INTRODUCTION
Explaining virulence (defined as parasite-induced host mortality) is fundamental to understanding parasitic organisms, arguably the most abundant life forms on earth (Poulin and Morand, Reference Poulin and Morand2000; Zimmer, Reference Zimmer2001). Although traditional views assumed that parasites should evolve to become benign (e.g. Burnet and White, Reference Burnet and White1972), host-parasite models indicate that high virulence can evolve and be maintained in natural populations (e.g. Anderson and May, Reference Anderson and May1982; Antia et al. Reference Antia, Levin and May1994; Frank, Reference Frank1996; Levin, Reference Levin1996; Ebert, Reference Ebert and Stearns1999; Stearns and Ebert, Reference Stearns and Ebert2001).
Two important assumptions underlying much of this theory are that virulence is a direct product of parasite reproduction in the host, and that parasite genotypes differ in the virulence they cause. However, many other factors could affect virulence, and hence its evolution. These include host characteristics such as genotype and condition (Mackinnon et al. Reference Mackinnon, Gaffney and Read2002; Krist et al. Reference Krist, Jokela, Wiehn and Lively2004; Grech et al. Reference Grech, Watt and Read2006), environmental factors (Imhoof and Schmid-Hempel, Reference Imhoof and Schmid-Hempel1998; Carius et al. Reference Carius, Little and Ebert2001; Cory and Myers, Reference Cory and Myers2004; Hodgson et al. Reference Hodgson, Hitchman, Vanbergen, Hails, Possee and Cory2004) and the dose of the parasite inoculum (Van Beek et al. Reference Van Beek, Wood and Hughes1988; Agnew and Koella, Reference Agnew and Koella1999; Ebert et al. Reference Ebert, Zschokke-Rohringer and Carius2000; Timms et al. Reference Timms, Colegrave, Chan and Read2001; Hughes et al. Reference Hughes, Petersen, Ugelvig, Pedersen, Thomsen, Poulsen and Boomsma2004; Osnas and Lively, Reference Osnas and Lively2004; Brunner et al. Reference Brunner, Richards and Collins2005).
We studied the monarch butterfly, Danaus plexippus, and its obligate protozoan parasite Ophryocystis elektroscirrha (McLaughlin and Myers, Reference McLaughlin and Myers1970). This parasite naturally occurs in monarch populations (Leong et al. Reference Leong, Yoshimura and Kaya1997b; Altizer et al. Reference Altizer, Oberhauser and Brower2000), and has received recent attention for its potential impacts on the migratory behaviour and conservation of monarch butterflies (Brower et al. Reference Brower, Fink, Brower, Leong, Oberhauser, Altizer, Taylor, Vickerman, Calvert, Van Hook, Alonsomejia, Malcolm, Owen and Zalucki1995; Harvell et al. Reference Harvell, Mitchell, Ward, Altizer, Dobson, Ostfeld and Samuel2002; Bradley and Altizer, Reference Bradley and Altizer2005). Previous studies have shown that heavy parasite infections can reduce host fitness (Leong et al. Reference Leong, Yoshimura, Kaya and Williams1997a; Altizer and Oberhauser, Reference Altizer and Oberhauser1999), but the mechanisms by which the parasite harms its host have yet to be examined. Here, we used 4 wild-collected O. elektroscirrha isolates to examine the relationship between parasite infection load and virulence, as well as the effects of parasite genotype, host age and sex and inoculum size.
