Numerous industrial activities such as those of refineries and textile, chemical, plastic and food processing produce considerable amounts of wastewater contaminated by a range of organic species, many of which are recalcitrant and toxic. Removal of those pollutants from aqueous streams has become a crucial problem over recent decades because environmental regulations and water-quality standards have become increasingly stringent, while conventional wastewater treatments do not provide a safe solution. Phenolic compounds are among the most representative toxic species listed by the European Commission and the US Environmental Protection Agency (EPA) as priority pollutants since the mid-1970s (EPA, 1982; European Parliament, 2008). They are ubiquitous in the effluents from various industries, including refineries, coking operations, coal processing, pulp mills and petrochemical industries, among others.
Advanced oxidation processes may provide useful solutions when working in near-ambient conditions. Among them, the Fenton process is the most widely used at full scale, and several Fenton-like technologies have been developed. These include the so-called catalytic wet peroxide oxidation (CWPO), which is the heterogeneous version of the Fenton system. The main advantage of CWPO with respect to the conventional homogeneous process is that it avoids or drastically reduces the continuous loss of iron in the effluent and the subsequent need for dealing with the sludge resulting from iron precipitation upon the neutralization step preceding final discharge. The CWPO of phenol and 4-CP has been much reported in the literature using a diverse range of catalysts mostly based on iron, but also with some other transition metals supported on various materials (Carriazo et al., Reference Carriazo, Guelou, Barrault, Tatibouët, Molina and Moreno2005; Garcia-Molina et al., Reference Garcıa-Molina, Lopez-Arias, Florczyk, Chamarro and Esplugas2005; Valverde et al., Reference Valverde, Romero, Romero, García, Sánchez and Asencio2005; Zazo et al., Reference Zazo, Casas, Mohedano and Rodriguez2006; Peraira et al., Reference Peraira, Tavares, Fabris, Lago, Murad and Criscuolo2007; Valkaj et al., Reference Valkaj, Katovic and Zrncevic2007; Molina et al., Reference Molina, Zazo, Casas and Rodrıguez2010, Reference Molina, Casas, Pizarro, Rodriguez, Humphrey and Boyd2011; Catrinescu et al., Reference Catrinescu, Arsene and Teodosiu2011, Reference Catrinescu, Arsene, Apopei and Teodosiu2012; Hailing et al., Reference Hailing, Pingxiao, Zhi, Nengwu and Ping2011; Zhou et al., Reference Zhou, Gu, Qian, Xu and Xia2011, Reference Zhou, Zhang, Hu, Wang, Xu, Xia, Zhang and Song2014; Inchaurrondo et al., Reference Inchaurrondo, Massa, Fenoglio, Font and Haure2012; Pan et al., Reference Pan, Fang and Xin2012; Khanikar & Bhattacharyya, Reference Khanikar and Bhattacharyya2013; Munoz et al., Reference Munoz, de Pedro, Casas and Rodriguez2013; Dominguez et al., Reference Dominguez, Quintanilla, Casas and Rodriguez2014; Duan et al., Reference Duan, Yang, Li, Cao, Wang and Zhang2014; Kurian et al., Reference Kurian, Nair and Rahnamol2014; Deka & Bhattacharyya, Reference Deka and Bhattacharya2015; Oxana et al., Reference Oxana, Artemiy, Olga, Prosvirin, Isupova and Parmon2016; Tomul, Reference Tomul2016; Wei et al., Reference Wei, Li, Zhang, Li, Deng, Shao and Mo2017; Leal et al., Reference Leal, Lourenço, Brandao, da Silva, Souza and Souza2018), including raw and modified clays. So far, kaolin has attracted limited attention, probably due to its less developed porous texture compared to other clays such as bentonite (Jozefaciuk & Bowanko, Reference Jozefaciuk and Bowanko2002). However, its textural properties may be improved upon appropriate treatment (Yavuz & Saka, Reference Yavuz and Saka2013; Gao et al., Reference Gao, Li, Wu, Zhao, Deligeer and Asuha2015, Reference Gao, Zhao, Wu, Deligeer and Asuha2016; Boukhemkhem & Rida, Reference Boukhemkhem and Rida2017).
This work is focused on the application of Tamazert kaolin (KT), a naturally occurring mineral, as a precursor in the preparation of an inexpensive catalyst for CWPO after various treatments followed by impregnation with FeCl3. The catalyst has been tested in the temperature range of 25–55°C using phenol and 4-CP as target pollutants. The evolution of the starting compounds, TOC and reaction by-products, as well as iron leaching, was monitored.
Materials
Catalyst preparation
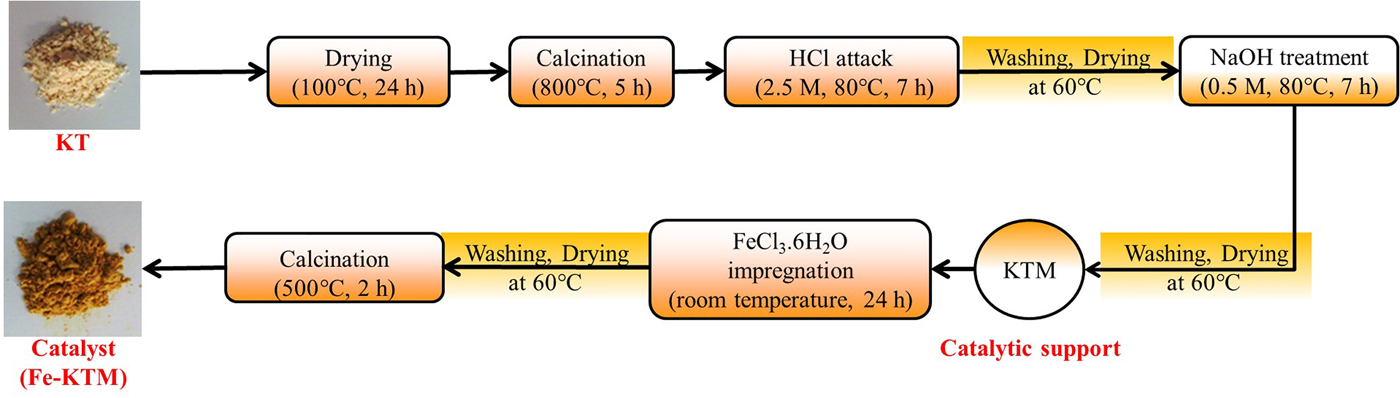
The KT was supplied by the National Society of Sanitary Ceramics located in the region of El-Milia, Jijel Province, east Algeria. The KT was subjected to successive treatments, previously reported elsewhere (Boukhemkhem & Rida, Reference Boukhemkhem and Rida2017), to obtain the modified kaolin (KTM) used as the catalytic support in this work. Next, 2 g of KTM was added to 100 mL of FeCl3·6H2O aqueous solution (37 mM) under vigorous stirring for 24 h. Then, the solid was washed, dried and calcined at 500°C (Fig. 1). The resulting catalyst contained 9.4 mass% of iron.
