Myotragus rely on the shared deep, slender and closed U-shaped outline of the groove placed between the proximal trochleae when seen in dorsal view, and the not very rounded plantar articular facet for the calcaneus. In other caprines this groove is more V-shaped (Budorcas, Capra, Ovis, Nesogoral) or open U-shaped (Capricornis, Oreamnos, Nemorhaedus, Ovibos, Gallogoral) and the facet for the calcaneus is clearly rounded (P. Bover, unpub. Ph.D. thesis, Univ. Illes Balears, 2004).
4.c.4. Cubonavicular
Material. IMEDEA 90141, left (Fig. 3e).
This bone shows the dorsal margin fragmented. It has a square outline in proximal view and is slightly compressed proximo-distally. All attachment areas of ligaments are robust. The medial and central processes are partially eroded. In plantar view, the attachment area of the plantar ligament is large and rounded. A longitudinal groove located centrally along the plantar margin can be observed. Although it is partially abraded, the postero-external metatarsal process is blunt and robust. In distal view, it displays a deep and narrow groove to accommodate the tendon of the musculus peroneus longus that continues obliquely along the lateral margin of the bone and contacts the calcaneal articular facet. The antero-external metatarsal facet and the entocuneiform facet are rounded and concave, the former being almost half the size of the latter. The posterior cuneal facet is flat and very small. The postero-external metatarsal facet is also rounded. The calcaneal articular facet is broad medio-laterally and the internal and external astragal articular facets are deep and very concave, with a sharp crest between them. There is a deep and elongated notch on the planto-lateral part of the internal astragal articular facet.
Characters shared by the CdR caprine and Myotragus are the robust attachment area for ligaments, the general proximo-distal compression of the bone, the shape of the distal articular facets, and the similar level at which the antero-external metatarsal facet and the entocuneiform facet are placed when seen in dorsal view.
4.c.5. Metatarsal
Material. IMEDEA 90140, right (Fig. 3f)
The metatarsal is a very short and wide bone (Fig. 4). It is more compressed dorso-plantarly than transversally. The plantar surface of the diaphysis is fairly flat and wide, and a shallow plantar longitudinal groove can be observed on the proximal and distal extremes, close to the epiphyses. The dorsal surface is also almost flat, and the dorsal longitudinal groove, wide and deep, can just be observed on the distal 1/3 of the diaphysis, especially on the area close to the intertrochlear incision.

Figure 4. Metatarsals in dorsal view of (a) Myotragus palomboi n. sp. IMEDEA 90140 (holotype); (b) Myotragus pepgonellae MNIB 59204 and (c) Myotragus balearicus IMEDEA 85651. Dashed lines indicate the anatomical separation of metatarsal and cubonavicular (fused in the more modern species of Myotragus). Scale bar 2 cm.
In dorsal and plantar views, the transverse diameter is practically the same along all the diaphysis (that is, the lateral and medial margins of the diaphysis are parallel).
The proximal articular surface is almost flat, and the proximal metatarsal groove is deep, mainly at its central part. The posterior subfacet of the cubonavicular facet is abraded and does not allow us to conclude if it is high. The other facets and projections are also eroded. The morphology of the insertion of the lateral and medial extensor tendons cannot be fully resolved.
Although it is poorly preserved, the articulation surface of the trochlea is not as restricted as in Myotragus (Leinders, Reference Leinders1979).
The index of metatarsal robustness (TD/GL × 100; see Table 1) of the CdR caprine (22.8) is larger than in almost all fossil and extant caprines studied, except Myotragus. Among extant caprines, only Budorcas taxicolor has a similar, even higher, robustness index (mean = 23.7; n = 4; range = 22.2–24.6), but its morphology is completely different. While the tranverse diameters of the distal and proximal epiphyses in the CdR fossil are similar or slightly larger than the transverse diameter at midshaft, Budorcas displays a broad difference in transverse diameters, those of distal and proximal epiphyses being clearly wider than at midshaft (P. Bover, unpub. Ph.D. thesis, Univ. Illes Balears, 2004).
Among the fossils, only the Balearic caprines show a similar morphology and similar or larger robustness index. Two partially reconstructed metatarsals of M. pepgonellae have a robustness index of 32.6 and 36.2 (MNIB 59204 and 59205, respectively) and display almost parallel margins in dorsal view (Fig. 4). Identical morphology is shown by a metacarpal of the caprine from Ses Fontanelles, which has a robustness index of 27. Caprines show similar proportions in hind and forelimb metapodials. Thus, it is probable that the metatarsals of the caprine from Ses Fontanelles had a similar robustness index to the CdR caprine.