MATERIALS AND METHODS
The host-parasite system
Monarch butterflies inhabit islands and continents worldwide and occupy a subset of the range of their host plants in the family Asclepiadaceae (Ackery and Vane-Wright, Reference Ackery and Vane-Wright1984). In North America, monarchs are best known for the population east of the Rocky Mountains that migrates annually to overwinter in central Mexico (Brower, Reference Brower1995). A second population inhabits the continental plateau west of the Rocky Mountains and annually migrates to the Californian coast (Nagano et al. Reference Nagano, Sakai, Malcolm, Cockrell, Donahue, Brower and Zalucki1993). Two non-migratory populations inhabit South Florida and Hawaii, and monarchs in these populations are reported to breed year-round (Ackery and Vane-Wright, Reference Ackery and Vane-Wright1984; Knight, Reference Knight1998). The neogregarine O. elektroscirrha (McLaughlin and Myers, Reference McLaughlin and Myers1970) is an apicomplexan protozoan that infects monarchs throughout their range (Leong et al. Reference Leong, Yoshimura and Kaya1997b; Altizer et al. Reference Altizer, Oberhauser and Brower2000). Parasite prevalence differs between populations, with less than 5% of the monarchs infected in the eastern US, and up to 80% infected in South Florida (Leong et al. Reference Leong, Yoshimura and Kaya1997b; Altizer et al. Reference Altizer, Oberhauser and Brower2000).
Because transmission occurs from adult butterflies to larvae, parasites require that hosts survive to deposit spores as adults (McLaughlin and Myers, Reference McLaughlin and Myers1970; Leong et al. Reference Leong, Yoshimura, Kaya and Williams1997a; Altizer et al. Reference Altizer, Oberhauser, Geurts, Oberhauser and Solensky2004). Infections occur when caterpillars ingest spores from contaminated eggs or leaves of the host plant (Asclepias spp.); spores lyse in the larval gut and parasites penetrate the intestinal wall to undergo asexual replication in the hypoderm. Vegetative replication occurs by schizogony, whereby each parent cell can give rise to large numbers of daughter cells through multiplicative fission (McLaughlin and Myers, Reference McLaughlin and Myers1970). After host pupation, the parasite undergoes a sexual phase and forms spores around the scales of the developing host, such that adult butterflies emerge covered with dormant spores on the outsides of their bodies. Parasites do not continue to replicate on adults, and spores must be ingested by larvae to cause new infections.
Parasites are mainly transmitted vertically, from infected butterflies to their progeny, but they can also transmit horizontally: either when infected males transfer spores to females during mating (Altizer et al. Reference Altizer, Oberhauser, Geurts, Oberhauser and Solensky2004) – which can then be transferred to the female's offspring – or by scattering spores in the environment, which are then ingested by unrelated caterpillars (Vickerman et al. Reference Vickerman, Michels and Burrowes1999). This horizontal parasite transmission presumably occurs more often in non-migratory monarch populations, where milkweed occurs throughout the year and parasites can build up on host plants and be ingested by caterpillars.
Host and parasite sources
Monarchs used in this study were the grand-progeny of a cross between wild-caught migrating butterflies obtained from Virginia, USA (Sept. 2004) and wintering monarchs in Central Mexico (Feb. 2005), thus representing monarchs from the eastern US migratory population. To obtain eggs, 10 females were mated to a total of 6 males using a breeding design that eliminated the possibility of full-sib matings. Adult monarchs were kept in 0·6 m3 mesh cages, held at 26°C and fed ad libitum with a 10% honey solution. Mated females were pooled into a new cage supplied with potted greenhouse-grown Asclepias incarnata (swamp milkweed) for oviposition. Plants with eggs were replaced daily and transferred to a laboratory maintained at 24°C.
Parasites were derived from 4 wild-caught parasitized monarchs from different populations, and were denoted E1 (Eastern US migratory population, Ithaca, New York), C2 (Western US migratory population, Santa Barbara, California), F3 (Miami, Florida) and H1 (Oahu, Hawaii). Spores used to initiate infections were harvested from lab-reared butterflies that were infected with 100 wild-type spores to propagate the strains.
Experimental design
First and second instar monarch larvae were inoculated with 1 of 4 parasite doses per parasite isolate. The experiment was fully factorial, with larval instar, parasite isolate (E1, C2, F3 or H1) and infectious dose (1, 5, 10 or 100 spores per larva) as experimental factors (Table 1). Initially all instar-by-parasite isolate-by-infectious dose treatments consisted of 20 replicate larvae, except for the 100-spore dose treatments, which had 15 replicate larvae (smaller replicate size was chosen because 100% infection probability was expected for this highest dose). Larvae were allocated randomly to experimental treatments. Host sex was determined at the adult stage and included as a factor in the analyses.