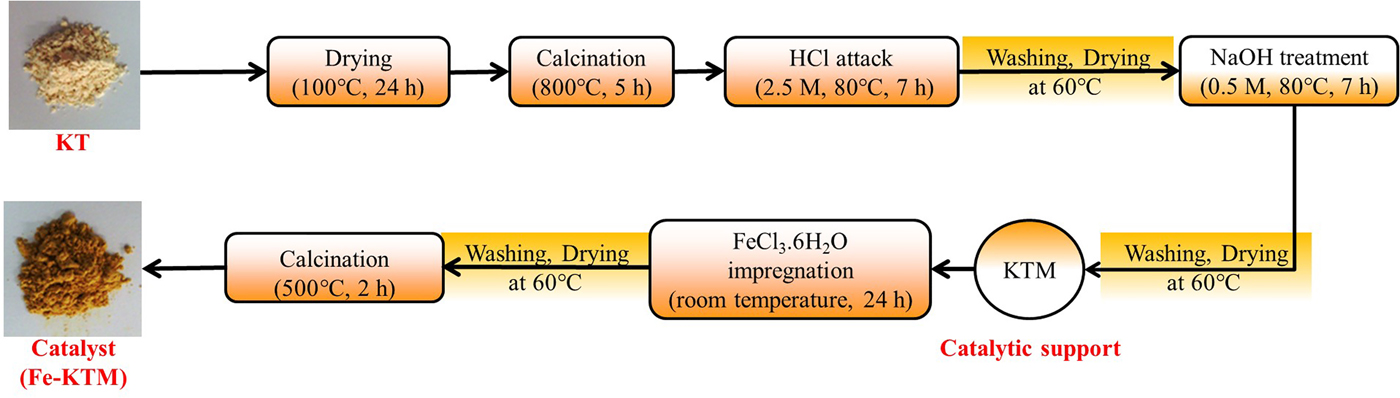
Fig. 1. Catalyst preparation steps.
Experimental
Catalyst characterization
The porous texture of the raw material and the resulting catalyst was characterized by N2 adsorption–desorption at –196°C using a Micromeritics Tristar 3020 instrument. Before measurements were taken, the samples were outgassed overnight at 160°C and a residual pressure of 5 × 10–3 Torr. The specific surface area (S BET) was calculated by applying the Brunauer–Emmett–Teller (BET) equation in the linear region of the isotherms in the relative pressure range up to 0.35. The micropore volume (V μp) was calculated by the t-plot method. The Fe content of the samples was determined by total-reflection X-ray fluorescence (TXRF) spectroscopy with a TXRF EXTRA-II spectrometer (Rich and Seifert, Germany).
CWPO tests
The CWPO tests were conducted in a thermostatically controlled batch glass reactor under stirring conditions. To evaluate the contribution of a non-catalytic mechanism to the reaction, preliminary experiments under the same operation conditions as the catalytic tests were performed. In the presence of catalyst (Fe-KTM) and the absence of H2O2, the disappearance of phenol or 4-CP after 4 h due to adsorption was marginal; in the presence of H2O2 and the absence of catalyst, no conversion of pollutants was observed.
After stabilization of the temperature, the corresponding amount of catalyst (0.2 g) was added to 0.2 L of aqueous phenol or 4-CP solutions (100 mg/L initial concentration). The initial pH value was 3.3 (adjusted with 1 M HCl), the optimum value for Fenton oxidation (Tatibouët et al., Reference Tatibouët, Guélou and Fournier2005). Stirring was maintained for 15 min to ensure wetting of the solid. Then, the stoichiometric amount of H2O2 (500 and 344 mg/L for phenol and 4-CP, respectively) was added from a 33 mass% H2O2 aqueous solution. The experiments were carried out at 25, 40 and 55°C. The samples containing the reaction medium (25 mL) were collected at various reaction times from 15 min to 4 h, filtered using 0.45 µm pore size polytetrafluoroethylene (PTFE) filters and analysed.
The CWPO of phenol and 4-CP produces aromatic compounds as intermediate by-products and short-chain carboxylic acids as final products, which may undergo further oxidation to CO2 and H2O (Molina et al., Reference Molina, Pizarro, Monsalvo, Polo, Mohedano and Rodrıguez2010b). The phenol and 4-CP concentrations in each sample collected from the reaction medium were analysed by high-performance liquid chromatography (HPLC; Varian Pro-Start 240) with a diode array detector at 280 nm wavelength (UV–Vis detector) and a C18 column (Microsorb-MV) as the stationary phase. Milli-Q water and acetonitrile (50/50 v/v) at a flow rate of 1 mL/min were used as the mobile phases. Intermediate by-products (hydroquinone, benzoquinone and catechol) in the final effluents were also analysed by HPLC, but with a different mobile phase; namely, 4 mM aqueous H2SO4 solution at a flow rate of 1 mL/min. Short-chain carboxylic acids were identified by anionic suppression ion chromatography (Metrohm 790 IC) using a conductivity detector. A Metrosep A Supp 5-250 column (25 cm length, 4 mm diameter) was employed as the stationary phase, while the mobile phase was a mixture of 3.2 mM Na2CO3 and 1 mM NaHCO3 at a flow rate of 0.7 mL/min. The TOC was measured with a TOC analyser (Shimadzu, model 5000 A) equipped with an auto-sampler. The H2O2 and iron concentrations in the collected samples were quantified by colourimetry with a Cary 60 UV–Vis spectrophotometer (Agilent Technologies) using the titanium oxisulfate (Eisenberg, Reference Eisenberg1943) and ortho-fenantroline methods (Saywell & Cunningham, Reference Saywell and Cunningham1937), respectively.