A similar morphology and robustness index to the CdR caprine is found in a metacarpal of a Bovidae indet. from the Baccinello-Cinigiano Basin (Tusco-Sardinian palaeoprovince, Late Miocene, Italy) that appears figured in Abbazzi et al. (Reference Abbazzi, Delfino, Gallai, Trebini and Rook2008, text-fig. 18). According to measurements taken from this figure, its robustness index falls around 22.7 and it also displays parallel margins in dorsal view. Unfortunately, no taxonomical assignment can be achieved yet regarding this bone, but the authors suggest that the metacarpal (and another one, not figured) could belong to Etruria viallii (?Antilopinae) or Turritragus casteanensis (Subfamily indet.).
4.c.6. Proximal phalanx
Material. IMEDEA 90132, 90133 and 90274 (Fig. 3g–i).
Since none of the preserved CdR caprine phalanges can be assigned to the hind or the forelimb, all phalanges of recent and fossil caprines (when available) have been used for comparative purposes.
This is a robust phalanx. No remarkable differences between the transverse diameter of the epiphysis and the diaphysis can be observed. The medial and lateral walls of the diaphysis are parallel in dorsal view.
The proximal articular surface is broad, and the incision for the metapodial verticillus is shallow (as in Myotragus). In lateral view, it is concave with a rounded articular area (suggesting that joint movement was slightly restricted, but not as in Myotragus).
The dorsal surface of the diaphysis is convex, and the insertion area for the interdigital ligament is prominent and located slightly distal to the midshaft. There is no groove for the interosseum tendon.
The plantar/palmar surface is slightly concave in transverse section. The distal articular facets do not extend onto the plantar/palmar surface and their margins are flat-V shaped in this view. Sesamoidean facets are small and the insertion areas for the sesamoidean ligaments are also small and are oriented proximo-plantarly/palmarly.
No proximal phalanges of M. pepgonellae have been recovered as yet. Thus, for comparative analysis, the proximal phalanges of the more recent species M. antiquus have been used (Fig. 5). No proximal phalanges of the caprine from Ses Fontanelles or of Aragoral were available for study.
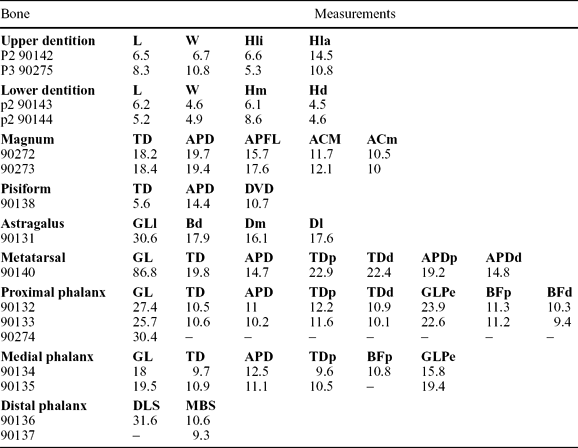
Figure 5. Comparison between phalanges of Myotragus palomboi n. sp. (a–c) and Myotragus antiquus (d–f). (a) Proximal phalanx IMEDEA 90132: 1 – axial view; 2 – dorsal view; (b) medial phalanx IMEDEA 90134: 1 – abaxial view; 2 – palmar/plantar view; 3 – axial view; (c) distal phalanx IMEDEA 90136: 1 – axial view; 2 – solar view; (d) proximal phalanx: 1 – MNIB 57902: medial view; 2 – MNIB 57901: reversed, dorsal view; (e) medial phalanx: 1 – MNIB 57935, reversed, abaxial view; 2 – MNIB 57922, reversed, palmar/plantar view; 3 – MNIB 57928, axial view; (f) distal phalanx: 1 – MNIB 57950, axial view; 2 – MNIB 57951, reversed, solar view. Scale bar 2 cm.
When compared to other caprines, the most relevant feature of the Caló den Rafelino phalanges is their great robustness (Mean Robustness Index, RI (TD/GL × 100; measurements following von den Driesch, Reference von den Driesch1976) = 39.8; n = 2 (one of the three phalanges, IMEDEA 90274, is fragmented); range = 38.2–41.4). Among recent caprines, only Budorcas and Ovibos display larger or similar RI (48.7 (n = 1) and mean = 39.4 (n = 5; range = 36.7–44.9), respectively), while among the fossils, only Myotragus antiquus shows larger values (even larger than 50; mean = 48; n = 5; range = 43.9–51.8).