Table 1. Number of surviving monarchs per parasite and dose treatment and the number and proportion (in parentheses) of animals that became infected

To inoculate newly-hatched first instar larvae, eggs were transferred to individual 10 cm Petri-dishes and precise numbers of parasite spores – which are roughly 14 μm long – were manually deposited on eggs using a drawn-out glass capillary tube. Upon hatching, larvae consumed their egg-shells, ingesting parasite spores, and were then provided with single A. incarnata leaves. Second instar larvae were inoculated in a similar way by feeding them 1 cm2 milkweed pieces onto which parasite spores were manually deposited. Upon reaching third instar, all larvae were transferred to 0·47 litre plastic containers with meshed lids, where they were reared singly and fed greenhouse-grown A. incarnata cuttings in florist tubes. Containers were checked daily and provided with fresh milkweed cuttings and paper linings every 1–3 days. Following host pupation, containers were transferred to an adjacent laboratory maintained at 26°C. After adult butterflies emerged from their pupal cases, they were transferred to individual glassine envelopes. We recorded the sex and date of emergence for each butterfly, and placed butterflies in glassine envelopes in a 14°C controlled environment chamber. They were left unfed and checked daily to determine their time of death.
Quantifying parasite loads and virulence
We assessed the proportion of animals that became infected within each treatment group. Upon death, monarch wings were clipped off and bodies vortexed in 5 ml of de-ionized H2O at high speed for 5 min. A haemocytometer counting chamber was used to estimate the number of spores per butterfly based on replicate counts from 5 grids per sample.
Parasite replication rates were quantified in 2 ways: first, by calculating the growth rate from inoculation to adult emergence as [log10(sporeload)- log10 (dose)]/host development time; and second, by simply dividing the final spore load by the initial dose, thus giving an estimate of the per capita replication rates of individual parasites entering an infection. Because host development times from inoculation to adult emergence did not differ between animals infected with different doses (see Results section) and because no parasite replication occurs during the adult stage, these 2 measures provided identical patterns and results. We therefore only report on results based on the second measure, as these are intuitively easier to interpret.
Virulence was measured in 4 principal ways. First, we quantified parasite effects on larval survival by calculating the proportion of animals from each treatment group that died before the adult stage. Second, we measured host development time (in days) between hatching and adult emergence. Third, for monarchs that survived to the adult stage, we measured adult longevity in days. Finally, we recorded 2 measures of adult body size: total weight was measured to the nearest mg 1 day post-eclosion, and wing size was quantified by scanning and digitally measuring the area of forewings as described elsewhere (Davis et al. Reference Davis, Farrey and Altizer2005). Adult longevity and body size should be positively correlated and both are important predictors for monarch fitness (Oberhauser, Reference Oberhauser1997): larger females may live longer and thereby have a higher life-time fecundity (Oberhauser, Reference Oberhauser1997), while larger males may live longer and hence be able to mate more frequently. Male body size may also directly increase fitness, for example by providing greater amounts of sperm per mating, as observed in another butterfly species (Wiklund and Kaitala, Reference Wiklund and Kaitala1995).
Statistical analysis
Data were analysed in SPSS 13.0 (2004) and R 2.2.1 (R Development Core Team 2006). First, we examined the effect of design variables and sex on the proportion of animals infected (using logistic regression) and on final spore load and parasite replication rate (using analysis of covariance). In these analyses, dose was treated as a continuous variable, and parasite isolate, host stage at inoculation (instar) and host sex were treated as categorical explanatory variables; we included all possible 2-way interactions in the full models (Table 2). Dose was fitted as a continuous variable to investigate the shape of the relationships; because visual inspection of the data suggested a nonlinear relationship with dose, we included both Log10(dose) and (Log10(dose))2 in the full models. Analyses of proportions of animals infected were performed separately for larvae inoculated as first versus second instars because the shapes of their relationships with dose appeared to differ (Fig. 1A, B). Analyses that examined spore load and parasite replication rate were performed using data from infected animals only.