Results and discussion
Catalyst characterization
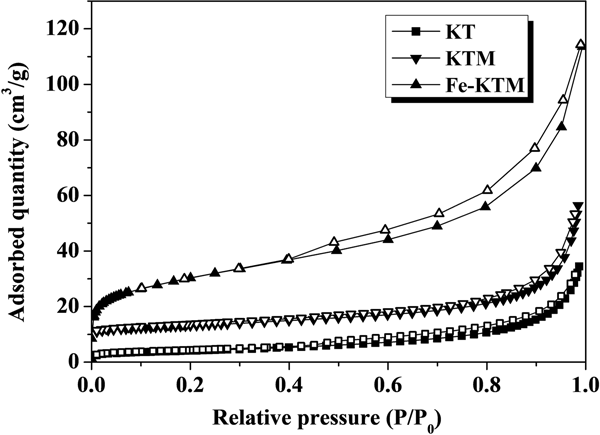
The KT, KTM and Fe-KTM showed type IV isotherms with H3 hysteresis loops due to the capillary condensation of N2 in mesopores (Fig. 2). This type of isotherm is typically obtained for aggregates of plate-like particles, giving rise to the slit-shaped pores typical of clay minerals (Horikawa et al., Reference Horikawa, Do and Nicholson2011; Auta & Hameed, Reference Auta and Hameed2012).
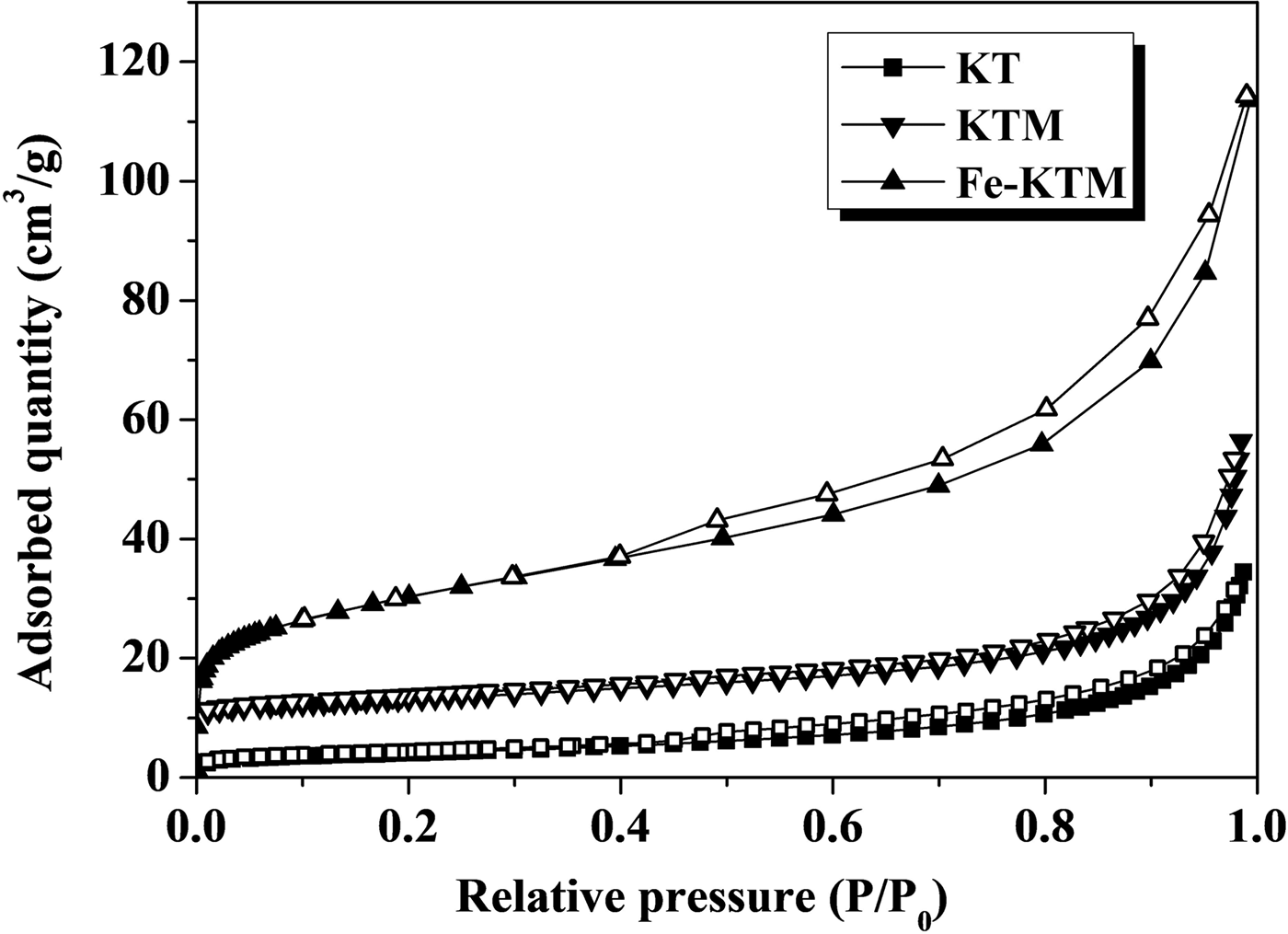
Fig. 2. The –196°C N2 adsorption–desorption isotherms of KT, KTM and Fe-KTM.
