Hazardous chemicals are utilized widely nowadays for various industrial applications. Chemical spills occur during the production, storage and transportation of hazardous substances. These may pose a severe threat to the public and the environment. The risk of hazardous liquids has been reduced by various techniques, including dispersion (Kujawinski et al., Reference Kujawinski, Kido Soule, Valentine, Boysen, Longnecker and Redmond2011), in situ burning (Fritz, Reference Fritz2003), sorption (Melvold & Gibson, Reference Melvold and Gibson1988; Chabot et al., Reference Chabot, Higgins, Yu, Xiao, Chen and Zhang2014) and bioremediation (Singh et al., Reference Singh, Bhattacharya, Channashettar, Jeyaseelan, Gupta, Sarma, Mandal and Lal2012). Most of these methods produce secondary chemical pollutants. However, sorption may clean up these hazards without serious side effects. The use of inert sorbents is an efficient and promising approach for the mitigation of hazardous spillages.
A great number of sorbents have been investigated for cleaning up spills of petroleum products using natural minerals such as zeolites, clays and diatomaceous materials (Bastani et al., Reference Bastani, Safekordi, Alihosseini and Taghikhani2006; Medeiros et al., Reference Medeiros, Sansiviero, Araújo and Lago2009; Bandura et al., Reference Bandura, Woszuk, Kolodynska and Fronus2017), natural organics such as wood fibres, feathers and wool (Radetic et al., Reference Radetic, Ilic, Radojevic, Miladinovic, Jocic and Jovancic2008; Rajaković-Ognjanović et al., Reference Rajaković-Ognjanović, Aleksić and Rajaković2008), synthetic polymers (Zhu et al., Reference Zhu, Qiu, Jiang, Wu and Zhang2011; Yati et al., Reference Yati, Aydin and Sonmez2016) and macroporous carbon materials (Bi et al., Reference Bi, Xie, Yin, Zhou, Wan, He, Xu, Banhart, Sun and Ruoff2012; Wu et al., Reference Wu, Li, Liang, Zhang, Wang, Chen and Yu2014; Ge et al., Reference Ge, Shi, Wang, Zhao, Yao, Zhu, Zhang, Zhu, Wu and Yu2017; Ren et al., Reference Ren, Li and Lv2017). Although advanced carbon materials exhibited a greater sorption capacity for oil and organic chemicals than other sorbents, there are still several technical and practical issues relating to their application. Most carbon sorbents are unsatisfactory at sorption of aqueous chemicals due to the highly hydrophobic nature of carbon. Their macroporous structures are destroyed by the fires and explosions that often accompany chemical accidents, and they become inactive under high-temperature conditions. The problem of burning is more serious when using organic-based sorbents such as natural organics and synthetic polymers. Furthermore, cost-effective sorbents are required in large quantities to mitigate large-scale chemical accidents. Some advanced sorbents based on carbon macrostructures (Bi et al., Reference Bi, Xie, Yin, Zhou, Wan, He, Xu, Banhart, Sun and Ruoff2012; Wu et al., Reference Wu, Li, Liang, Zhang, Wang, Chen and Yu2014; Ren et al., Reference Ren, Li and Lv2017) and synthetic polymer composites (Zhu et al., Reference Zhu, Qiu, Jiang, Wu and Zhang2011) with excellent sorption capabilities are available in sponge or mat forms. Nevertheless, expensive equipment and long processing times hamper the large-scale production of these advanced sorbents. It is a challenge to develop affordable, environmentally harmless sorbents with acceptable sorption capacities and excellent thermal/chemical stabilities.
Vermiculite is a natural 2:1 phyllosilicate formed by the hydrothermal alteration of mica. Vermiculite flakes are exfoliated or expanded to produce concertina-shaped granules by thermal shock at ~1000°C (El Mouzdahir et al., Reference El Mouzdahir, Elmchaouri, Mahboub, Gil and Korili2009; Marcos et al., Reference Marcos, Arango and Rodriguez2009; Marcos & Rodríguez, Reference Marcos and Rodríguez2010). The exfoliation relies on an impulsive build-up of the steam produced by evaporating interlayer water within the mosaic-like multiphase lamellar structures (Hillier et al., Reference Hillier, Marwa and Rice2013). Expanded vermiculite has remarkable properties: it is lightweight and has low thermal conductivity, it is chemically inert, has a high melting point and good sorption ability due to its capillary pore structure and active silicate surface (Valášková & Martynková, Reference Valášková, Martynková, Valaškova and Martynkova2012; Rashad, Reference Rashad2016). These properties are required in order for ideal sorption of liquid hazards to work effectively under harsh conditions. While the absorption characteristics of expanded vermiculite have been investigated extensively for pollutant removal from aqueous solutions (Duman et al., Reference Duman, Tunç and Polat2015; Marcos & Rodríguez, Reference Marcos and Rodríguez2016; Bourliva et al., Reference Bourliva, Sikalidis, Papadoulou, Betsiou, Michailis, Sikalidis and Filippidis2018), there has been very limited study of the absorption mitigation of hazardous chemical spillages (Melvold & Gibson, Reference Melvold and Gibson1988). Recently, ultrathin layered silicates were incorporated into graphene oxide beads to produce a fire-retardant sorbent for cleaning up hazardous chemicals (Bao et al., Reference Bao, Bi, Zhang, Zhao, Wang, Yue and Yang2016). The hybrid graphene oxide beads were synthesized using a long coagulation process followed by freeze-drying. The graphene oxide ingredient was calcined at >550°C. Moreover, the involvement of coagulation agents may generate toxic, volatile by-products through chemical reactions with the absorbed hazards. Therefore, a cheap and inert sorbent is still desired for the mitigation of large-scale spillages of hazardous chemicals.
The main objective of this work is to study the relationship between the structural parameters (particle dimensions and textural properties) and sorption performance of chemically inert, thermally stable vermiculite for evaluating its potential as a general-purpose sorbent for land-based spillages of hazardous chemicals. To the best of our knowledge, the dependence of sorbent performance on structural features has not been studied in detail for expanded vermiculite, although the effects of the sorbent's dimensions and textural properties have been investigated in synthetic zeolite produced from fly ash (Bandura et al., Reference Bandura, Franus, Józefaciuk and Franus2015, Reference Bandura, Panek, Rotko and Franus2016). Here, commercially available Palabora vermiculite was further classified according to its particle size and thickness to prepare more homogeneous samples. The textural features of expanded vermiculite obtained at different heating temperatures were characterized for the size-selected samples using material analysis techniques. The sorption behaviour was investigated in terms of the sorption capacity, removal efficiency and imbibition rate to verify the importance of textural control in the sorption process. Furthermore, the expanded vermiculite with an optimized structure was confirmed to be a universal sorbent for various hazardous liquids (hydrophilic/hydrophobic organic chemicals and strongly acidic/basic aqueous solutions). The affordable vermiculite sorbent exhibited both a fast imbibition speed and an excellent removal efficiency through the optimization of particle dimensions and pore-size distribution.
Materials and methods
Materials and chemicals
Three different grades (medium, superfine and micro) of raw vermiculite (Palabora, South Africa) were obtained from Shingsung Mineral (South Korea). Ethanol, toluene, benzene, methyl ethyl ketone, furfuryl alcohol, acrylic acid, ethyl acetate, m-cresol and ammonium hydroxide (28 wt.%) were purchased from Daejung Chemicals & Metals (South Korea). Methanol and allyl chloride were obtained from Junsei Chemical (Tokyo, Japan). 2-Nitrotoluene and nitric acid (30 wt.%) were purchased form Acros Organics (India) and Merck Millipore (Germany), respectively. All chemicals were of reagent grade and used without further purification.