Fig. 1. Effects of parasite dose (number of spores per larva) on the proportion of animals that became infected (A, B), final parasite spore load (C, D) and per capita parasite replication rate (E, F). Data points in (C–F) depict individual animals, and lines show least-squares regressions fitted for the 4 parasite isolates. Panels (C–F) show data for infected animals only (hence the absence of data points for the 1 spore dose treatment in first instars). Left and right panels show data for animals inoculated as first and second instars, respectively.
Table 2. Analysis of proportion of animals infected, spore load and replication rate
(Proportions of animals infected were analysed using logistic regression, whereas spore loads and replication rates in infected animals were analysed using analysis of covariance. χ2, F- and P-values are shown only for those explanatory variables that needed to be retained in the minimal model; other variables are depicted as n.s. (not significant at the 0·05 level). For logistic regression analyses of parasite isolate, the significance tests reported here examined whether one or more isolates differed (d.f.=3). Although not shown, all possible 2-way interactions were included in the maximal models. Hyphens indicate variables that were not included in a particular analysis.)

Second, we investigated how host traits (pre-adult mortality, pre-adult development time, adult longevity, body weight and wing area) responded to parasite infection. We used analysis of variance with infection status as the sole explanatory variable, where infection status was defined by 5 levels: uninfected (data from all control monarchs combined with data from parasite-treated monarchs that did not become infected) or infected in the 1, 5, 10, or 100 spore dose treatments. Pre-adult development time was analysed for first instars only because hatching dates were not recorded for the second instar treatment groups.
Third, we analysed in more detail the effects of spore load, dose, host sex and larval instar on adult longevity and body weight. We used analysis of covariance with spore load as a covariate, and parasite isolate, larval instar, host sex and dose as categorical explanatory variables; all possible 2- and 3-way interaction terms were included in the full models (Table 3). Analyses were performed using data from infected animals only. Because per capita parasite replication rates are confounded with dose and final spore loads, we did not include replication rates in these analyses, but performed an additional analysis in which we examined the effect of replication rate on adult longevity within each dose treatment. We also investigated the relationship between longevity and body weight for males and females separately, using an analysis of covariance with body weight as a covariate, infection status as a categorical explanatory variable, and the 2-way interaction term.
Table 3. Analysis of adult monarch longevity and body weight
(Annotations as in Table 2.)
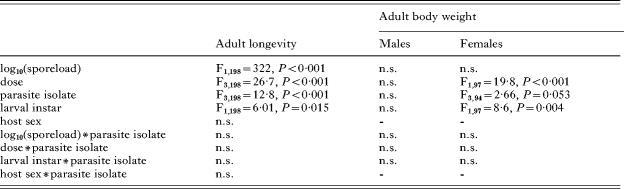
In all analyses, model simplification was performed by removing model terms and comparing the explanatory power of subsequent models (Agresti, Reference Agresti1996; Crawley, Reference Crawley2002). If removal of a term reduced the explanatory power of the entire model, it was retained in the minimal model. For these minimal models, we report Wald-statistics (logistic regression) and F-values (analyses of variance and covariance) and their associated p-values based on each effect retained in the final model. In the analyses of variance and covariance, residuals were tested for normality, and response and explanatory variables were log-transformed where necessary (in the case of final spore load, parasite replication rate, and dose).
RESULTS
A total of 406 inoculated animals and 24 control monarchs (71% and 60% of the larvae used in the experiment) survived to adulthood (Table 1). No control monarchs became infected. Analyses of proportions of animals infected, replication rates and spore loads focus on data from adult butterflies only.
Proportion infected, spore load and replication rate
A higher proportion of monarchs became infected when inoculated with higher parasite doses (Fig. 1A, B; Table 2). Fewer animals became infected when exposed as first versus second instar larvae (Table 2), and the shape of the relationship between dose and proportion infected also differed between instars, with an increasing relationship for first instars and a saturating relationship for second instars (Fig. 1A, B). Parasite isolates differed in the proportions of animals they infected (Table 2), with E1 generally infecting fewer animals than C2 and F3 (Fig. 1A, B). Proportions of animals infected were similar for male and female monarchs (Table 2).
