Introduction
Gene transformation technology and knowledge of genomics have allowed generating new genotypes more effectively in a short period of time. However, compared with our increasing capacity to generate new genotypes, our technologies for phenotyping and screening are still far behind to meet the needs of modern breeding due to the unavailability of high-throughput phenotyping methods under field conditions and high costs of field screening. The recent introduction of non-destructive phenotyping methods based on plant phenomics reveals technical possibilities of meeting the needs (Rascher et al., Reference Rascher, Blossfeld, Fiorani, Jahnke, Jansen, Kuhn, Matsubara, Märtin, Merchant and Metzner2011). Among the techniques introduced, thermal image and chlorophyll fluorescence analyses may be suitable for high-throughput screening (HTS) considering their implementation time and cost-effectiveness. Chen et al. (Reference Chen, Huang and Jackson2005) used thermal image analysis to study drought stress responses in corn and soybean. The results of previous studies suggest that thermal image analysis can be used for screening water stress tolerance in genetic resources and breeding water stress-tolerant varieties.
Soybean (Glycine max) is known to be susceptible to salt stress (Katerji et al., Reference Katerji, van Hoorn, Hamdy and Mastrorilli2000). Screening salt tolerance in soybean can be done using non-destructive methods that measure phenomic responses of soybean in response to salt stress. Among the non-destructive methods based on phenomic responses, thermal image analysis may be the most effective one as thermal response is directly related to water status in plant. Therefore, this study demonstrated the feasibility of phenomic evaluation of salt stress responses in soybean by establishing a non-destructive method based on plant thermal images for screening salt tolerance.
Materials and methods
Seedlings of soybean (G. max cv. Daewon) at the second trifoliate (V2) stage were transplanted into 250 ml plastic pots. Treatment with 0, 12.5, 25, 50 and 100 mM NaCl was carried out to induce salt stress. The experiment consisted of a randomized block with four replicates. Photosynthesis rate, stomatal conductance (Li-COR 6400, Li-COR, Lincoln, Nebraska, USA) and chlorophyll contents (SPAD-502, Minolta, Tokyo, Japan) were measured in every leaflet in the first trifoliate stage and chlorophyll fluorescence (Handy PEA, Hansatech Instrument, King's Lynn, Norfolk, UK) was measured after 1 h of dark adaption as ordinary physiological non-destructive parameters together with visual growth at 0, 1 and 2 d after NaCl treatment (DAT). Plant leaf temperature (T420, FLIR systems Inc., Täby, Sweden) was also measured at 0, 1 and 2 DAT. Thermal images were analysed using FLIR Tools 3.1 (FLIR systems Inc., USA) and MATLAB 8.1 (The MathWorks Inc., Natick, Massachusetts, USA) to determine soybean leaf temperature. All statistical analyses were carried out using SAS 9.3.
Results and discussion
Among the five physiological parameters, photosynthesis rate, stomatal conductance and chlorophyll fluorescence (F v/F m) were significantly decreased with an increase in NaCl concentration (Table S1, available online). A significant decrease was found at 50 and 100 mM NaCl concentrations from even 1 DAT, compared with the control, and it became much more significant at 2 DAT. No significant changes in chlorophyll contents and visual damage (data not shown) were observed until 2 DAT. Soybean leaf temperature increased significantly at 50 and 100 mM NaCl concentrations at 2 DAT (Fig. 1).

Fig. 1 Thermal images (A) and leaf temperature (B) of soybean at 2 d after NaCl treatment. The vertical line indicates the standard error of four replications.
Chlorophyll fluorescence is widely used for estimating the quantum yield in photosynthesis in vivo (Genty et al., Reference Genty, Briantais and Baker1989). Chlorophyll fluorescence values were significantly correlated with photosynthesis (P< 0.001; Table 1). Consequently, chlorophyll fluorescence can help determine the photosynthetic performance of soybean under salt stress.
Table 1 Summary of correlation analysis results for non-destructive parameters of soybean treated with NaCla
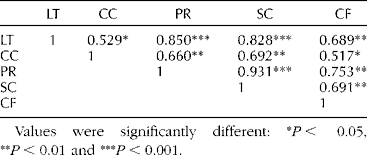
LT, leaf temperature; CC, chlorophyll contents; PR, photosynthesis rate; SC, stomatal conductance; and CF, chlorophyll fluorescence.
Values were significantly different: *P< 0.05, **P< 0.01 and ***P< 0.001.
a The parameters were measured at 2 d after NaCl treatment.
Leaf temperature was significantly correlated with other parameters such as photosynthesis rate and stomatal conductance (P< 0.001; Table 1). Leaf temperature is closely related to photosynthetic performance including stomatal movement (Jossier et al., Reference Jossier, Kroniewicz, Dalmas, Le Thiec, Ephritikhine, Thomine, Barbier-Brygoo, Vavasseur, Filleur and Leonhardt2010) and useful for estimating stomatal conductance (Jones, Reference Jones1999) and transpiration rate (Katul et al., Reference Katul, Ellsworth and Lai2000). Together, our findings and previous reports suggest leaf temperature to be an indicator of the whole plant status or physiological responses under salt stress.
Physiological parameters can be used for determining responses to salt stress within 2 DAT in soybean (Table S1, available online). Leaf temperature is a parameter that is easier and faster to measure than the other parameters, suggesting that it can be used for screening salt stress tolerance in soybean. Sirault et al. (Reference Sirault, James and Furbank2009) screened salt-tolerant wheat and barley using thermal image analysis. Furbank and Tester (Reference Furbank and Tester2011) emphasized that thermal imaging can be applied to HTS to save time and cost and thus to improve phenotyping and crop breeding.
Compared with genotyping technology (e.g. next-generation sequencing), phenotyping technology is still far behind in terms of its speed and capacity (White et al., Reference White, Andrade-Sanchez, Gore, Bronson, Coffelt, Conley, Feldmann, French, Heun, Hunsaker, Jenks, Kimball, Roth, Strand, Thorp, Wall and Wang2012) and thus does not meet the technical demands of phenotyping-based screening. Fortunately, many efforts have been made using phenomic screening methods, the so-called next-generation phenotyping (Cobb et al., Reference Cobb, DeClerck, Greenberg, Clark and McCouch2013). Jones et al. (Reference Jones, Serraj, Loveys, Xiong, Wheaton and Price2009) quantified the response of rice and grapevine to water deficit using thermal infrared image analyses at the field level. Balota et al. (Reference Balota, Payne, Evett and Peters2008) demonstrated canopy temperature depression (CTD) during day and night and the relationship between CTD and other physiological and morphological traits, resulting in the confirmation of drought tolerance in wheat lines at the field level. To acquire more accurate information of water use efficiency, McAusland et al. (Reference McAusland, Davey, Kanwal, Baker and Lawson2013) combined thermal and chlorophyll fluorescence image information. Recently, field-based phenotyping has been implemented using a semi-automated technology (Comar et al., Reference Comar, Burger, de Solan, Baret, Daumard and Hanocq2012). Physiological and phenomic responses of soybean to salt stress and close correlations between thermal images and other parameters suggest that thermal image analysis can be used for assessing salt tolerance in soybean genetic resources and for screening salt-tolerant soybean. Although further studies are required to be carried out, thermal responses of soybean to salt stress can contribute to HTS in soybean breeding programmes.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1479262114000422
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project title: Development of High Throughput Screening (HTS) Technology based on Crop Phenomics, Project No. PJ00907801), Rural Development Administration, Republic of Korea.




