Subaortic membranes can cause haemodynamically significant obstruction in children, particularly in the presence of concomitant CHD. While the development of subaortic membranes has been attributed to turbulent flow in the left-ventricular outflow tract due to associated congenital defects, an undefined portion of patients with subaortic membranes do not have additional congenital heart defects.Reference Cilliers 1 We sought to examine factors associated with reoperation and the degree of aortic valve regurgitation as a potential long-term source for reoperation.
Materials and methods
This single institution case–control study was approved by the Baylor College of Medicine Institutional Review Board, with waiver of informed consent.
Definitions
The diagnosis of subaortic stenosis was made by post-natal echocardiography with confirmation of membranous obstruction with intra-operative findings. Isolated subaortic membranes were defined as being in the absence of any other pathology that would independently lead to surgical intervention. For this reason, the presence of a patent foramen ovale did not exclude a patient from inclusion in this study. Cases were defined as patients requiring reoperation for subaortic membrane resection (n = 13). Controls included the remaining patients who underwent resection of an isolated subaortic membrane but have not required reoperation (n = 71). Indications for operation or reoperation for isolated subaortic membrane at our institution include: 1) mean left-ventricular outflow tract gradient >40 mmHg or 2) presence of left-ventricular hypertrophy, new aortic regurgitation, or development of symptoms.
Patients
Patients included all patients who had resection of an isolated subaortic membrane at Texas Children’s Hospital between October 1995 and April 2018 and were younger than 18 years old at the time of their first isolated subaortic membrane resection. In total, 84 patients met the inclusion criteria and were followed into adulthood (Fig 1). Measurements were obtained from transthoracic echocardiogram prior to the day of operation. Intra-operative findings were collected from the operative reports. During the study period, subaortic membranes were collected for structural analysis and septal muscle was sent to the biorepository; therefore, pathologic weights and measures were not available for retrospective analysis.

Figure 1. Establishment of study population.
Operation
A subaortic resection with a septal myectomy is performed in a consistent manner in our institution. After initiation of cardiopulmonary bypass and complete diastolic arrest of the heart, the left heart is vented through either the right upper pulmonary vein or a surgically created atrial septal defect. An oblique (hockey-stick-shaped) aortotomy is performed. A limited septal myectomy is carried out by creating parallel incisions in the ventricular septum starting at the nadir of the right coronary cusp, and a second incision at the left–right commissure. The septal muscle between these two incisions is then excised using scissors (about 1 × 1 cm). The subaortic membrane is then dissected from its attachments using a combination of blunt and sharp dissections and resected. The left-ventricular outflow tract is then inspected to assure a visually adequate resection and myectomy. The ventricular vent is discontinued, and the ventricle is irrigated with copious amounts of cold saline. The left ventricle is de-aired, and the aortotomy incision is closed using running polypropylene suture. Bypass is weaned, and a post-operative transesophageal echocardiogram is performed before decannulation and protamine administration.
Statistics
Statistical analysis was performed using R version 3.3.2 (“Sincere Pumpkin Patch,” Vienna, Austria 2016). Continuous variables were compared using Student’s t-test or Wilcoxon Rank Sum test, as appropriate. Categorical data were compared using Pearson’s χ2 Test or Fisher’s Exact Test, as appropriate. McNemar’s test was used to evaluate paired categorical data, such as the degree of aortic regurgitation at various time points. Univariate time-to-event analyses were performed using Kaplan–Meier methods with log-rank tests for categorical variables and univariate Cox models for continuous variables. Alpha was set at a value of 0.05.
Results
Median age at operation for the cohort was 6.6 years old (interquartile range [IQR] 4.0–11.1) with a median weight of 24.4 kg (IQR 16.5–45.7). Males accounted for 69% (n = 58) of the patient cohort. Median time from the first documented observation of a subaortic membrane to surgical resection was 1.4 (IQR 0.3–3.7) years. Median follow-up time for all patients was 4.5 years (IQR 1.2–8.8). Reoperation was required in 13 (15%) patients. In total, 98 operations were performed for the 84 patients with 12 patients requiring one reoperation and 1 patient requiring two reoperations. Median time to reoperation was 5.0 years (IQR 1.9–9.0). Table 1 outlines complete demographic data for the population.
Table 1. Baseline patient characteristics for the entire patient population.

