INTRODUCTION
Ciliated protozoa are essential components of the microbial communities and play an important role in the functioning of the microbial food loop (Corliss, Reference Corliss2002). Many ciliates can often inhabit environments that are unfavourable to most metazoans and some can tolerate what would be extremes of environmental conditions to macrofauna (Patterson et al., Reference Patterson, Larsen and Corliss1989). Furthermore, with their short life cycles, high species diversity and quicker responses to environmental changes than most other eukaryotic organisms, they can serve as bioindicators of water pollution (Cairns et al., Reference Cairns, Lanza and Parker1972; Foissner, Reference Foissner, Lee and Soldo1992; Coppellotti, Reference Coppellotti1998; Madoni & Braghiroli, Reference Madoni and Braghiroli2007).
Glass slides may be used as artificial substrates, allowing microorganisms to form a periphyton or biofilm, on which periphytic ciliates usually colonized in high abundance and richness (Zimmermann, 1961; Cairns & Yongue, Reference Cairns and Yongue1968; Foissner, Reference Foissner, Lee and Soldo1992; Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999). Compared to collecting periphytic ciliates from natural substrates, sampling by glass slides is more effective since most species can be observed, enumerated and even identified by living observation on the slides under an inverted or a stereomicroscope (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Gong et al., Reference Gong, Song and Warren2005). Moreover, the species richness of ciliate communities colonizing glass slides is almost as high as that of natural substrates exposed to the same environmental conditions (e.g. Foissner, Reference Foissner, Lee and Soldo1992; Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999).
Although the slide method has been widely used to host periphytic ciliates and/or to monitor freshwater quality, such studies on marine environment have rarely been carried out (Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999; Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000; Gong et al., Reference Gong, Song and Warren2005). The conventional slide method applied to marine water was previously shown to be less efficient than that to freshwater, mainly due to the presence of tidal current and circulation (Cairns & Yongue, Reference Cairns and Yongue1968; Foissner, Reference Foissner, Lee and Soldo1992; Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999). This warranted the need for modifications of the slide method to suit marine ecosystems. In this study, a modified system, designated the polyurethane foam enveloped slide (PFES) system, was used to host ciliate communities in coastal area. Our main aim is focus on: (1) the taxonomic composition, temporal species distribution of periphytic ciliates colonizing modified glass slides; and (2) comparing the community parameters with the conventional slide method, in order to find a more effective artificial substrate for monitoring water quality using periphytic ciliates in marine ecosystems.
MATERIALS AND METHODS
Study site and period
The study station was located at the coastal waters, near Incheon Harbour, Korea (Figure 1). This was a polluted coastal area, with a depth of about 8 m, having high turbidity due to its mud–sandy bottom. Investigations were carried out during the period of August–November 2007. During the exposure time of our glass slides, the water depths were usually changed from minimum value to the maximum in an interval of about 3.5 m by the strong tide wave and currents.

Fig. 1. Map of the sampling station, which was located at coastal waters near Incheon Harbour, Korea.
The conventional slide method
For the conventional slide (CS) method, 10 glass slides (2.5 × 7.5 cm) were clipped to a polyvinyl chloride (PVC) frame (14 × 8 × 7 cm), and immersed in water at a depth of 1 m below the surface (Figures 2A & 3). The slides were exposed as back-to-back pairs, such that they could be split and observed directly without cleaning. They were placed vertically in the frames and were collected every 10 days. A total of 8 samplings were carried out, in each of which all 10 slides were collected every 10 days, followed by a next 10-day exposure of the frame holding 10 sheets of slides.

Fig. 2. Anchoring system for glass slides: (A) conventional slide (CS) method; (B) schema of the polyurethane foam enveloped slide system.