In addition to their robustness, the phalanges of the CdR caprine and Myotragus share other morphological features (Fig. 5). The articular facets for the sesamoids are small. The protuberances where the ligaments between sesamoids and the proximal part of the phalanges are attached (sesamoidean ligaments, following Köhler, Reference Köhler1993) are also small and oriented in the proximo-plantar/palmar direction. In other caprines studied, these areas are rough surfaces oriented plantarly/palmarly and extend distalwards to approximately one-third of the diaphysis. Even in species with very robust proximal phalanges (Budorcas) this area is extended.
A characteristic feature of Myotragus (Leinders, Reference Leinders1979; Spoor, Reference Spoor1988) is the reduction of the articular movement between the proximal and medial phalanges, manifested in the flat morphology of the distal articular facet of the proximal phalanx (in lateral view). The caprine from Caló den Rafelino is similar to Norbertia, Gallogoral, Budorcas or Nesogoral in that respect, showing a rounded facet, but without an expansion of these facets on the dorsal and plantar/palmar surface of the diaphysis, as displayed by other caprine taxa (e.g. Capra and Ovis). In addition, the proximal articular surface is slightly reduced in the CdR caprine, Myotragus, Norbertia and Nesogoral (where it has a less concave morphology in lateral view), while in the other caprines it has a rounded outline.
4.c.7. Medial phalanx
Material. IMEDEA 90134 and 90135 (Fig. 3j, k).
Like the proximal phalanx, the second phalanx is short and robust. The post-articular plateau and the dorsal extensor process are very weak or almost absent. The articular fovea is concave.
No articular notches (in the sense of Köhler & Moyà-Solà, Reference Köhler and Moyà-Solà2001) can be observed on the distal and proximal articular surfaces. The distal articular lateral facet has a rounded outline, while the medial facet has a subtriangular outline in medial view (it is divided by a crest).
The distal articular facets are proximally expanded on the dorsal part and they tend to converge near the midshaft. On the plantar/palmar surface these facets extend to one-third of the distal extreme. While in one specimen (IMEDEA 90134) this surface is flat, in the other specimen (IMEDEA 90135) a sagittal groove along the whole diaphysis can be clearly observed. The lateral and medial extremes of the distal articular facets are approximately at the same level.
No medial phalanges of M. pepgonellae have been recovered as yet. Thus, for comparative analysis, medial phalanges of the derived species M. antiquus have been used (Fig. 5). No data for Budorcas, Aragoral or the caprine from Ses Fontanelles were available for study.
As in the proximal phalanx, the medial phalanx of the CdR caprine is very robust (mean RI = 55; n = 2; range = 54–56.1; calculated as in proximal phalange; Table 1). Among the available living caprines, only Ovibos has a similar RI (mean = 55.2; n = 5; range = 48.9–61.5). Again, Myotragus is the taxon with the greatest RI, with values close to 60 (mean = 58.6; n = 5; range = 50.3–70.3). The CdR caprine and Myotragus also share characteristic features not observed in the other caprines studied, such as the very weak morphology of the postarticular plateau and the dorsal extensor process, and an important reduction in the articular movements (as derived from the flat proximal and distal articular facets in lateral view) (Fig. 5). This morphology of the articular facet is also displayed by Ovibos. In both fossil taxa, the lateral and medial extremes of the distal articular facets are approximately at the same level, as in Nesogoral, Capricornis and Gallogoral.
4.c.8. Distal phalanx
Material. IMEDEA 90136 and 90137 (Fig. 3l, m).
Like other phalanges, the distal phalanx is short and robust. Both phalanges available are broken in the processus extensorius zone. The dorsal ridge (on the dorsal border) is rounded. On the wedge/interdigital margin of the plantar or palmar surface of the phalanx there is a prominent protuberance extended axially (clearly visible in IMEDEA 90137, but IMEDEA 90136 is abraded and this protuberance cannot be observed). The solar (distal) border is flat or slightly concave. The border of the plantar wedge (where the flexor digitorum profundus inserts) is poorly developed. In the coronary (proximal) border, the articular surface is broad (in accordance with the size of the articular surface in the medial phalanx) and the crest that separates the two facets is axially curved. The articular surface is open (that is, not very rounded). The apex is blunt. As IMEDEA 90136 is abraded, the protuberance for the extensor insertion is not visible and the dorsal border is also eroded. In this specimen a central notch can be observed on the articular facets, while in IMEDEA 90137 it is absent.