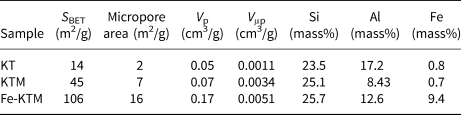
The treatment of the starting kaolin led to an increase in pore volume and specific surface area (Table 1), mainly due to the dissolution of aluminium atoms located in the octahedral sites during the acid attack of KT after calcination (Volzone & Ortiga, Reference Volzone and Ortiga2006). Sample Fe-KTM presented the best textural properties, being characterized by a significant increase in the specific surface area (from 45 m2/g for KTM to 106 m2/g for Fe-KTM), with an increase in pore and micropore volumes. Development in mesopores was also observed; the hysteresis loop appears more pronounced in the case of Fe-KTM, although some contribution of microporosity may also be observed from the isotherm. The specific surface area of Fe-KTM is higher than the corresponding values reported in the literature for other iron catalysts supported on modified kaolin and also used in oxidation processes, ranging between 33 and 45 m2/g (Ayodele et al., Reference Ayodele, Lim and Hameed2012; Kosa et al., Reference Kosa, El Maksod, Alkhateeb and Hegazy2012).
Table 1. Textural properties and chemical compositions of the starting materials and the prepared catalyst.
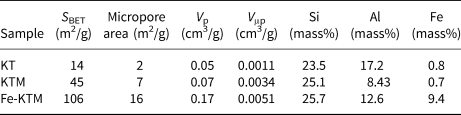
The chemical composition of these materials (Table 1) showed that the iron content of the sample increased substantially as expected from the FeCl3 impregnation. In contrast, the Al/Si ratio decreased, confirming the above-mentioned dealumination during the acid attack.
Catalytic activity
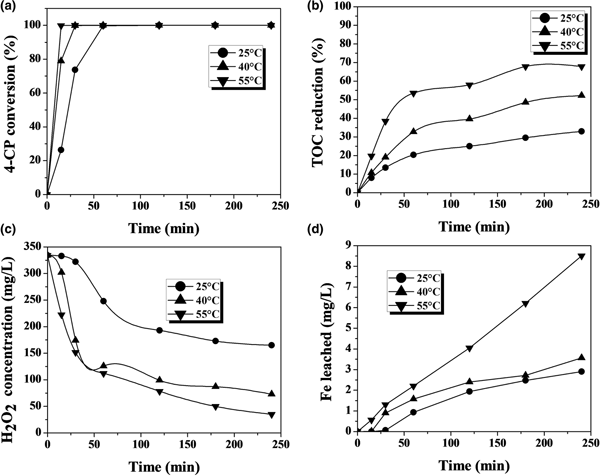
The CWPO of phenol and 4-CP with the Fe-KTM catalyst at various temperatures suggests that the complete conversion of the starting compound was achieved at all temperatures (Figs 3, 4). Temperature barely affected the rate of disappearance of phenol, but it had a more significant effect on 4-CP. The latter is converted more rapidly due to the presence of the Cl atoms in its molecule, favouring the attack of the OH· radicals (present in the reaction medium from H2O2) on the aromatic ring of the 4-CP.
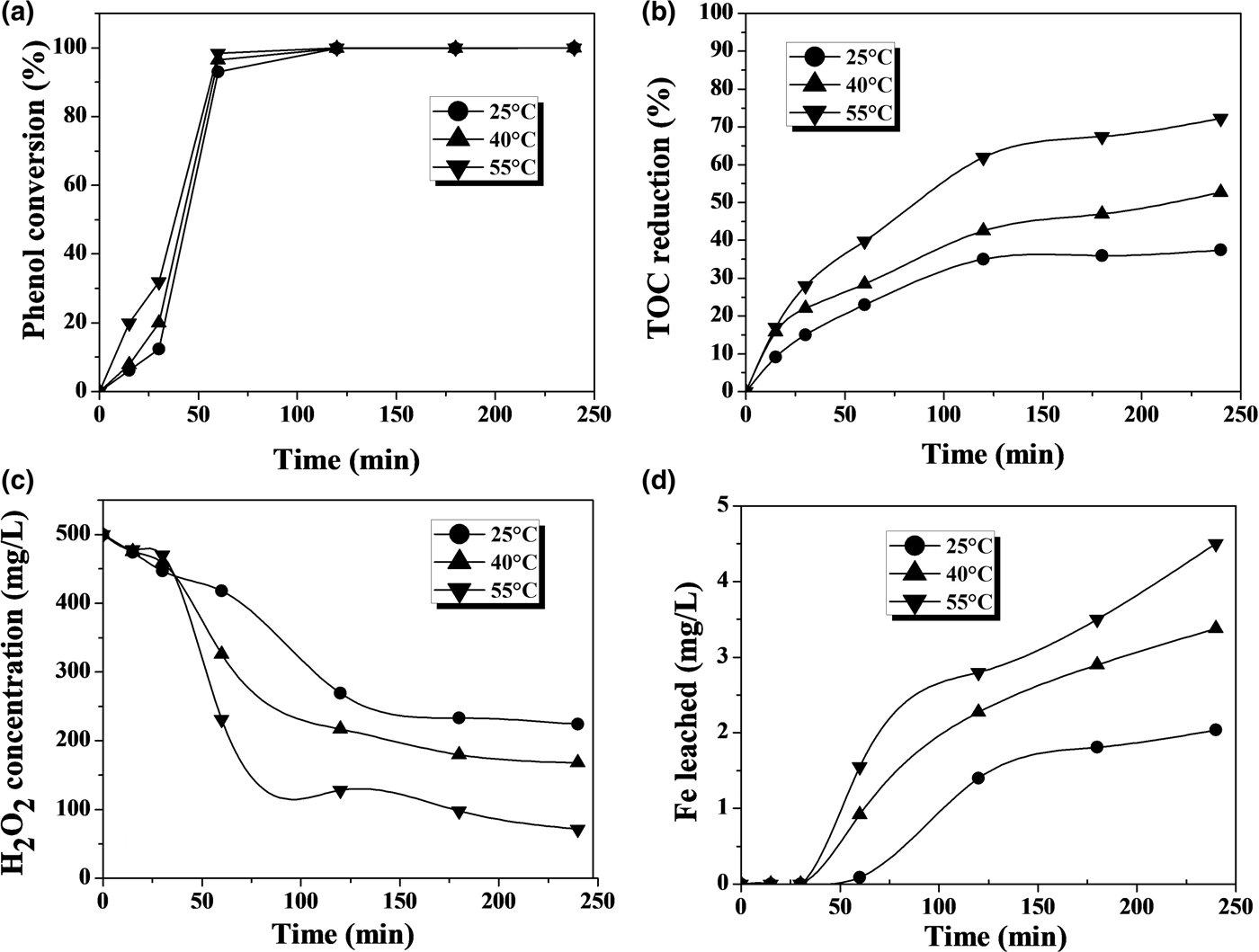
Fig. 3. CWPO of phenol with the Fe-KTM catalyst at various temperatures: (a) phenol conversion, (b) TOC reduction, (c) H2O2 concentration and (d) Fe leaching ([phenol]0: 100 mg/L; [H2O2]0: 500 mg/L; [Fe-KTM]: 1 g/L; pH0: 3.3).