Among hosts that became infected, increasing doses resulted in higher final spore loads (Fig. 1C, D; Table 2). Across all doses, animals infected as first instar larvae had lower spore loads than those infected at second instar, and female butterflies had higher spore loads than males (Table 2). Parasite isolates also differed (Table 2), with H1 and C2 causing higher spore loads than E1 and F3 (Fig. 1C, D). These parasite differences were maintained across dose treatments, larval instars and host sex (all 2-way interactions with parasite isolate non-significant: Table 2).
Per capita replication rates of O. elektroscirrha were as high as 106 parasites per ingested spore. Average replication rates were highest in the 1-spore treatment and decreased with increasing doses (Fig. 1E, F; Table 2), such that increases in final spore loads were disproportionately lower than increases in initial doses. Parasites from animals infected as first instars showed lower per capita replication rates than those from animals infected as second instars, and parasites in females had higher replication rates than those in males. Moreover, parasite isolates differed in their replication rates (Table 2), such that H1 and C2 had higher rates than E1 and F3 (Fig. 1E, F). All these differences accounted for the observed differences in final spore loads (Fig. 1C, D). Parasite isolates maintained their differences across dose, instar and sex (all 2-way interactions with parasite isolate non-significant: Table 2).
Host development time, longevity, and body size
Neither pre-adult mortality (40% mortality in control animals, 29% mortality in inoculated animals: χ2=1·85, d.f.=1, P=0·17) nor development time from egg to adult (Fig. 2A) differed between inoculated and control animals. In contrast, adult longevity was greatly reduced by parasite infection (Fig. 2B). As compared with the control group, monarchs infected with 1, 5, 10, and 100 spores experienced reductions in adult longevity of roughly 25, 30, 50, and 75%, respectively. Parasitism also reduced adult body weight, but to a lesser extent than adult longevity (Fig. 2C). There was no consistent decline in wing area with increasing parasite doses (Fig. 2D).

Fig. 2. Effects of parasite infection and dose on pre-adult development time (A), adult longevity (B), adult body weight (C), and adult forewing area (D) (mean±1 s.e.m.). F-values, P-values and R2-values are based on analyses of variance with infection status (uninfected, or infected with parasites in the 1, 5, 10 or 100 spore dose treatments) as the sole explanatory variable. Thus, analyses were performed by pooling data across parasite isolates, larval instar and sex. Letters above each bar indicate significant differences (P<0·05) based on pair-wise comparisons. Because exact hatching dates were recorded for animals infected as first instars only, the analysis of pre-adult development time (A) was carried out on a reduced dataset. This explains the lack of a development time for animals infected with a single spore (A), as no animals inoculated as first instars became infected in this treatment group.
The observed shorter host longevity with increasing parasite doses was largely explained by the continuous effect of higher spore loads (Table 3). In other words, higher doses resulted in higher spore loads, which caused shorter longevity (Fig. 3). However, dose was retained in the final statistical model (Table 3), such that for a given spore load, higher initial doses caused shorter longevity (Fig. 3A). Similarly, animals infected as second instar larvae experienced shorter longevity for a given spore load than animals infected as first instar larvae (Table 3). The relationship between spore load and longevity also differed among parasite isolates (Table 3), with E1 causing shorter longevity than H1 for a given spore load (Fig. 3B). Thus, although E1 caused lowest spore loads and hence least virulence of the parasite isolates tested, it caused most virulence on a per parasite basis. Differences between parasite isolates were maintained across all doses and larval instars (all 2-way and 3-way interactions with parasite isolate non-significant).

Fig. 3. Effects of final parasite spore load on adult longevity. Data points depict individual animals, and least-squares regression lines are shown for the 4 dose treatments (A) or parasite isolates (B).