In lateral view the dorsal border is strongly convex, almost rounded. The axial surface is concave and has a small crest on the anterior part, running in the dorso-plantar direction, and the axial foramen is at the same level as the dorsal extreme of the crest on the articular facet.
No distal phalanges of M. pepgonellae have been recovered as yet. Thus, for comparative analysis, distal phalanges of the derived species M. antiquus have been used (Fig. 5), in addition to distal phalanges of Norbertia (fragmented), Nesogoral, Gallogoral, Capra, Ovis, Oreamnos, Nemorhaedus and Ovibos.
The robustness of the distal phalanx in the CdR caprine is 33.6 (n = 1; phalanx IMEDEA 90137 is fragmented); measured as the ratio middle breadth of the sole/diagonal length of the sole (MBS/DLS × 100; Table 1), following von den Driesch, Reference von den Driesch1976). Only Ovibos (mean = 33.2; n = 5; range = 30.6–35.2) and Myotragus (mean = 39.7; n = 5; range = 35.2–42) display similar or greater robustness. The CdR caprine shares with the other studied caprines an almost rounded shape of the distal part of the dorsal ridge. In Myotragus, a prominent protuberance extended axially on the wedge of the phalanx can also be observed, although it is not as developed as in the CdR caprine (Fig. 5). In the other caprines this protuberance is a weak and elongated rough area (except in Ovibos, where this protuberance projects axially from the broad articular facet). Broad articular facets and practically flat soles are shared by Ovibos, Myotragus and the CdR caprine.
5. Systematic palaeontology
The CdR caprine and Myotragus share the following combination of characters: (1) an extremely short and robust metatarsal, which is columnar and has parallel walls; (2) great robustness in all postcranial remains studied; (3) similar shape of P2 and P3 (compared to M. pepgonellae and M. antiquus); (4) cubonavicular compressed proximo-distally, with robust attachment area for ligaments; shape of distal articular facets, with antero-external metatarsal and entocuneiform facets placed at same level when seen in dorsal view; (5) astragalus with deeper, more slender and closed U-shaped outline of the groove placed between the proximal trochleae in dorsal view, and with plantar articular facet for the calcaneus not very rounded; (6) in proximal phalanges, articular facets for sesamoids small, and areas where sesamoidean ligaments insert also small and oriented, as a blunt protuberance, in proximo-plantar/palmar direction; (7) in medial phalanges, very weak postarticular plateau and dorsal extensor process, and important reduction in articular movements (manifested in flattened proximal and distal articular facets in lateral view); (8) in distal phalanges, presence of a prominent protuberance extended axially beyond the wedge of the phalanx.
In accordance with all of these similarities, the CdR caprine is here included in the genus Myotragus. Nevertheless, there are remarkable differences between the Myotragus from Caló den Rafelino and M. pepgonellae (and also the rest of species of Myotragus), namely: (1) lower robustness index in all available postcranial remains (metatarsal, phalanges, astragalus, etc.) in the CdR Myotragus; (2) i3 of ‘normal’ shape, that is, with a more rectangular wear surface, and with enamel surrounding all the crown, and not expanded to the root; (3) presence of a larger p2, with a reduced paraconid–parastylid complex. These differences warrant the description of a new species of Myotragus, as follows.
Class MAMMALIA Linnaeus, Reference Linnaeus1758
Order ARTIODACTYLA Owen, Reference Owen1848
Family bovidae Gray, Reference Gray1821
Subfamily caprinae Gray, Reference Gray1821
Genus Myotragus Bate, Reference Bate1909
Myotragus palomboi n.sp.
Holotype. Right metatarsal (IMEDEA 90140).
Paratypes. The remaining material described herein.
Type locality. Caló den Rafelino, Manacor (Mallorca; Balearic Islands, Spain).
Age. Earliest Early Pliocene.
Etymology. The species name is honouring Prof. Maria Rita Palombo, singular contributor to the development of palaeontological studies on Mediterranean mammals.
Diagnosis. Myotragus with short and robust metapodials, longer than in other representatives of the genus; i3 neither hypsodont nor ever-growing, chisel-shaped and with enamel just covering the erupted area of the tooth (that is, not elongated nor with enamel covering the labial part of the root); p2 relatively large, displaying important reduction of paraconid–parastylid complex.