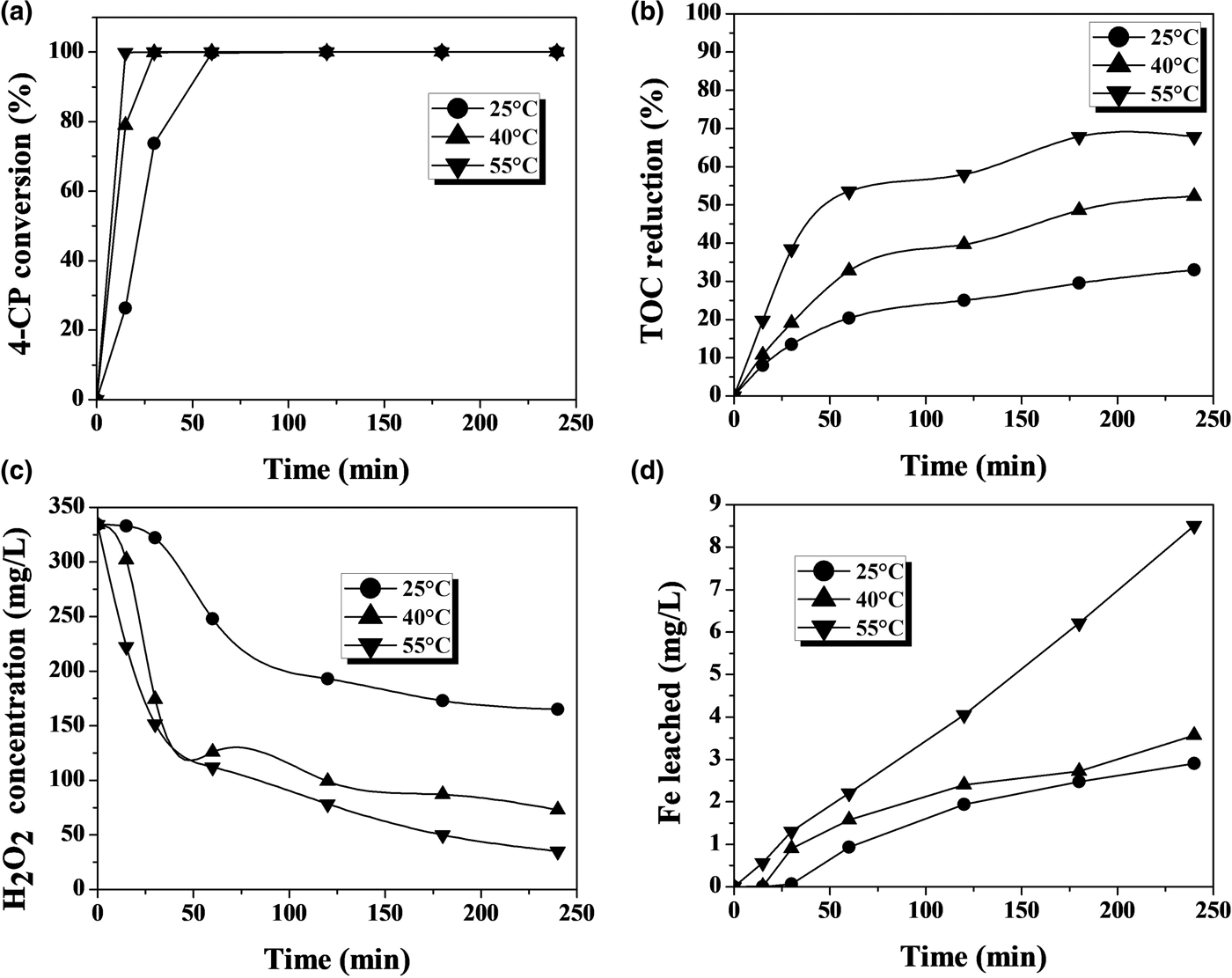
Fig. 4. CWPO of 4-CP with the Fe-KTM catalyst at various temperatures: (a) phenol conversion, (b) TOC reduction, (c) H2O2 concentration and (d) Fe leaching ([4-CP]0: 100 mg/L; [H2O2]0: 344 mg/L; [Fe-KTM]: 1 g/L; pH0: 3.3).
Temperature affected significantly the extent and rate of TOC reduction, with a dramatic improvement observed at 55°C compared to at ambient-like temperature (25°C). Again, a higher rate of TOC removal was observed in the case of 4-CP, although quite similar TOC removal percentages were finally achieved for both starting materials. This higher rate of TOC removal is consistent with the more rapid decomposition of H2O2 in the experiments with 4-CP. This may be due in part to the faster and greater iron leaching, which may lead to a somewhat greater contribution from the homogeneous reaction, which is promoted by dissolved iron. The effect is more pronounced at the highest temperature tested.