Preparation of expanded vermiculite
The commercially available vermiculites had a broad particle-size distribution (micro = 0.25–0.71 mm; superfine = 0.35–1.00 mm; medium 1.40–4.00 mm). To improve dimensional homogeneity, the vermiculite was classified according to its planar size using a sieving machine and was then further categorized by thickness through a weight-based separation process. The dimensions of the separated samples are summarized in Table 1. The planar size and thickness were determined as average values obtained by measuring 50 vermiculite flakes. Thermal expansion experiments were performed by using a tube furnace at temperatures of 400°C, 700°C and 1000°C. To generate a thermal shock, a crucible containing vermiculite was loaded quickly into the furnace stabilized at a pre-set temperature and removed after 5 min. The expanded vermiculite was stored in a desiccating cabinet and dried at 150°C in a vacuum oven for 1 h before use. The density of the sample was measured using a balance (GX-200, A&D, Japan) equipped with a density-determination kit (GX-13, A&D). The expansion ratio was calculated using Eq. (1).
where D 0 and D are the densities of crude and expanded vermiculite, respectively. The expansion ratio was determined by averaging the values obtained from three measurements.
Table 1. Summary of the average dimensions of raw vermiculite used in this work.

a The mean and standard deviation values were obtained from measuring 50 samples.
Material characterization
The microstructure and atomic composition were determined by scanning electron microscopy (SEM; Hitachi S-4800), using a SEM device equipped with an energy-dispersive X-ray spectrometer (EDS). To probe crystalline structures, X-ray diffraction (XRD) traces were obtained by using an X-ray diffractometer (Rigaku D/MAX-2500) with Cu-Kα1 (λ = 1.54056 Å) radiation at 40 kV, 100 mA and a scanning step of 0.02°2θ. Mercury porosimetry (AutoPore IV 9500, Micromeritics, USA) was used to characterize the specific surface area, pore volume and pore-size distribution.
Evaluation of sorption performance
The sorption was characterized in terms of the sorption capacity, removal efficiency and imbibition rate of liquid chemicals. To determine the sorption quantity, 1.0 g vermiculite samples were placed in a meshed stainless steel cell with square openings of 0.5 mm. The cell was fully immersed in a vessel containing a test solvent and then shaken at a rate of 60 rpm for 30 min, permitting the liquid chemical to penetrate into the pores of the vermiculite. Then it was lifted out from the container and held until the interval between successive drops was >10 s. Subsequently, the meshed cell was placed on absorbing paper to further remove excess solvent. The vermiculite absorbent was discharged from the cell and spread over a polyethylene terephthalate film to eliminate the excess liquid that is present in the voids among the particles. Shortly afterwards, the weight of the sample absorbed (M abs) was recorded using a balance, and the dry mass (M dry) was quantified after drying in a vacuum oven at 150°C for 1 h. Finally, the sorption capacity, defined by the relative weight of absorbed liquid to sorbent, was calculated using Eq. (2).
Three measurements were performed to obtain the average sorption capacity and its variation.
The removal efficiency was quantified using the percentage weight of removed liquid substance with respect to the initial amount. For this purpose, 10 g of vermiculite and 20 g of liquid chemical were used. The liquid was poured into a cylindrical reservoir and its initial weight (M 0) was measured precisely using a balance. A stainless steel mesh container with a 0.5 mm gap was filled with a sample and was then introduced into the chemical-containing vessel for 10 min. The sorbent container was lifted out and held until the interval between successive drops was >10 s. The weight of the remaining solvent (M) was recorded. The removal efficiency was calculated using Eq. (3).
The efficiency depends on the dimensions of both the liquid container and the sample cell. In this work, the outside diameter of the cell was ~10 mm smaller than the inside hole of the reservoir (diameter ~55 mm).
The imbibition rate was evaluated from the capillary rise speed of water in a vertical configuration along a glass tube (diameter = 8 mm) packed with expanded vermiculite (Kirdponpattara et al., Reference Kirdponpattara, Phisalaphong and Newby2013). To achieve a densely packed column, 0.5 g of vermiculite was placed manually in a glass tube blocked with a Parafilm® layer at the bottom, and the sample-containing tube was dropped 100 times from a height of ~20 cm. This procedure was repeated three more times for the packing of a 2 g sample. After detaching the Parafilm, the vermiculite-packed column was mounted vertically on a scaffold connected to a balance (GX-200, A&D). A suspended water vessel was raised manually using a jack until the bottom of the column was just in contact with the water. The uptake mass of spontaneously imbibed water was recorded as a function of time.
Results and discussion
Thermal expansion behaviours
A photograph of vermiculite samples thermally treated at 400°C, 700°C and 1000°C is shown in Fig. 1a, together with an untreated sample. They were prepared using the same amount of sample S1 with a planar size of 0.84 ± 0.14 mm and a thickness of 150 ± 50 µm. A distinct volume increase at >700°C was noted in terms of the expansion or exfoliation of raw vermiculite, as has been previously reported for vermiculite with a layered structure (El Mouzdahir et al., Reference El Mouzdahir, Elmchaouri, Mahboub, Gil and Korili2009; Marcos & Rodríguez, Reference Marcos and Rodríguez2010). To quantify the degree of expansion, the expansion ratio was determined for various samples with dissimilar dimensions at different treatment temperatures. Figure 1b shows the temperature dependence of the expansion ratio for vermiculite with three different dimensions (samples S1–S3 in Table 1), exhibiting greater expansion both for larger samples and at higher temperatures. The highest value was 7.1 for sample S3 heated at 1000°C with an average planar size of 4.2 mm. The temperature and size dependences of the expansion ratio may be explained by the exfoliation mechanism proposed by Hillier et al. (Reference Hillier, Marwa and Rice2013): the expansion results from a build-up of steam pressure formed by the release of interlayer water in mosaic-like intergrown vermiculite during rapid heating. The evaporation of interlayer water was confirmed by XRD, as described below. Because larger vermiculite has more mosaic-layer structures acting as a maze for the escaping gaseous molecules, it may expand intensively due to the effective retention of the steam generated. The thermal expansion of vermiculite takes place when the build-up of pressure exceeds the bonding forces between the layers.