6. Discussion and conclusions
This new species of Myotragus represents the earliest record of the genus. No bovid remains are known from the Middle Miocene of the Balearic Islands (Mein & Adrover, Reference Mein and Adrover1982; Adrover et al. Reference Adrover, Agustí, Moyà and Pons1985; Quintana & Agustí, Reference Quintana and Agustí2007). The ancestor of the CdR caprine presumably reached the island during the Messinian Salinity Crisis (MSC) (5.6–5.32 Ma ago; e.g. Clauzon et al. Reference Clauzon, Suc, Gautier, Berger and Loutre1996; Krijgsman et al. Reference Krijgsman, Hilgen, Raffi, Sierro and Wilson1999). Myotragus palomboi n.sp. should be considered as the first representative of the Myotragus phylogenetic lineage (Fig. 6), in which a progressive reduction in length of metapodials and a reduction in number of incisiforms and premolars took place (e.g. Alcover, Moyà-Solà & Pons-Moyà, Reference Alcover, Moyà-Solà and Pons-Moya1981). Accordingly, M. palomboi displays a metapodial longer than the previously considered earliest known representative of the genus, M. pepgonellae, but it is shorter than the condition exhibited in other fossil (and extant) European caprines. It should be considered as the direct ancestor of M. pepgonellae. This is also suggested by the reduction in size of p2 (promoted by the reduction of the paraconid–parastylid complex). This feature agrees with the evolutionary scenario for the genus, with a presumed extremely reduced p2 (or remains of dp2) in M. pepgonellae and complete loss of this tooth in their descendants (Alcover, Moyà-Solà & Pons-Moyà, Reference Alcover, Moyà-Solà and Pons-Moya1981). The chronology proposed for the taxon from Caló den Rafelino also fits into the proposed palaeobiogeographical scenario: while M. pepgonellae has been considered to be Early/Middle Pliocene in age (Moyà-Solà & Pons-Moyà, Reference Moyà-Solà and Pons-Moyà1982; Pons-Moyà, Reference Pons-Moyà1990), M. palomboi is attributed to the beginning of the Early Pliocene.
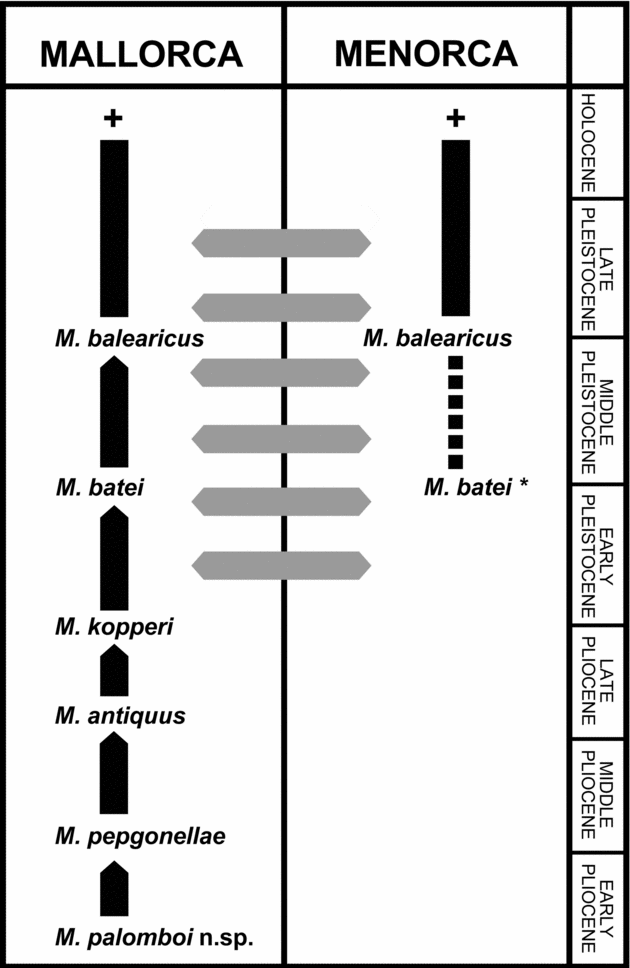
Figure 6. Phylogenetic relationships among the different chronospecies of the Myotragus lineage. * = the species formerly described as M. binigausensis by Moyà-Solà & Pons-Moyà (Reference Moyà-Solà and Pons-Moyà1980) was synonymized to M. batei by Bover & Alcover (Reference Bover and Alcover2000) but this view is currently under discussion (see Moyà-Solà et al. Reference Moyà-Solà, Köhler, Alba, Pons-Moyà, Pons and Vicens2007). Grey lines indicate contact and faunal exchange between Mallorca and Menorca during Quaternary glaciations. + = extinction of the lineage.