Fig. 3. Diagram of the polyurethane foam enveloped slide system.
According to Wilbert (Reference Wilbert1969), there are no significant differences between ciliate communities colonizing slides within the same frame. Thus, for every sampling date 10 glass slides were collected, 7 of which were randomly selected for studying. The slides were transferred into Petri dishes containing water from the sampling site, and then stored in a cooling box before transporting to the laboratory within 12 hours for identification and counting.
The polyurethane foam enveloped slide (PFES) system
The PFES apparatus comprised a slide-frame system enveloped within two caved polyurethane foam blocks of 2 cm-thickness walls (Figures 2B & 3). The slide-frame used in this study was similar to a conventional one in size. The polyurethane foam block was about 18 × 14 × 7 cm in size (Figure 3). The PFES and the CS systems were placed at the same depth and sampled simultaneously. Microscopic examinations of both the PFES and the CS system were performed in the same way.
Identification and enumeration
Species were first examined at a 45-fold magnification using a stereomicroscope (Olympus SZH10 research stereo) to observe the behaviour and movement of the cells. They were then transferred using a micropipette onto a clean glass slide and placed under a microscope (Leica DM2500) at 100- to 1250-fold magnification to reveal details of the cell size and other morphological characters (Foissner et al., Reference Foissner, Berger and Schaumburg1999). Over 30 cells of each morphotype were isolated with the micropipette and then identified to the species level using the protargol method (Wilbert, Reference Wilbert1975). Species identifications were made following published references to keys and guides such as Song et al. (Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999, Reference Song, Zhao, Xu, Hu and Gong2003) and Gong et al. (Reference Gong, Kim, Kim, Min, Roberts, Warren and Choi2007). The taxonomic scheme used was according to Corliss (Reference Corliss1979). The designation of species as being sessile, vagile or planktonic was made in terms of their mobility and the ecological niches they occupied. This approach has previously been used in other studies including those by Foissner (Reference Foissner, Lee and Soldo1992), Foissner et al. (Reference Foissner, Berger and Schaumburg1999), Coppellotti & Matarazzo (Reference Coppellotti and Matarazzo2000) and Gong et al. (Reference Gong, Song and Warren2005).
The enumeration of ciliates in vivo was carried out under an inverted microscope as soon as possible after sampling (generally within 2 to 4 hours) in order to prevent significant changes in species number and composition. Using bright field illumination, 20 fields of view per slide were randomly chosen for counting. The ciliate abundances were calculated from all 7 replicate slides to confirm the average cell density (cell cm−2).
Data analysis of samples
Species diversity (H′), evenness (J) and species richness (d) of samples were calculated as follows:

where H′ = observed diversity index; Pi = proportion of the total count arising from the i th species; S = total number of species; and N = total number of individuals.
The community structures of samples were analysed using the PRIMER (Plymouth Routines in Multivariate Ecological Research) package (Clarke & Warwick, Reference Clarke and Warwick1994). The multivariate analysis of cluster and multidimensional scaling (MDS) ordination were used to summarize species distribution, using the Bray–Curtis similarity from the log-transformed data of species abundance (Clarke & Warwick, Reference Clarke and Warwick1994). Differences between the PFES and the CS community samples were tested by the PRIMER program ANOSIM. The contribution of each species to the average Bray–Curtis dissimilarity between groups of samples, as well as to the similarity within a group was examined by the SIMPER analysis program.
For statistical analyses the community parameter data were log-transformed. The calculations were carried out with the statistical program SPSS (version 11.5). The paired t-test was used to test for differences of ecological parameters between the PFES and the CS ciliate communities at the 0.05 level.
RESULTS
Taxonomic composition and diversity
The ciliate taxa recovered from the glass slides of both the PFES and the CS system are listed in Table 1. A total of 41 ciliate species were identified in the communities of colonizing glass substrates submerged in the coastal area around Incheon, Korea from August to November 2007. The sessile taxa belonged to Peritrichida (Zoothaminium duplicatum, Zoothamnium plumula and Vaginicola sp.), Suctoria (Corynophyra lyngbyi) and Heterotrichida (Folliculinopsis producta). All other ciliates represented vagile and planktonic assemblages, with species mainly belonging to Hypotrichida, Cyrtophorida, Pleurostomatida, Heterotrichida, Haptorida, Prostomatida and Oligotrichia (Table 1). It should be noted that the planktonic ciliates detected may be generally transported to the slides from the water.
Table 1. List of the species of ciliates recorded in a total of 16 (8 PFES and 8 CS) samples, including their biohabits, degree of average abundance and occurrence.

PFES, polyurethane foam enveloped slide; CS, conventional slide; Occurr., occurrence; Abund., abundance; Se, sessile; V, vagile; P, planktonic; +, 0–10 cells cm−2; ++, 10–100 cells cm−2; +++, 100–400 cells cm−2; ++++, over 400 cells cm−2.
The taxonomic compositions of ciliate colonization showed similar patterns between the two systems. In the PFES system, a total of 37 ciliate species representing 9 orders and 27 genera were found (Table 1). Hypotrichida and Cyrtophorida were the 2 orders that represented the most species, accounting for 40.5% and 16.2% respectively; each of the other 7 orders had a comparatively low number of species (Figure 4A). In the CS system, a total of 38 species belonging to 8 orders and 27 genera were recorded during this period (Table 1; Figure 4B). Hypotrichida and Cyrtophorida were the 2 orders that constituted the most number of species, accounting for 50.0% and 15.8%; the other 6 orders represented comparatively lower numbers of species. The vagile ciliates represented the majority of species number, accounting for 72.5% and 71.1% of the cumulative total species number from the slides of the PFES and the CS system respectively; the other two components had a comparatively lower number of species (Figure 5A, B). However, the number of sessile taxa of the PFES system was higher than that of the CS system (15.7% versus 7.9%) while the percentage of planktonic species of the PFES system was lower than that of the CS system (11.8% versus 21.0%).