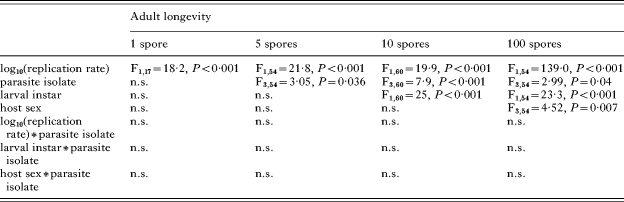
We chose to analyse the relationship between spore load and host longevity, rather than that between replication rate and longevity, because replication rates were confounded with spore load and inoculation dose. To demonstrate, however, that higher replication rates resulted in shorter longevity, we analysed the replication rate within each dose treatment (the 4 regression lines in Fig. 3A), confirming the negative relationship between replication rate and host longevity (Table 4).
Table 4. Analysis of adult monarch longevity as a function of parasite replication rate
(Annotations as in Table 2.)
Longevity and body weight in male and female butterflies
Although male and female butterflies showed similar longevity in response to infection, the effect of parasite infection on adult body weight was much smaller for males than females (Fig. 4A, B). Infected males weighed slightly less than uninfected males, but there was no additional effect of spore load, dose, parasite isolate, or larval instar on male body weight (Table 3). Among females, higher parasite doses resulted in lower body weight (Fig. 4B; Table 3), and females infected as second instars weighed less than females infected as first instars (Table 3). Weight differed among females infected with different parasite isolates, but this effect was only marginally significant (Table 3). Continuous measures of spore load did not explain further reductions in female body weight when dose was included in the final model (Table 3).

Fig. 4. Effects of parasite infection and dose on adult body weight (A, B) and relationship between body weight and adult longevity (C, D). Bars and error bars in panels (A) and (B) show mean±1 s.e.m. for uninfected animals and animals that became infected in the 1, 5, 10 or 100 spores treatment groups. Data points in panels (C) and (D) depict individual animals, and least-squares regression lines are fitted for uninfected and infected animals. Left and right panels show data for males and females respectively.
Because spore load had a major effect on adult longevity but not on adult body weight, we assessed their correlation. For both males and females that emerged with and without parasite infections, longevity and body weight were positively correlated (Fig. 4C, D; males: F1,217=44·7, P<0·001; females: F1,204=78, P<0·001). However, infected animals lived much shorter for a given body weight than uninfected animals (Fig. 4C, D; males: F1,217=360, P<0·001; females: F1,204=336; Fig. 4F). Comparing their respective adjusted Sums of Squares revealed that parasite infection explained more of the variation in these models than did body weight (males: 3437 vs. 558; females: 1948 vs. 789 for infection and weight respectively), suggesting that parasite infection overshadowed the effect of body weight on longevity.
DISCUSSION
Parasites showed high rates of replication within monarch butterflies, with some singly ingested O. elektroscirrha spores producing more than a million offspring on the bodies of infected adults. These high spore loads caused significant virulence in the host, particularly with respect to adult longevity. Monarchs infected with just a single spore experienced a 25% reduction in adult longevity, and those inoculated with 10 spores lived only half as long as uninfected animals.
High parasite loads will have important consequences for the fitness of both host and parasite. Female monarch butterflies mate and lay eggs throughout their life-time, and shorter longevity and body weight translate into lower lifetime fecundity and mating success (Oberhauser, Reference Oberhauser1997). For males, shorter longevity should also reduce fitness due to the reduction of mating opportunities and hence the number of offspring they could sire. The effects of high spore loads for parasite fitness are less clear. The main transmission route of O. elektroscirrha is from infected females to their offspring, when parasite spores from the female's body are deposited on eggs and surrounding leaf material during oviposition. Therefore, high parasite loads could result in higher rates of transfer of spores onto eggs and milkweed leaves, and hence higher infection probabilities. However, the shorter life-spans and reduced life-time fecundity of infected hosts should also reduce parasite transmission opportunities. In terms of sexual horizontal transmission, high spore loads on males could result in higher spore transfer to females during mating, but again, a reduction in mating opportunities would reduce the number of times spores were transferred. Understanding the relative importance of these costs and benefits for parasite fitness is necessary to predict evolutionary optimal levels of virulence in this system (e.g. Anderson and May, Reference Anderson and May1982; Antia et al. Reference Antia, Levin and May1994; Frank, Reference Frank1996; Ebert, Reference Ebert and Stearns1999; Stearns and Ebert, Reference Stearns and Ebert2001), and will be part of future studies.