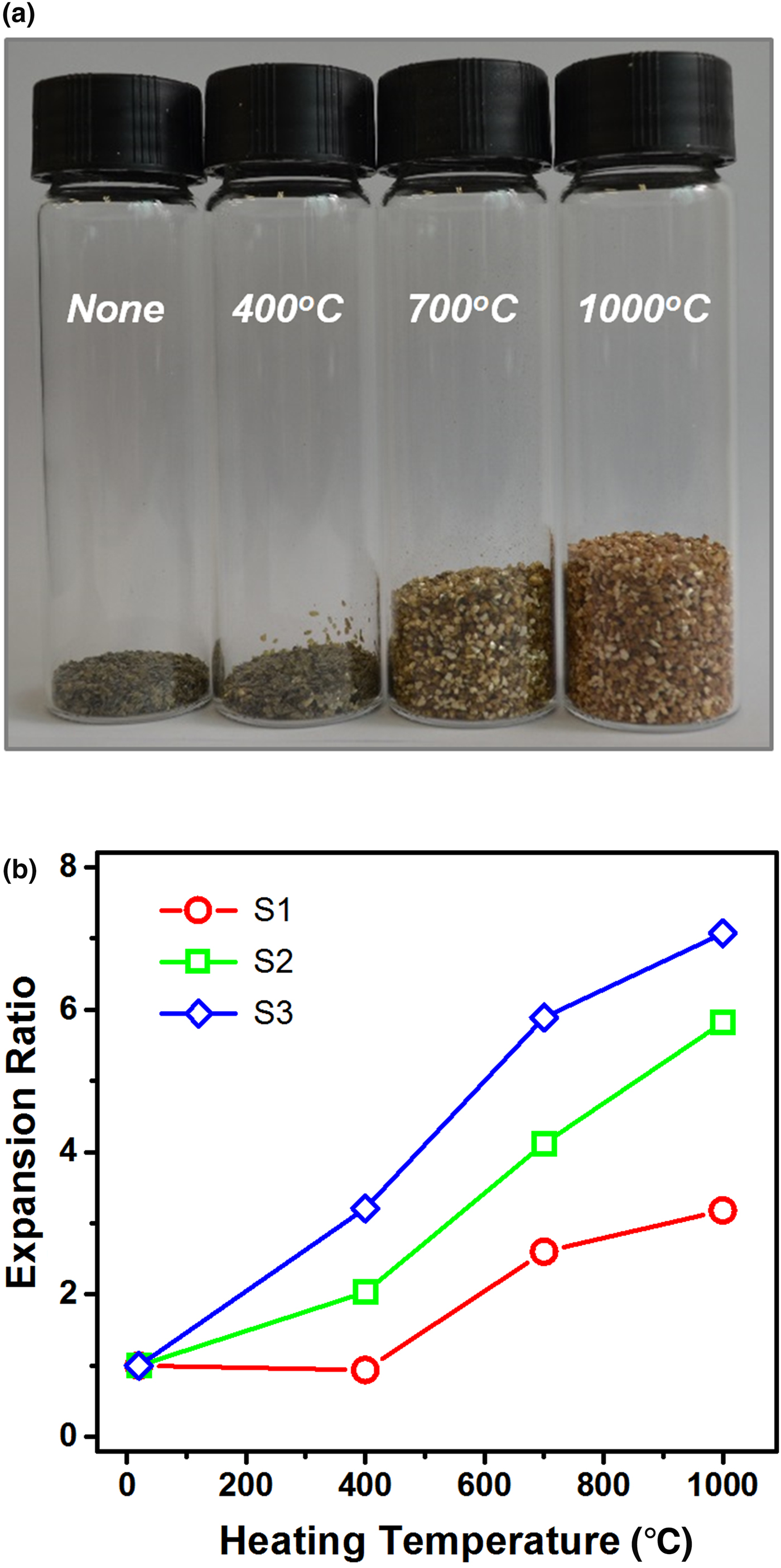
Fig. 1. (a) Photograph of thermally treated vermiculite at the designated temperatures using sample S1. (b) Curves of the expansion ratios as a function of the heating temperature for the three samples S1–S3 with different dimensions. Each expansion ratio was determined by averaging the values obtained from three measurements.
Material characterization
Material properties of expanded vermiculite were examined to understand the exfoliation process in detail. Figure 2a shows a typical SEM image of one vermiculite particle (sample S1) expanded at 1000°C, illustrating a delaminated structure with different gaps. The delaminated morphology with slit-shaped porosity clearly indicates the intensive volume expansion in terms of thickness without fragmentation via thermal shock. The degree of expansion was significantly heterogeneous from particle to particle. The chemical composition of the vermiculite used was probed by EDS analysis (Fig. 2b). The composition obtained is summarized in the inset table in Fig. 2b, which agrees with previously reported results (Marcos et al., Reference Marcos, Arango and Rodriguez2009; Marcos & Rodríguez, Reference Marcos and Rodríguez2010). The high K2O content at 8 wt.% suggests that the dominant phase of Palabora vermiculite corresponds to the interstratification of mica and vermiculite layers (hydrobiotite) rather than pure vermiculite. The hydrobiotite had been reported to provide a greater propensity for exfoliation than true vermiculite (Hillier et al., Reference Hillier, Marwa and Rice2013). The high degree of expansion may be attributed to the interstratified structure that is responsive to the extensive formation of the mosaic-like intergrown layer.
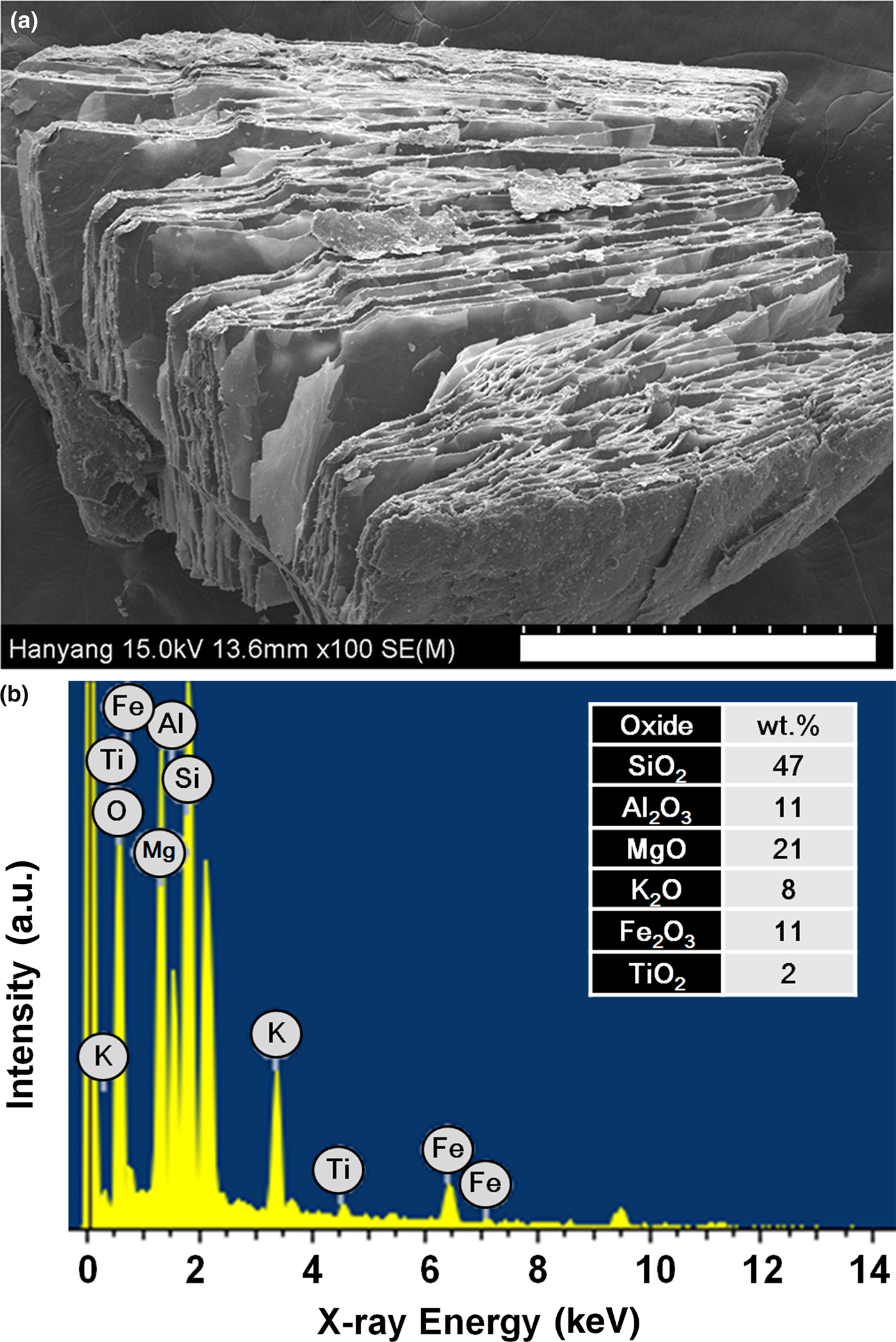
Fig. 2. (a) SEM image and (b) EDS spectrum of vermiculite expanded at 1000°C using sample S1. The scale bar in (a) corresponds to 500 µm.