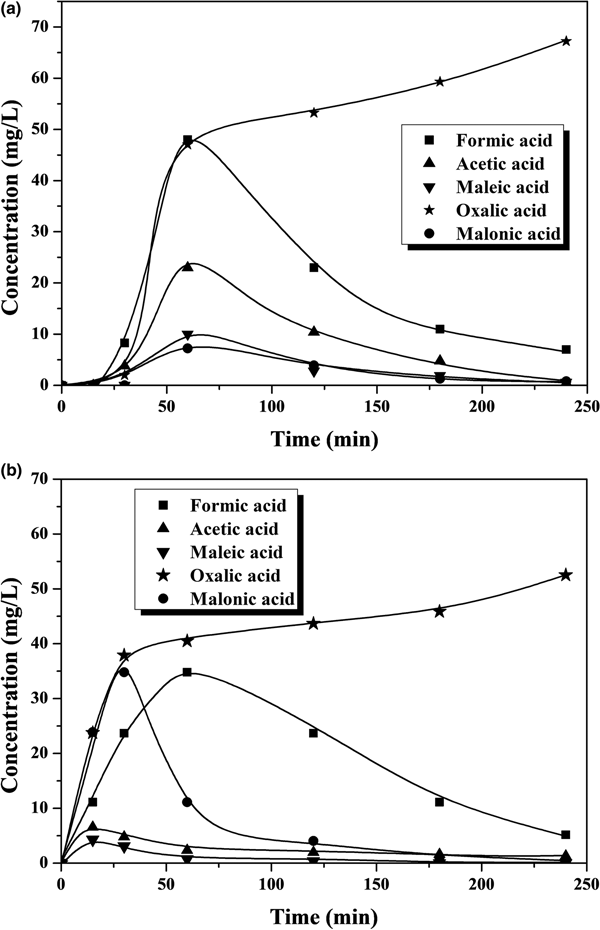
Iron leaching is a crucial issue regarding the stability of the catalyst, which is the main challenge so far in CWPO because the active catalysts developed have fairly poor durability in general. This work also showed evidence of iron leaching, which increases with temperature (Figs 3, 4). At the highest temperature tested, the iron loss was ~5% and ~9% of the initial load of the catalyst after 4 h of reaction with phenol and 4-CP, respectively. This is an important outcome, which, in previous works with various catalysts, has been attributed as a main cause of the presence of oxalic acid among the reaction products (Zazo et al., Reference Zazo, Casas, Mohedano and Rodriguez2006, Reference Zazo, Pliego, Blasco, Casas and Rodriguez2011; Molina et al., Reference Molina, Pizarro, Monsalvo, Polo, Mohedano and Rodrıguez2010, Reference Molina, Casas, Pizarro, Rodriguez, Humphrey and Boyd2011; Munoz et al., Reference Munoz, Dominguez, de Pedro, Casas and Rodriguez2017). In our case, this acid was the main by-product remaining in the reaction medium at reasonably high concentration values (Figs 5, 6). A blank experiment carried out with only the Fe-KTM material in water did not demonstrate iron leaching.
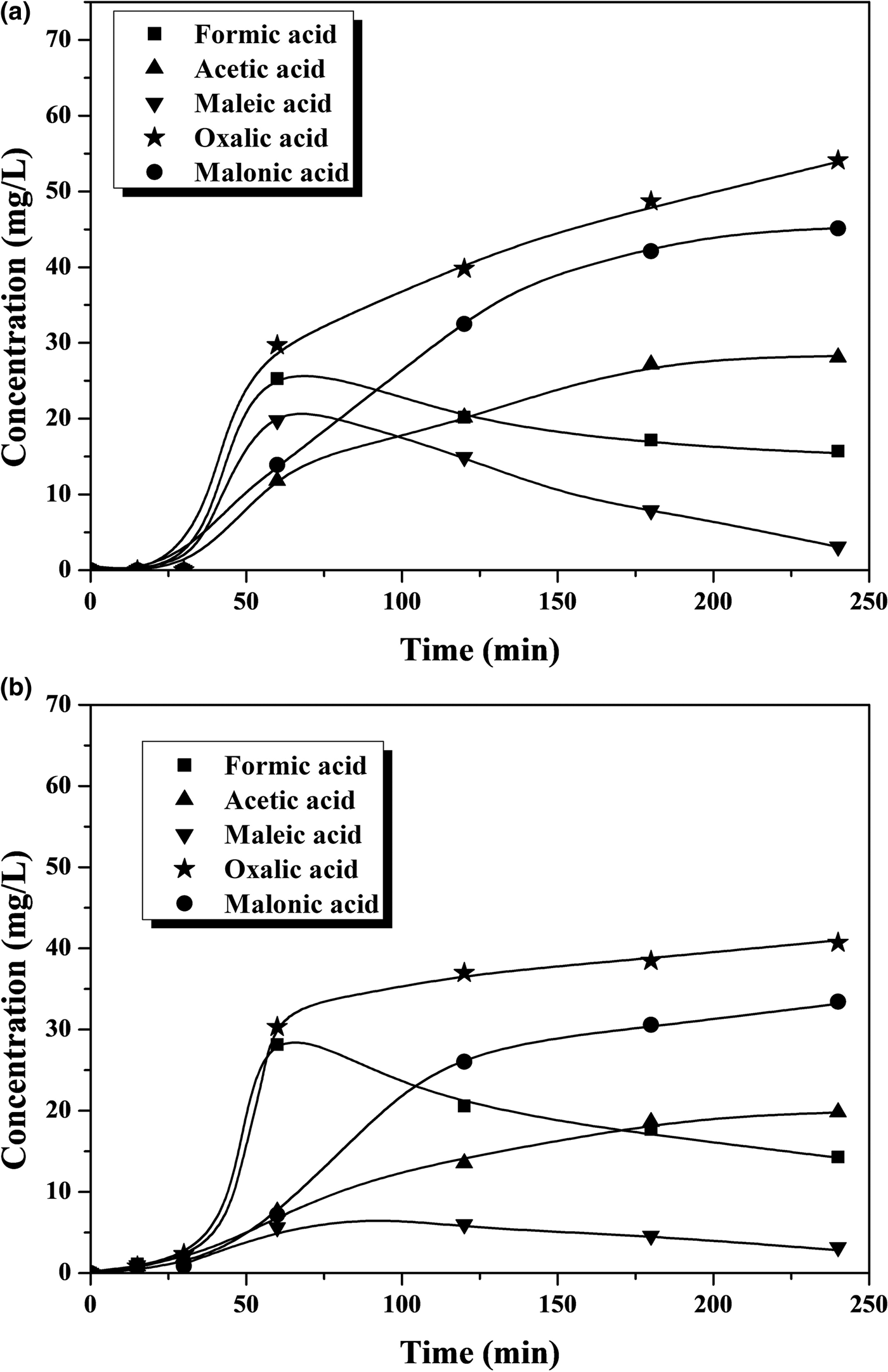
Fig. 5. Time course of short-chain carboxylic acids upon CWPO at 25°C of (a) phenol and (b) 4-CP.