IQR = interquartile range.
In comparing baseline characteristics, patients requiring reoperation were less likely to be males (38% versus 75%, p = 0.02) and more likely to be younger (3.6 years versus 7.6 years, p = 0.0003) and smaller (weight in kg 16.2 versus 27.5, p = 0.002) at the time of their initial operation compared to patients who did not require reoperation. Patients requiring reoperation tended to be younger at the time of initial diagnosis, with decreased time from diagnosis to initial operation, relative to patients who did not require reoperation. Baseline demographic comparisons are available in Table 2.
Table 2. Comparison of baseline characteristics between patients who required reoperation and those who did not. Patients requiring reoperation were more likely to be females, were younger at operation, and weighed less than patients who did not require reoperation. Patients requiring reoperation had a non-significant trend towards being younger at diagnosis and having less time between diagnosis and surgery than patients who did not require reoperation.

IQR = interquartile range.
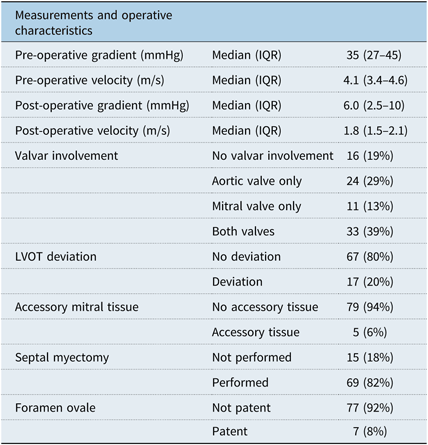
The median left-ventricular outflow tract gradient was 35.0 (IQR 27.0–45.0) mmHg pre-operatively and 6.0 (IQR 2.5–10.0) mmHg post-operatively. There was not a significant linear relationship between pre-operative and post-operative gradients for the whole group (r2 = 0.20, p = 0.06), but the relationships in the myectomy (r2 = 0.24, p = 0.052) and non-myectomy (r2 = −0.25, p = 0.38) subgroups were inversed (Fig 2). Membrane involvement of the aortic or mitral valves was present in 24 (29%) and 11 (13%) patients, respectively, with both valves involved in 33 (39%) patients. A septal myectomy was performed in 69 (82%) patients. A patent foramen ovale was present in seven (8%) patients. A summary of all intra-operative findings is located in Table 3. One patient (1%) required a temporary pacemaker post-operatively and no patients have required a permanent pacemaker.

Figure 2. Scatter plot of pre-operative and post-operative mean left-ventricular outflow tract gradients. Black circles are the non-myectomy group, while gray triangles are the myectomy group. Lines of best fit are overlaid.
Table 3. Pre- and post-operative measurements and intra-operative characteristics for the entire patient population.
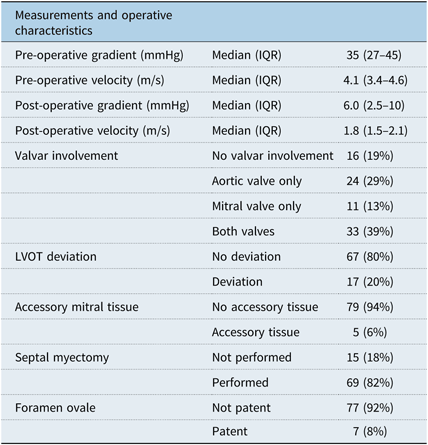
IQR = interquartile range; LVOT = left ventricular outflow tract.
There was no difference between the two groups in pre-operative gradient, valvar involvement of the membrane, or septal hypertrophy. Patients requiring reoperation were more likely to have a patent foramen ovale (31% versus 4%, p = 0.0098) than patients who did not require reoperation. Comparison of intra-operative findings is included in Table 4.
Table 4. Comparison of pre- and post-operative measurements and intra-operative characteristics between patients who required reoperation and those who did not. Patients requiring reoperation had more patent foramen ovales. Patients requiring reoperation had a trend towards higher post-operative left-ventricular outflow tract gradients and fewer myectomies; however, there was not enough data to conclude there was a true difference between the groups.

IQR = interquartile range; LVOT = left ventricular outflow tract.
Freedom from reoperation
At the median follow-up time of 4.5 years, actuarial freedom from reoperation was 90% (Fig 3). By univariate time-to-event analysis, being male (p = 0.03) and having a septal myectomy (p = 0.004) were associated with greater freedom from reoperation. The presence of a patent foramen ovale (p < 0.0001) was associated with decreased freedom from reoperation.