The amount of interlayer water was monitored by means of the first-order basal spacing of layered vermiculite. Powder XRD traces of crude and expanded vermiculite (sample S1) in a low 2θ angle region are shown in Fig. 3. The raw material exhibits three intense reflections at 6.13°, 7.00° and 7.43° and a weak peak at 8.73°, corresponding to the interplanar distances of 1.44, 1.26, 1.19 and 1.01 nm, respectively. They had previously been assigned to different water-layer hydration states (WLHS): 2-WLHS for 1.44 nm, 2/1-WLHS for 1.26 nm, 1-WLHS for 1.19 nm and 1/0-WLHS for 1.01 nm (Suzuki et al., Reference Suzuki, Wada, Hines and Whittingham1987; Udoudo et al., Reference Udoudo, Folorunso, Dodds, Kingman and Ure2015). Therefore, the crude sample may be interpreted as showing the coexistence of highly hydrated states, while the 1000°C sample is dominated by the less hydrated 1/0-WLHS. The dehydrated peak is significantly enhanced at >700°C, which is in good agreement with the temperature dependence of the expansion ratio (Fig. 1b). These observations support the expansion mechanism based on the thermal dehydration of interlayer water.

Fig. 3. Powder XRD traces of vermiculite expanded at different temperatures of 400°C, 700°C and 1000°C together with the crude form. Sample S1 was used for these experiments.
The size distribution of slit holes in expanded vermiculite is a key factor in many useful applications, such as thermal insulation, water retention and the absorption of soundwaves. The pore-size distributions characterized by Hg porosimetry are shown in Fig. 4. Sample S1 (planar size = 0.84 mm) heated at 400°C exhibits one dominant peak centred at ~250 µm, while sample S1 heated at 1000°C displays a bimodal distribution with an additional maximum at 1.9 µm. Because the 400°C sample displayed negligible exfoliation, a pore diameter of >100 µm was assigned to artificial pores caused by the void spaces between particles (Giesche, Reference Giesche2006). The interparticle pore diameters are frequently associated with the packing conditions of the particles. On the other hand, the 1.9 µm peak at 1000°C can be interpreted as the void gap between delaminated layers in expanded vermiculite. A featureless population in the middle region of 5–100 µm is also regarded as being a result of the slit holes with conical openings, indicating a broad macroporous size distribution within expanded vermiculite. A pore-size distribution of sample S2 with a planar size of 2.4 mm was characterized in order to examine the effect of vermiculite particle size. The larger vermiculite particles exhibited a stronger peak at the larger pore size of ~3 µm. The pore volumes and areas obtained for various samples by the Hg porosimeter are summarized in Table 2. Extensive separation among the silicate layers is possible when large vermiculite flakes expand at high temperatures.

Fig. 4. Distributions of the pore size probed by Hg porosimetry for different samples and temperatures: sample S1 expanded at 400°C, sample S1 expanded at 1000°C and sample S2 expanded at 1000°C.
Table 2. Summary of the pore volumes and pore areas determined by Hg porosimetry.

Liquid-absorbing characteristics of expanded vermiculite
Fundamental absorption characteristics were quantified in terms of the sorption capacity, removal efficiency and imbibition rate for non-toxic ethanol. Figure 5a shows the variation curves of the sorption capacity as a function of heating temperature for three samples with different particle dimensions (samples S1–S3). The sorption capacity increased steadily for all samples when the temperature increased, as observed in the expansion ratio (Fig. 1b). However, the temperature dependence of the sorption capacity was quantitatively different from that of the expansion ratio for the samples with a different size. For example, the sorption capacities of the samples prepared at 1000°C are similar at ~1.9 g g−1, while the expansion ratio of sample S3 is 2.2 times larger than that of sample S1. Because during absorption the liquid is taken inside the spaces of the expanded vermiculite, the sorption capacity can be more directly correlated with the pore volume determined by Hg porosimetry. Figure 5b presents the variation in sorption capacity with respect to pore volume, which displays a good proportional relationship for the smallest sample S1. This result implies that the sorption capacity depends heavily on the total pore volume of a sample, including intraparticle and interparticle voids. Nevertheless, the dependence of the sorption capacity on pore volume became weak for samples of different sizes, suggesting the involvement of additional factors in the sorption process.
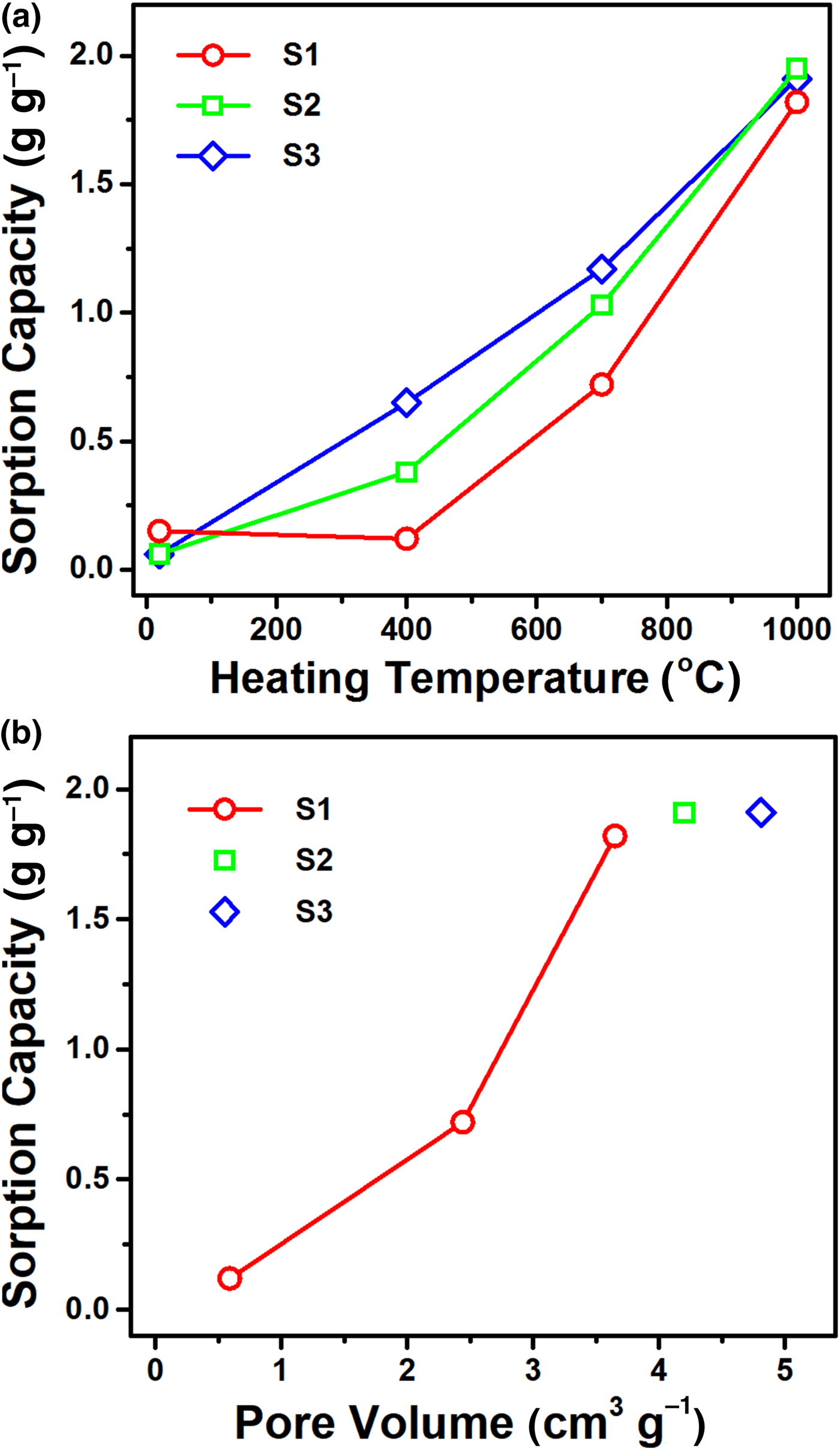
Fig. 5. (a) Plots of the sorption capacities as a function of heating temperature for samples S1–S3. (b) Variation of the sorption capacities with respect to pore volume. Three measurements were performed to determine average values of the sorption capacities.