The similarities between the faunal assemblages of the Late Miocene/Early Pliocene site at Ses Fontanelles (Eivissa: Moyà-Solà et al. Reference Moyà-Solà, Quintana, Alcover, Köhler, Rössner and Heissig1999) and Caló den Rafelino could suggest that they are closely related. Ses Fontanelles has rendered two bovids (the caprine reported here and an Antilopini), two rodents (a gerbillid Protatera sp. and a glirid Eliomys sp.), an insectivore and a leporid (Hypolagus sp. according to Quintana et al. in press), as well as several reptiles (Podarcis sp. and a tortoise). In the Caló den Rafelino deposit, at least a caprine (here described as M. palomboi), a glirid, an insectivore, a Hypolagus, a tortoise and a small lacertid have been recorded, together with other mammalian and reptilian species (Bover et al. Reference Bover, Quintana, Agustí, Bailon and Alcover2007). Thus, it can be suggested that these species reached Mallorca and Eivissa from the mainland during the Messinian Salinity Crisis.
Some remarkable similarities can be traced between the metatarsal of M. palomboi and the metacarpal of an unidentified bovid from Baccinello figured in Abbazzi et al. (Reference Abbazzi, Delfino, Gallai, Trebini and Rook2008), although it is difficult to go further than Palombo et al. (Reference Palombo, Bover, Valli and Alcover2006a) in the establishment of a clear relationship between Myotragus and the Italian insular bovids. The same holds when trying to establish the mainland phylogenetic origin of Myotragus. As previously mentioned, both Aragoral (Upper Vallesian, MN10) and Norbertia (Turolian–Ruscinian boundary, MN13/14) display robust metapodials, weak upper premolar styles and reduced p2 (Köhler, Moyà-Solà & Morales, Reference Köhler, Moyà-Solà and Morales1995; Alcalá & Morales, Reference Alcalá and Morales1997), and they could be considered as putative ancestors of Myotragus, but their relationships cannot be clearly established on the basis of current evidence.
The reduction in size of Myotragus postcranial long bones and the progressive loss of teeth have been related to a process of evolution in insular conditions (e.g. Alcover, Moyà-Solà & Pons-Moyà, Reference Alcover, Moyà-Solà and Pons-Moya1981; Moyà-Solà & Pons-Moyà, Reference Moyà-Solà and Pons-Moyà1982). In M. palomboi, the reduction in size of the metatarsal and second lower premolar suggests that this species represents a first stage in this process. The faunal assemblage from Caló den Rafelino, with a reduced number of species, all of them endemic, and the lack of important ecological categories (as carnivores) among them, also suggest some kind of insular effect, or at least the presence of important barriers for dispersal of mainland taxa. Despite all of this evidence, the similar evolutionary pattern observed in mainland caprines such as Aragoral and Norbertia (Köhler, Moyà-Solà & Morales, Reference Köhler, Moyà-Solà and Morales1995; Alcalá & Morales, Reference Alcalá and Morales1997), and the presence of fossil insular bovids with long and slender metapodials and dentition like Nesogoral, do not allow conclusions to be formed on this subject. The caprine that arrived in the Balearics may already have displayed short metapodials before the colonization event took place, as Marcus (Reference Marcus1998) suggested. Nevertheless, its extremely short metapodials and teeth reduction are probably best explained as a result of island evolutionary processes (see Van der Made, Reference Van der Made, Alcover and Bover2005 for documentation and interpretation of the extent of the pattern of feet shortening of artiodactyls on islands).
Future findings and work are necessary in order to understand the taxonomy of the earlier species of Myotragus, the systematics of insular Mediterranean bovids and to understand evolutionary patterns on these islands.
Acknowledgements
Thanks are due to Maria Rita Palombo (Roma, Italy), Bienvenido Martínez Navarro (Tarragona, Spain), Jan Van der Made (Madrid, Spain), and an anonymous referee, who reviewed the manuscript. Miquel Trias (Palma, Spain) provided the topography of the deposit. This work is a contribution to the project CGL2007-62047/BTE of Dirección General de Investigación, Ministerio de Ciencia e Innovación. One of the authors (PB) benefits of a JAE-DOC (CSIC) contract (Junta para la Ampliación de Estudios).