Our results have several implications for virulence evolution. First, we found natural variation in replication rates and virulence in this parasite, even among the small number of samples tested in this study. Such genetic variation has been found in other parasites (e.g. Diffley et al. Reference Diffley, Scott, Mama and Tsen1987; Turner et al. Reference Turner, Aslam and Dye1995; Mackinnon and Read, Reference Mackinnon and Read1999; Hughes et al. Reference Hughes, Petersen, Ugelvig, Pedersen, Thomsen, Poulsen and Boomsma2004), and is a crucial prerequisite for natural selection to operate on virulence. The relative differences in virulence among strains were maintained across ecologically relevant variables including infective doses (see also Timms et al. Reference Timms, Colegrave, Chan and Read2001), host age at the time of infection (i.e. larval instar) and host sex. This suggests that for O. elektroscirrha, virulence is a robust genetically determined parasite trait.
Second, many studies have argued that vertically transmitted parasites (those transferred from parent to offspring) should evolve to be avirulent because of the strong link between host and parasite survival (e.g. Ewald, Reference Ewald1983; Bull et al. Reference Bull, Molineux and Rice1991; Herre, Reference Herre1993; Stewart et al. Reference Stewart, Logsdon and Kelley2005). However, our study suggests that this may not always be the case. One possible explanation for high virulence in this vertically transmitted parasite is that the transfer of spores during oviposition is so inefficient that high spore loads are required for successful infection of monarch offspring. Alternatively, high virulence may evolve because parasites can also transmit horizontally. This can happen when infected males transfer spores to the abdomens of healthy females during mating (Altizer et al. Reference Altizer, Oberhauser, Geurts, Oberhauser and Solensky2004), or when butterflies scatter spores in the environment (Vickerman et al. Reference Vickerman, Michels and Burrowes1999) which can be ingested by unrelated caterpillars (Altizer et al. Reference Altizer, Oberhauser, Geurts, Oberhauser and Solensky2004). Thus, it is conceivable that this horizontal component of transmission favours higher virulence than would be optimal for a purely vertically transmitted parasite (Lipsitch et al. Reference Lipsitch, Siller and Nowak1996). One more potential explanation for the observed high virulence is that we may have tested our parasite genotypes in novel host genetic backgrounds, to which the parasites had not fully adapted (only parasite genotype E1 was derived from the same population as the monarchs we tested our parasites in). Thus, if local adaptation of parasites to hosts occurs in this system, we may have observed lower virulence in sympatric rather than allopatric host-parasite combinations (Dybdahl and Storfer, Reference Dybdahl and Storfer2003).
Third, our results demonstrate that not all host traits respond similarly to infection, nor are equally useful for addressing virulence evolution theory. We found no effect of infection and inoculation dose on pre-adult mortality, development time or adult wing area, and only limited effects on body weight. In contrast, adult longevity declined in direct response to increasing spore loads. Even though our sample of 4 parasite genotypes was not large enough to assess a genetic relationship between parasite exploitation rate and virulence, the clear phenotypic relationship between host longevity and parasite exploitation nonetheless suggests that this is probably the most appropriate measure to study virulence evolution in this system. More generally, this finding suggests that even though surrogate measures of virulence – such as weight loss or body condition – may often be easier to obtain than host death rates in experimental studies, special care should be taken when interpreting these measures against theoretical models (see also Day, Reference Day2002).