In order to further clarify the dimension-dependent change, the expansion ratio and the sorption capacity at 1000°C were determined for vermiculite samples SS1–SS6, having different planar sizes in the range of 0.9–6 mm and similar thicknesses of ~270 µm. Figure 6 shows the variations in the expansion ratio and sorption capacity with respect to planar size, demonstrating a weak size dependence of the sorption capacity despite the steady increase in the expansion ratio. A plausible explanation for this may be the different efficiencies of liquid uptake according to the particle size. Interparticle spaces >1 mm not spontaneously infilled by ethanol were observed in large samples (planar size >4 mm), which implies a low uptake efficiency into very large pores. The thermal shock produces numerous slit pores with a triangle-like inward shape. When the planar particle size was large, ethanol would be partially infilled into the triangular pores and mainly retained at the corners of these pores due to the surface tension of the liquid (Mason & Morrow, Reference Mason and Morrow1991). Therefore, the dimensional features of intraparticle/interparticle pores are considered to play a key role in controlling the sorption behaviour.
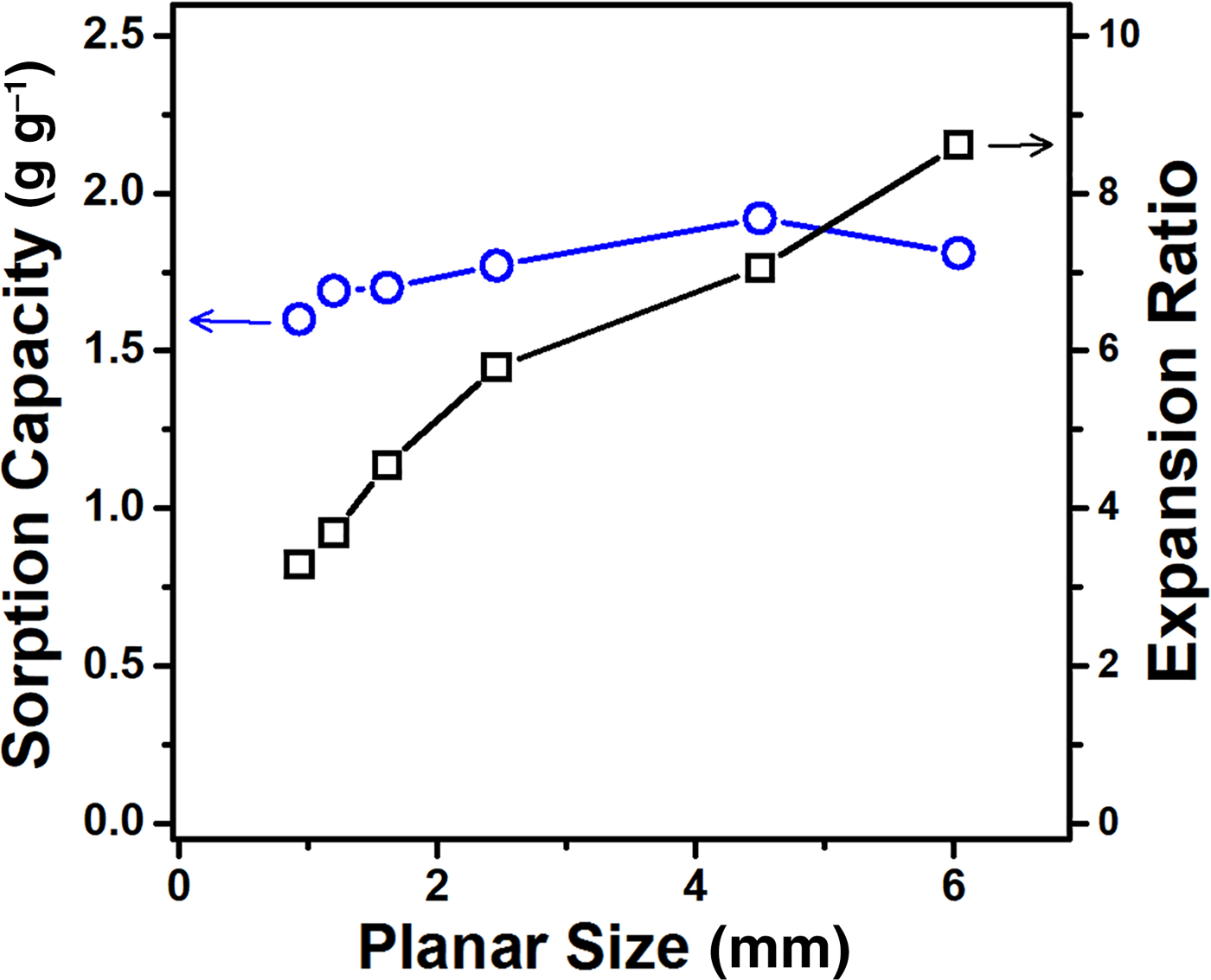
Fig. 6. Variations of the sorption capacities and the expansion ratios with respect to the planar size of vermiculite. Three measurements were performed to determine average values of the sorption capacities and the expansion ratios.
The removal efficiency was examined in order to evaluate the applicability of expanded vermiculite to the fast, spontaneous mitigation of hazardous chemicals via sorption. Figure 7a shows the variations in removal efficiency of ethanol as a function of the heating temperature, demonstrating a maximum value for the smallest sample S1 expanded at 1000°C. These variations indicate that the removal efficiency is heavily dependent on the particle size together with the heating temperature. The enhancement of removal efficiency at higher temperatures may be explained by the additional generation of intraparticle pores, resulting in larger pore volumes. At 1000°C, the removal efficiencies were 92.3 wt.% for sample S1, 47.8 wt.% for sample S2 and 38.0 wt.% for sample S3. The thermally untreated vermiculite has considerable uptake efficiency in the range of 12–26 wt.%. These results suggest the involvement of the void spaces between vermiculite particles for liquid adsorption. The excellent removal efficiency of small vermiculite particles suggests the occurrence of capillarity-driven sorption into interparticle/intraparticle pores. In order to examine the size dependence of sorption in more detail, the removal efficiency was also evaluated for samples SS1–SS6 expanded at 1000°C. Figure 7b shows a plot of removal efficiency vs. planar size, which exhibits a reciprocal-like curve. The size-dependent variation might be correlated with the capillary pressure (P c):
where r eff is the effective radius of the void channels in expanded vermiculite, γ is the surface tension and θ is the contact angle. Because tiny vermiculite particles develop intraparticle/interparticle pores with small radii, a strong capillary force may result in a high removal efficiency. The size-dependent variation is at odds with the trend observed in the sorption capacity, which may be attributed to the difference in the key controlling factors: pore volume for the sorption capacity and pore size for the removal efficiency.