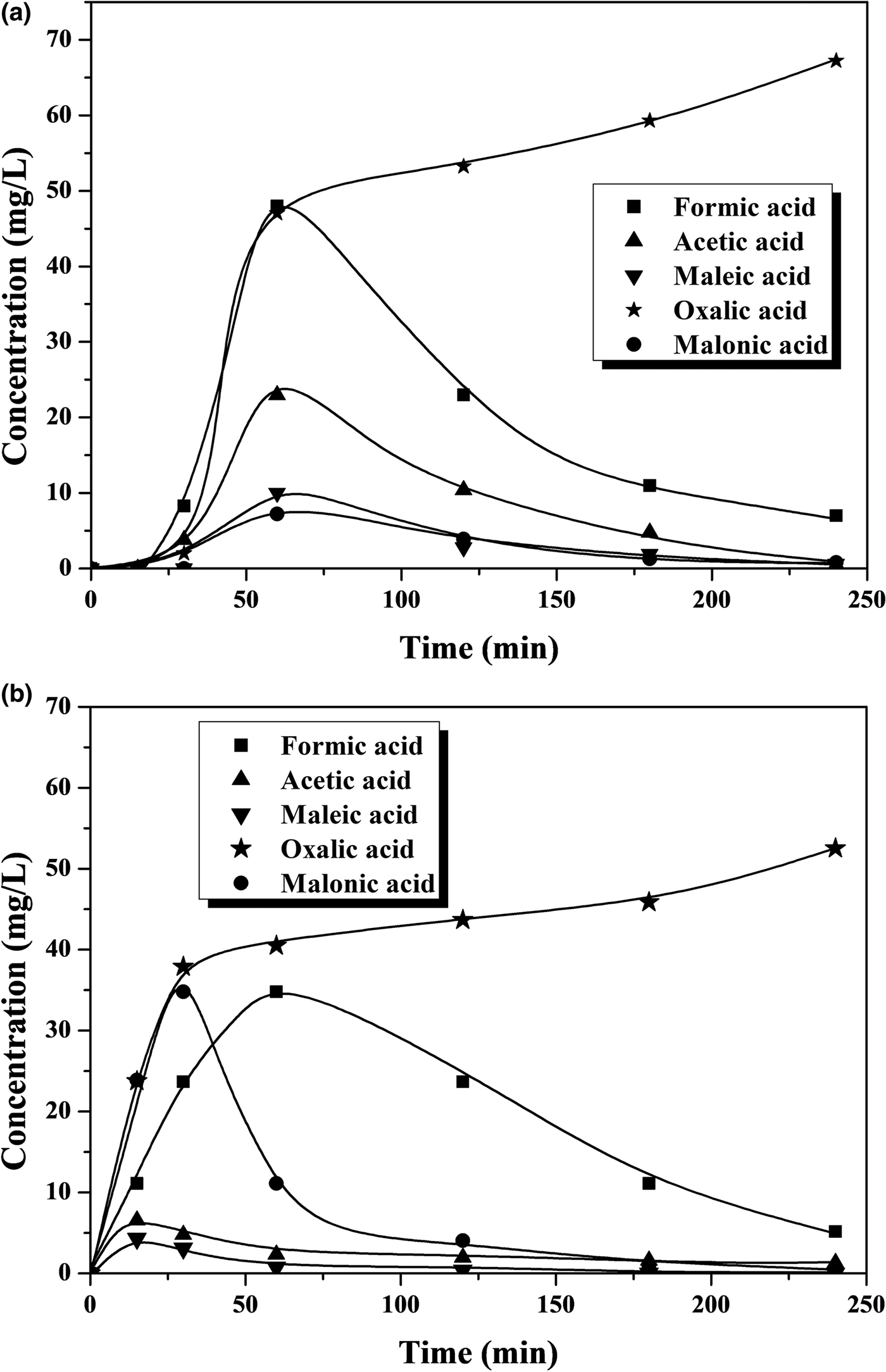
Fig. 6. Time course of short-chain carboxylic acids upon CWPO at 55°C of (a) phenol and (b) 4-CP.
The aromatic species commonly identified as intermediates from the Fenton process and CWPO of phenol and phenolic compounds (Zazo et al., Reference Zazo, Casas, Mohedano, Gilarranz and Rodriguez2005, Reference Zazo, Casas, Mohedano and Rodriguez2006) were not detected in the HPLC analyses of the final effluents in this study. Particular attention was paid to the highly toxic hydroquinone and p-benzoquinone, which were not detected even at the lowest temperature in the final samples after 4 h of reaction time (Figs 3, 4). In fact, even at that lowest temperature, the amount of carboxylic acids identified (Fig. 5) accounted for as much as 96% of the remaining TOC, thus indicating that the residual concentration of oligomeric condensation by-products should have been very low, if present at all. At 55°C, the amount of organic acids analysed after 4 h of experiments fitted almost completely with the residual TOC (Fig. 6). These findings are important given the very low ecotoxicity of those species (Zazo et al., Reference Zazo, Casas, Molina, Quintanilla and Rodriguez2007). From the results of this work, the reaction scheme proposed for CWPO of phenol and 4-CP with catalysts based on kaolin agree with previous findings for CWPO of these compounds (Molina et al., Reference Molina, Zazo, Casas and Rodrıguez2010; Catrinescu et al., Reference Catrinescu, Arsene and Teodosiu2011), namely oxidation of phenol or 4-CP to intermediate by-products (hydroquinone, benzoquinone and catechol) and further oxidation to short-chain carboxylic acids, CO2 and H2O.