Figure 3. Freedom from reoperation for the entire patient population. Actuarial freedom from reintervention at the median follow-up time of 4.5 years was 90%.
Change in aortic valve regurgitation
Pre-operatively, 69 (82%) patients had mild (65, 77%) or moderate (4, 5%) aortic valve regurgitation, which improved on post-operative echocardiogram to 48 (57%) patients with mild aortic valve regurgitation and only 1 patient (1%) with moderate aortic regurgitation (p = 0.0007 for effect of treatment). The patient with moderate aortic regurgitation was a 3-year-old girl, whose regurgitation had decreased to mild after discharge and has remained mild at 3.5 years post-operatively. At last follow-up, only 22 (26%) patients remained without any aortic valve regurgitation and 8 (10%) patients had moderate regurgitation. There was overall worsening of aortic valve regurgitation to last follow-up from post-operatively (p = 0.01); however, the degree of aortic valve regurgitation was not different from pre-operatively (p = 0.18). Overall, 18 (21%) patients had improvement in the degree of aortic valve regurgitation at last follow-up from pre-operatively and 14 (17%) had worsening in the degree of aortic valve regurgitation at last follow-up from pre-operatively (Fig 4). The presence of moderate aortic valve regurgitation was independent of the performance of a myectomy during subaortic membrane resection (p = 0.62)

Figure 4. Stacked bar plot of changes in aortic valve regurgitation at the pre-operative, post-operative, and last follow-up time points. Overall, patients had lessened degrees of aortic valve regurgitation in the post-operative period than pre-operatively or at last follow-up, with no overall difference between pre-operative and last follow-up.
Conclusions
In this study, we aimed to evaluate risk factors for reoperation in patients with isolated subaortic membranes. Performing a septal myectomy was associated with longer freedom from reoperation. It was also noted that the degree of aortic valve regurgitation improved post-operatively when compared to the pre-operative state, with return to pre-operative condition on long-term follow-up. The objective of identifying risk factors for operation or reoperation for subaortic stenosis, including isolated subaortic membrane, has been viewed in a variety of ways. Our experience adds operative findings and intra-operative decision-making into the model for associations with reoperation, including the performance of a septal myectomy. Septal myectomy relieves the offending obstruction and reduces turbulent flow in the left-ventricular outflow tract and restores the normal left-ventricular outflow tract geometry, possible etiologic components of subaortic membranes.Reference Cilliers 1 While a septal myectomy increases the freedom from reoperation, there is an associated risk of conduction disruption. Therefore, it is the decision of the operating surgeon if a septal myetomy is to be performed and how much tissue to remove, resulting in an 82% myectomy rate in our sample.
Our study is contradictory to several prior reports. Although the authors in one study found an association between septal myectomy and increased freedom from reoperation, the authors had concluded that performance of a septal myectomy also improved the function of the aortic valve.Reference Tefera, Gedlu and Bezabih 2 In our patient population, we failed to identify an association between a septal myectomy and long-term aortic valve function, suggesting that all patients remain at risk for long-term aortic valve deterioration. Additionally, other reports suggest an increased pre-operative gradient as having an association with reoperation, Reference Karamlou, Gurofsky and Bojcevski3–Reference Ruzmetov5 but we were unable to replicate this finding in our data. As there is no correlation between pre-operative and post-operative mean left-ventricular outflow tract gradients, this association is difficult to reconcile.
Previous authors have suggested no correlation between pre-operative and post-operative gradients.Reference Tefera, Gedlu and Bezabih 2 While we also failed to identify a correlation between pre-operative and post-operative gradients, we identified an inverse relationship between the correlations for patients with myectomy compared to patients without myectomy and would suggest the same inverse relationship likely exists in the prior author’s data, based on the plot presented in their manuscript. Our study additionally noted the association of a patent foramen ovale with a decrease in freedom from reoperation. We suspect that this is due to an artificial decrease in the left-ventricular outflow tract gradient from a potential left to right shunt, resulting in underestimation of the degree of obstruction. Finally, the involvement of the mitral and aortic valves has been cited as having an association with reoperation. Reference Karamlou, Gurofsky and Bojcevski3,Reference Geva, McMahon, Gauvreau, Mohammed, del Nido and Geva4 We did not find an association between involvement of the mitral and aortic valves, separately and combined, as having an association with reoperation.
The primary limitation of this study was its retrospective nature, limiting the uniformity of data collection. Furthermore, the heterogeneity of all patients undergoing resection of a subaortic membrane allowed data manipulation through covariate selection. We attempted to increase homogeneity by restricting our population to isolated membranes; however, with only 13 events in a population of 84, the power to detect meaningful associations was limited.
In patients with isolated subaortic membranes, performance of a septal myectomy can minimise risk for reoperation when controlling for age. Patients should be serially monitored for degradation of the aortic valve, even if aortic regurgitation is not present post-operatively.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional review board of the Baylor College of Medicine.