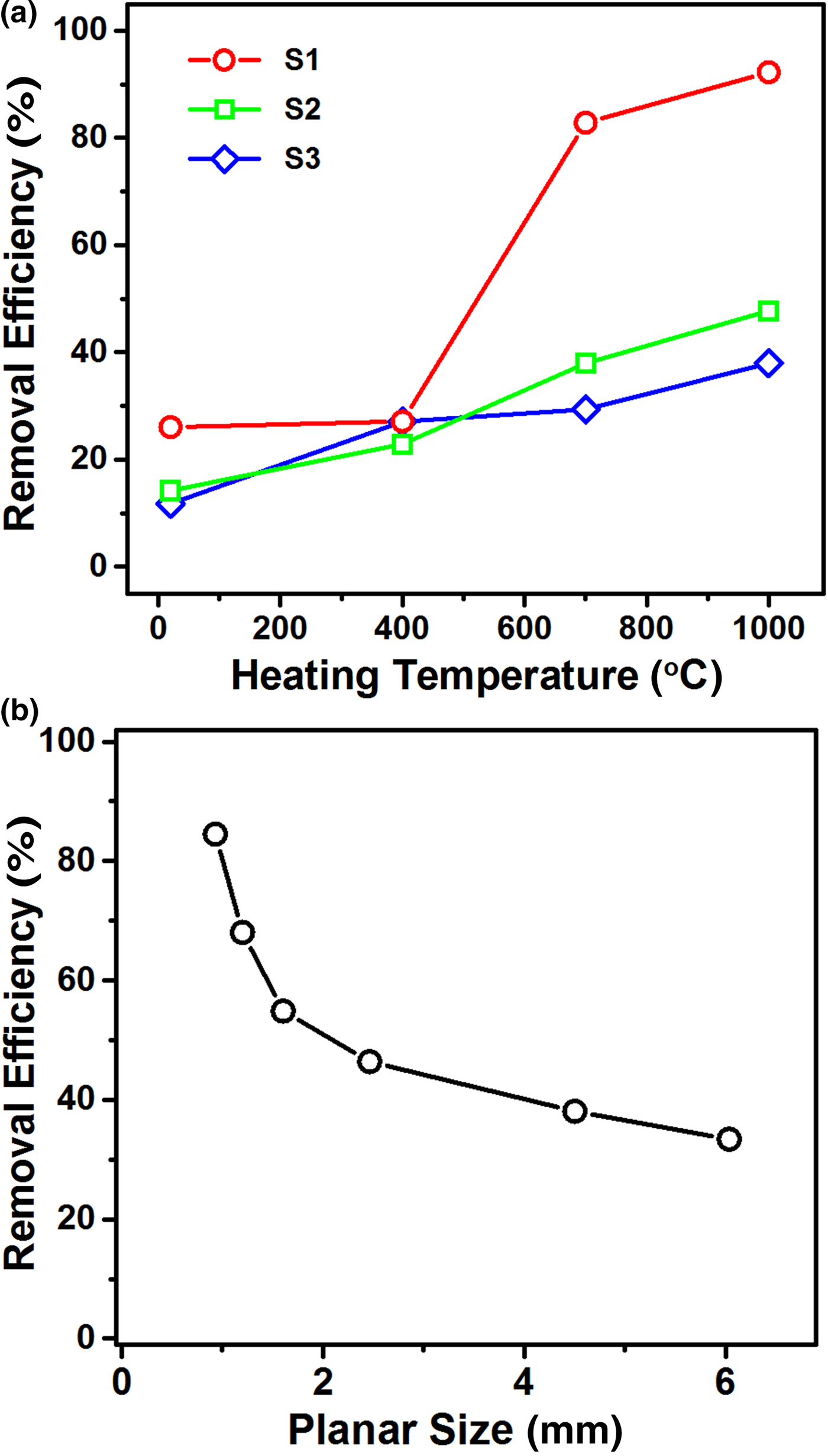
Fig. 7. (a) Plots of removal efficiencies as a function of the heating temperature for samples S1–S3. (b) Curve of the removal efficiency with respect to the planar size of expanded vermiculite.
In order to better understand the size dependence of removal efficiency, the wicking dynamics of water were studied using a glass column packed with expanded vermiculite. Figure 8 shows the time-dependent variations of a squared water uptake mass for samples S1–S3, which display a linear increase in squared mass only during an initial period of ~10 s. Afterwards, the wicking rate was greatly reduced and the uptake mass approached a saturated value within several minutes. The smallest sample S1 exhibited faster imbibition than samples S2 and S3, indicating a strong dependence of the uptake rate on the sample dimension. The saturated water uptake weights after 15 min were 2.6 g for the small sample S1, 1.1 g for the medium sample S2 and 0.6 g for the large sample S3. A stabilized uptake mass (m s) is possible under balanced conditions between the capillary pressure (P c) and the hydrostatic pressure (P h = ρgh). The stabilized m s value can be expressed by the relationship m = ρAhε as:
where A is the cross-sectional area of the glass tube, ε is the porosity of the packed expanded vermiculite, ρ is the liquid density and g is the gravitational acceleration. In separated experiments, the porosity was quantified to show little variation (<5 vol.%) for the three different columns. Assuming that the density and the contact angle are constant for the same type of liquid and sorbent, the greater uptake mass of sample S1 is predominantly ascribed to the narrower effective radius (r eff) for water flow. Furthermore, the wicking kinetics into porous expanded vermiculite is described by the modified Lucas–Washburn equation (Kirdponpattara et al., Reference Kirdponpattara, Phisalaphong and Newby2013):
where m is the weight of an imbibed liquid, C is the geometric factor, η is the viscosity of the liquid and t is the elapsed time after the contact with water. The Lucas–Washburn theory may explain the linear change of the squared uptake mass in the first short interval. The deviation from the model equation for a long period of elapsed time may be interpreted as being due to the wicking kinetics, which in turn are strongly affected by parameters such as a broad size distribution of the voids (Kirdponpattara et al., Reference Kirdponpattara, Phisalaphong and Newby2013), a delaminated slit-like pore shape (Nishi et al., Reference Nishi, Iwashita, Sawada and Inagaki2002) and heterogeneity in the packing density (Dang-Vu & Hupka, Reference Dang-Vu and Hupka2005). The consecutive packing processes created heterogeneity in the column density, along with loose connections at the top and large voids near the tube wall due to a wall effect (Mehta & Hawley, Reference Mehta and Hawley1969). As a result, the pore-size distribution formed by expanded vermiculite is a critical factor in regulating the liquid removal process based on spontaneous wicking.