Although parasite loads were important, they did not entirely account for the observed virulence in infected animals, as evidenced by comparison of the 4 parasite genotypes used. Parasite E1 caused least virulence overall, but actually caused more virulence on a per parasite basis. We also found that higher doses were more virulent on a per parasite basis, and that animals infected as second instar larvae suffered higher virulence on a per parasite basis than those infected as first instar larvae. Several explanations could account for these findings. First, parasite genotypes may differ in their ability to translate host resources into reproduction, such that isolate E1 needed more host resources than other isolates to produce the same number of offspring; alternatively, between-strain differences in virulence could arise if host immune responses to some strains are more costly to mount. Differential effects of dose on virulence could arise early in the infection process when sporozoites penetrate the gut wall and migrate to the hypoderm (McLaughlin and Myers, Reference McLaughlin and Myers1970). Higher parasite doses could lead to greater gut damage, reducing host digestion and nutrient intake, or allowing gut bacteria to invade the body cavity and cause secondary infections. Finally, differences between larval instars could arise from differences in the effective dose they received. Even at the highest dose tested, fewer animals became infected when inoculated as newly-hatched first instars. Perhaps this was because the gut chemistry of first instar larvae (that have not yet fed on milkweed) is less suitable for parasite infection: spore lysis and release of sporozoites into the host gut hinges upon digestive juices dissolving the polar plug of the parasite spore (Tanada and Kaya, Reference Tanada and Kaya1993).
Differences in effective dose may also explain why parasites had lower per capita replication rates at higher doses. It is possible that the larval gut has a limited number of parasite entry sites, such that the effective dose was lower than the given dose, thus reducing the per parasite spore load. Alternatively, if all parasites in a given dose did infect the host, they might still experience lower per capita replication rates due to limited host resources and within-host competition. Either way, the lower per capita replication rates at higher doses suggest density dependence for parasite reproduction in this system (see also Keymer, Reference Keymer1982; Ebert et al. Reference Ebert, Zschokke-Rohringer and Carius2000; Dezfuli et al. Reference Dezfuli, Volponi, Beltrami and Poulin2002; Hughes et al. Reference Hughes, Petersen, Ugelvig, Pedersen, Thomsen, Poulsen and Boomsma2004), which may play a role in regulating parasite prevalence and infection loads in wild monarch populations (Anderson and May, Reference Anderson and May1978, Reference Anderson and May1992; but see Jaenike, Reference Jaenike1996).
A final finding from this study is that the effect of parasite infection on the body weight of adult butterflies was much stronger for females than males. Females were also smaller, on average, and had greater spore loads than males, even though both sexes showed similar longevity. It is possible that the higher spore loads of females translated into greater weight loss rather than in additional reductions in longevity, raising the question as to whether reduced body weight is equally detrimental to the fitness of female monarchs as shortened life-spans. In experimental settings, for example, smaller females have lower egg-laying potential (Oberhauser, Reference Oberhauser1997), such that reduced life-time fecundity could arise from both shorter longevity and reduced egg production.
In conclusion, we showed that the virulence of a protozoan parasite, as measured by reduced longevity among infected butterflies, is phenotypically related to infection loads. Using a sample of wild-collected parasite isolates, we also showed that virulence is genetically determined and robust across infective doses, larval stages at infection, and host sex. Surprisingly, there was little correlation between different measures of virulence in this host-parasite system, suggesting that great care should be taken in choosing host fitness measures to test evolution of virulence theory. Our results indicate that the definition of the word ‘virulence’ is more than a semantic issue, and can have important implications for understanding selective pressures that operate on parasite life-history (Read, Reference Read1994; Poulin and Combes, Reference Poulin and Combes1999; Day, Reference Day2002).
We thank Zachary Baumann, Andrew Davis, Elizabeth Harp, Nicholas Vitone, and Lisa Sharling for help with the experiments, and Marc Choisy, Kristin Harper, Byron Ledbetter, Karen Oberhauser, Amy Pedersen, Pejman Rohani, Helen Wearing and two anonymous referees for helpful comments on the manuscript. Andrew Davis scanned and measured butterfly wings, Les Real provided greenhouse space and Michelle Solensky and Karen Oberhauser provided monarch butterflies to initiate experiments. The study was funded by Emory University (SA) and a Netherlands Organization for Scientific Research (NWO) TALENT fellowship to J.C.deR.