Finally, CWPO of phenol and 4-CP has been assessed using various catalysts and experimental conditions (Table 2). Complete phenol conversion is reached in most of these cases, while 4-CP shows conversion values of between 40% and 100%. In this work, complete conversion of both pollutants was achieved. The TOC was reduced by 20–80%, and in this work ~70% of TOC conversion was achieved with both reagents. The active-phase leaching (generally iron, but also copper or cobalt) is higher in the CWPO of 4-CP, and in previous experiments, a substantial amount of metal leaching has been reported. In this work, 5% and 9% of leached iron were quantified in the CWPO of phenol and 4-CP, respectively; these values are similar to those reported in the literature. Previous experiments on CWPO with Fe-Al2O3 catalysts demonstrated that iron leaching is produced mainly during the first hours of treatment (Bautista et al., Reference Bautista, Mohedano, Casas, Zazo and Rodriguez2011; Munoz et al., Reference Munoz, Dominguez, de Pedro, Casas and Rodriguez2017). This iron leaching is associated with the weak interaction between some iron phases and the support. Therefore, modified kaolin displayed good catalytic activity compared with previous works, and its use as a support in CWPO seems to be promising due to its high availability and low cost.
Table 2. Summary of the CWPO of phenol and 4-CP with various catalysts.
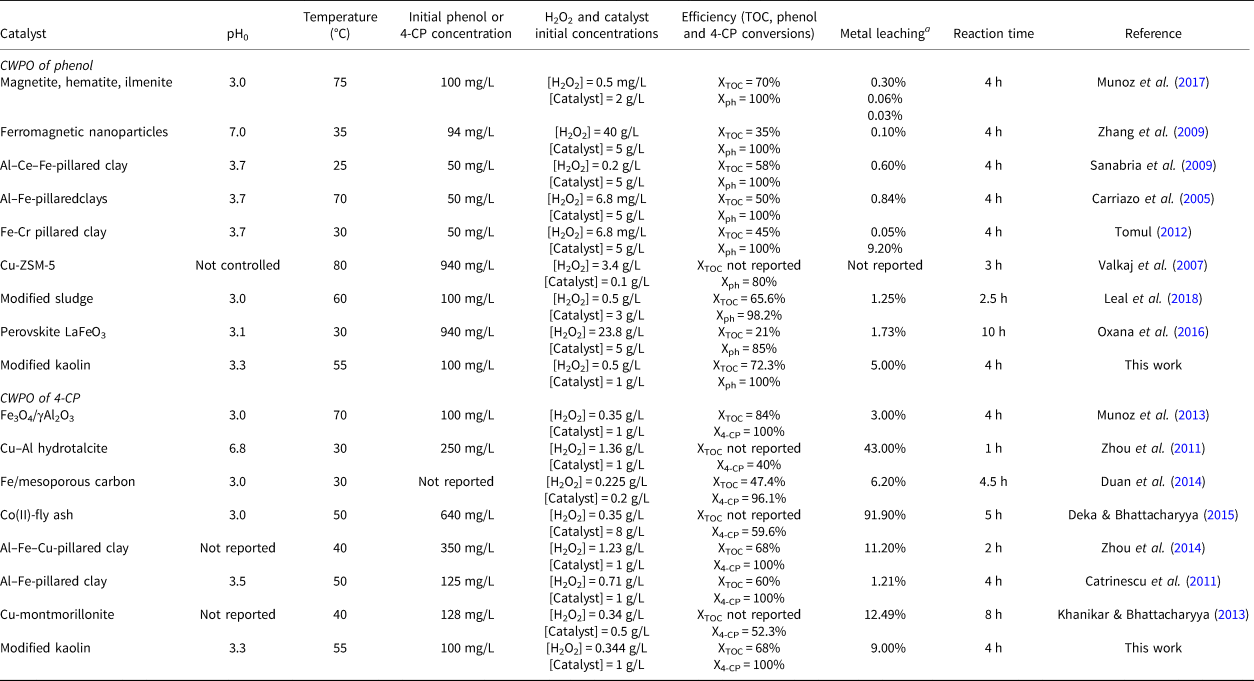
a Data refer to an initial catalyst concentration of 1 g/L.
Conclusions
The naturally occurring KT may be considered as a potential raw material for the preparation of inexpensive and environmentally friendly CWPO catalysts. Physical and chemical treatments, including calcination, followed by acidic and alkaline attack, allowed us to obtain a substrate with high S BET (>100 m2/g) corresponding mainly to mesopores. Further impregnation with FeCl3 yielded an active material for CWPO. By using phenol and 4-CP (100 mg/L initial concentration) as target compounds, ~70% TOC reduction was achieved after 4 h of reaction time at 55°C with the stoichiometric amount of H2O2. At that temperature, iron leaching from the catalyst took place at moderate but significant rates (5% and 9% of the initial iron load after 4 h in the experiments with phenol and 4-CP, respectively). Further research is needed to obtain greater knowledge regarding the stability of the catalyst for improving this critical feature. In addition, experience should be gained with other hazardous target pollutants and representative industrial wastewaters so as to assess the potential application of this catalyst in CWPO.
Author ORCIDs
Carmen Belen Molina, 0000-0001-8006-3359.
Acknowledgements
The authors are grateful for the financial support from the Spanish MINECO through project CTQ2016-78576-R and from the Ministère Algérien de l'Enseignement Supérieur et de la Recherche Scientifique. The authors also thank Daniel Collado for the English-language revision.