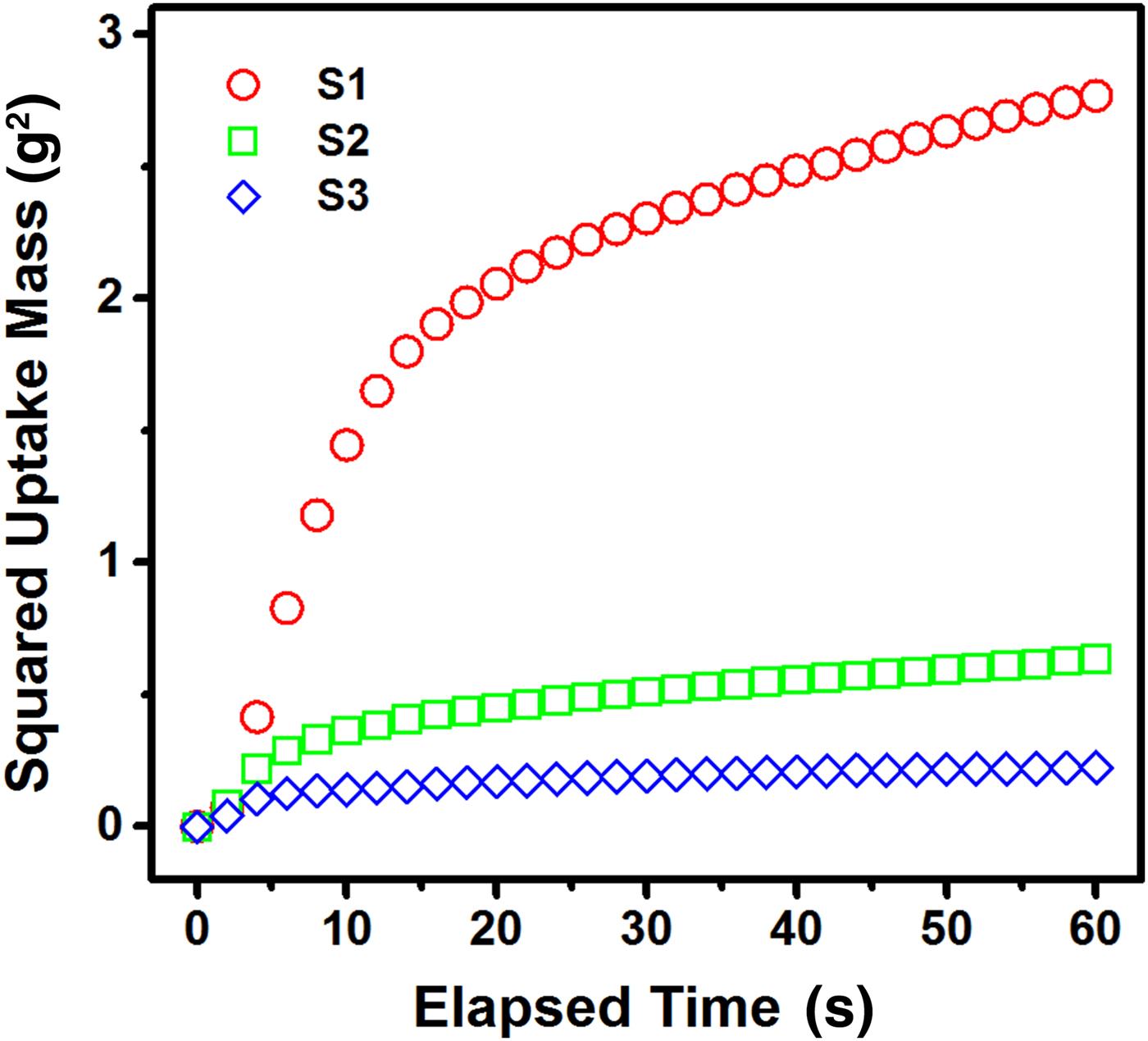
Fig. 8. Time-profiled curves of the squared mass of imbibed water along the glass tubes packed with vermiculite expanded at 1000°C using samples S1–S3. Each curve was obtained from three replicated experiments.
Absorptive mitigation testing of hazardous chemicals
The sorption capacity and the removal efficiency were determined for 12 hazardous liquid chemicals using sample S1 expanded at 1000°C. The liquids tested are representative hazardous substances classified as hydrophilic organic compounds, hydrophobic organic compounds and acidic/basic aqueous solutions. The sorption capacities obtained vary in the range 1.5–3.0 g g−1 (Fig. 9). These values are greater than the sorption capacities of 0.8–1.2 g g−1 that were previously reported for soybean, engine and diesel oils when using expanded vermiculite (Medeiros et al., Reference Medeiros, Sansiviero, Araújo and Lago2009). However, because the sorption capacity also relies on the physical properties of the sorbate, such as viscosity and density (Bandura et al., Reference Bandura, Franus, Józefaciuk and Franus2015; Ge et al., Reference Ge, Shi, Wang, Zhao, Yao, Zhu, Zhang, Zhu, Wu and Yu2017), these factors should be considered for a reasonable comparison. Our sorption capacities do not reveal any strong dependence on the different types of substances, indicating a weak relationship between the sorption capacity and the physicochemical properties of the hazardous liquid, such as hydrophilicity and molecular structure. This result indicates that expanded vermiculite may be utilized to mitigate various kinds of hazardous spillages without serious degradation or side effects. The sorption capacity was correlated significantly with the density of the hazardous chemical. Compounds with a density of >1 g cm−3 (furfuryl alcohol, acrylic acid, m-cresol, 2-nitrotolume and 30 wt.% HNO3(aq)) have higher sorption capacities, while the light methanol, methyl ethyl ketone and benzene have lower sorption capacities. This density-dependent increase in the sorption capacity was previously reported in the sorption of diesel and engine oils using zeolite sorbents (Bandura et al., Reference Bandura, Franus, Józefaciuk and Franus2015). When the sorption capacity was expressed by a hazard volume per unit weight of sorbent, the converted value was in the range of 2.0–2.6 cm3 g−1. This is smaller than the pore volume of 3.65 cm3 g−1 measured by Hg porosimetry, suggesting an incomplete filling of liquid chemicals into the interparticle/intraparticle pores, probably due to the very large pore size, the existence of trapped air and/or the incompatible interface property between vermiculite and the adsorbate. Consequently, a pore volume of acceptable size is regarded as a crucial parameter for determining the sorption capacity. In order to increase the sorption capacity of vermiculite, the formation of macroporous vermiculite structures via H2O2-based chemical expansion will be assessed in the future.
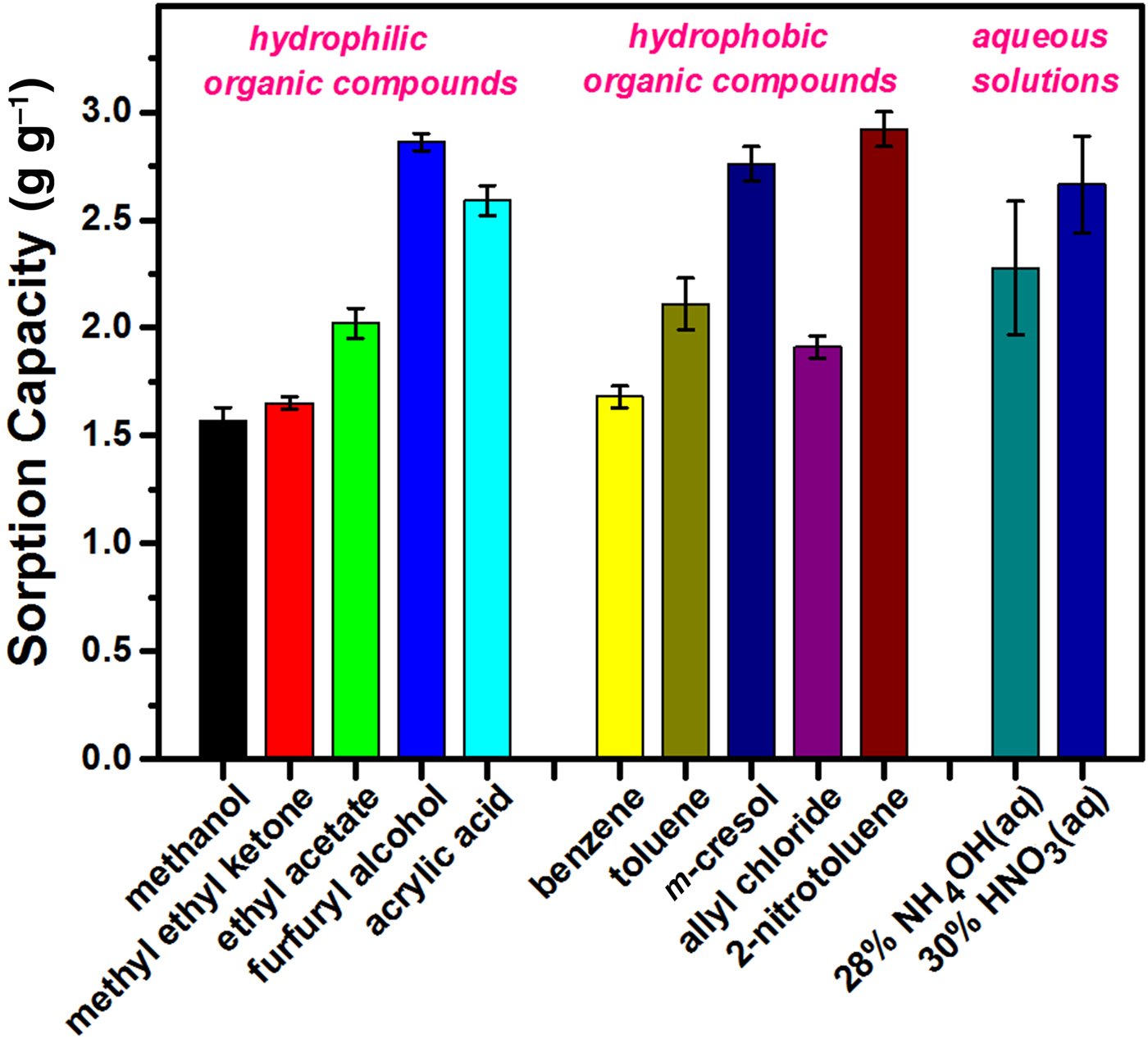
Fig. 9. Bar graph of the sorption capacities for various hazardous chemicals. The vertical error bars indicate variations of the sorption capacity obtained from triplicate measurements.
The removal efficiency was also quantified for the same harmful chemicals to evaluate their comparative spontaneously wicking ability. Figure 10 shows a bar graph of removal efficiencies grouped into the three hazard categories. All of them exhibit excellent removal efficiency, exceeding 94 wt.%. There is no distinct dependence on the types of absorbed chemicals, which supports the notion that the imbibition-based removal process is predominantly affected by physical features such as the pore-size distribution, as mentioned above, rather than chemical interactions between the liquid molecule and the sorbent.
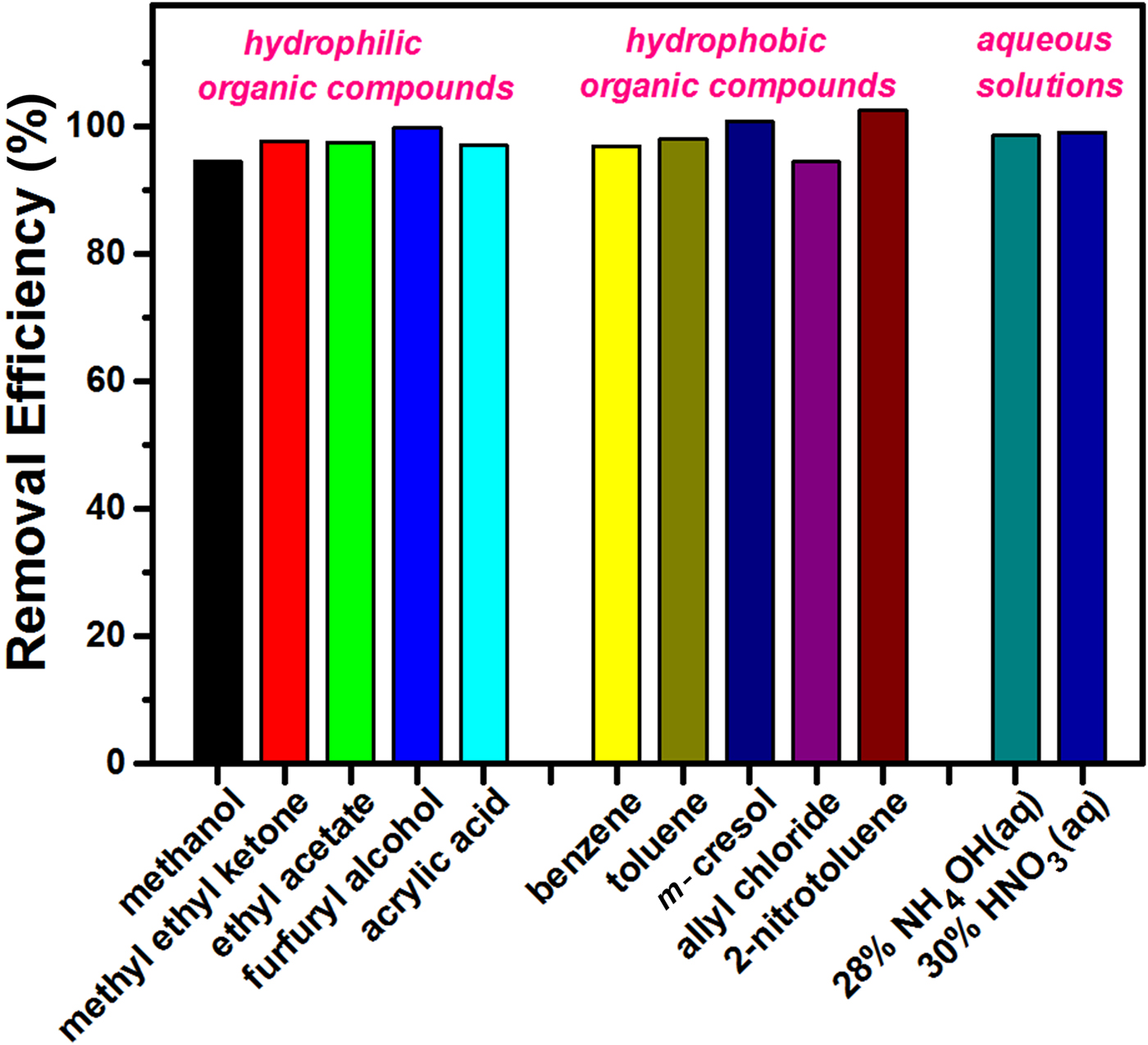
Fig. 10. Bar graph of the removal efficiencies for various hazardous chemicals.
Conclusions
A universal sorbent is needed to mitigate large-scale hazard spillages under harsh conditions (including high temperature and strongly acidic and alkaline conditions) without environmental risk or leading to secondary pollution. To achieve this goal, the sorbent must be cheap, thermally stable, chemically inert, highly porous and mechanically durable. Even though some polymer-based materials and macroporous carbons have been reported to have a very high sorption capacity, there is no sorbent that satisfies all of the requirements perfectly. Palabora vermiculite was tested here and evaluated as a risk-free, general-purpose sorbent that is appropriate for most hazardous chemicals because it is naturally abundant, easy to prepare, chemically inert and thermally stable. The sorption capacity and the removal efficiency were optimized by controlling the dimensions and macroporous structures of the expanded vermiculite. The macroporous structure dependence was explained by capillarity-driven imbibition. These sorption performances were insensitive to the chemical properties of hazardous chemicals, demonstrating the capability of expanded vermiculite as a general-purpose sorbent, based on testing 12 types of liquids classified into different chemical classes. Furthermore, there exists an additional opportunity to improve the sorbent performances through improved expansion and/or surface modification of vermiculite. The advanced vermiculite-based clay sorbent could be used to mitigate petroleum oils spilled on seawater and impermeable land.
Acknowledgements
This research was supported both by the Korea Ministry of Environment (MOE) as the Environmental Technology Development Program of the ‘Chemical Accident Prevention Technology Development Project’ and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07041567).