Fig. 4. Comparison of species composition of ciliate communities between samples obtained from the (A) polyurethane foam enveloped slide and (B) conventional slide system.

Fig. 5. Proportions of cumulative species numbers and abundances of vagile, sessile and planktonic ciliates in the polyurethane foam enveloped slide (A, C) and conventional slide (B, D) samples from August to November 2007. (A, B) species number; (C, D) abundance.
The mean abundance in CS system (1046 cells per cm2) was about 8.3 times than that of PFES system (126 cell pre cm2) (Table 2). Figure 5C and D show the proportions of cumulative abundances of the vagile, sessile and planktonic components during the survey period. The sessile ciliates represented the most dominant assemblage, accounting for 91.1% and 99.2% of the total abundance for the PFES and the CS system respectively, while the vagile and planktonic forms represented comparatively low percentages (Figure 5C, D). However, it should be noted that the proportion of vagile ciliates of the PFES system was considerably higher than that of the CS system. Statistical analysis revealed that the abundances of sessile ciliates represented a significant difference between the two sampling systems (t value = –3.29; P = 0.011), while those of the other components did not.
Table 2. Comparison of ciliate community characterization between 8 PFES and 8 CS samples. Mean values of variables and results of paired sample t-test.

*, significant difference at the 0.05 level; PFES, polyurethane foam enveloped slide; CS, conventional slide.
The cumulative species number, abundance, species richness, species evenness and species diversity of ciliate colonization in both the PFES and the CS system from August to November 2007 are shown in Table 2. Except for the species number and richness, other parameters showed significant differences between the two colonizing systems. The species diversity and evenness of ciliate colonization of the PFES system were both significantly higher than that of the CS system, whereas the abundance of these parameters was significantly lower in the PFES system than in the CS system.
Species distribution and similarity analysis
Tables 3 and 4 summarize the contribution of each species to the average Bray–Curtis similarity within the PFES and the CS samples respectively using the SIMPER analysis. In the PFES system, 27 ciliates exhibited 52.58% of average Bray–Curtis similarity during the three-month period (Table 3); the top 6 contributors were Zoothamnium duplicatum, Aspidisca leptaspis, Aspidisca steini, Folliculinopsis producta, Euplotes minuta and Amphileptus litonotiformis. In the CS system, at least 26 species presented high average similarity (74.20%); the top 6 of contributors included Protogastrostyla pulchra but not Euplotes minuta (Tables 3 & 4).
Table 3. Contribution of each species to average Bray–Curtis similarity (52.58%) within 8 samples from the PFES system.

Av. Sim., average similiarity; Contrib., contribution; Cum., cumulation; PFES, polyurethane foam enveloped slide.
Table 4. Contribution of each species to the average Bray–Curtis similarity (74.20%) within 8 samples from the conventional slide system.

See Table 3 for abbreviations.
Two dendrograms of the species distribution in the samples of both the PFES and the CS system respectively were plotted using group-average clustering from the Bray–Curtis similarities on log-transformed abundances (Figures 6 & 7). The cluster analysis resulted in the 37 ciliates of the PFES samples falling into seven groups (I–VII) at a 40% similarity level: group I was composed of 11 dominant ciliates with high abundance and/or occurrence, and the other 6 groups (II–VII) represented the assemblages with low abundance and/or occurrence (Figure 6). In the CS samples, 38 ciliate taxa were also assigned to 6 groups (I–VI): group I showed a similar pattern to that of PFES samples, and included 12 dominant species, whereas the other groups (II–VI) represented considerable differences from those of the PFES samples (Figure 7).
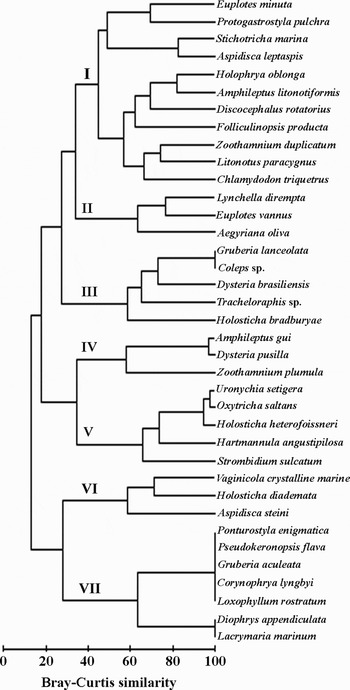
Fig. 6. Dendrogram of the species distribution using group-average clustering from Bray–Curtis similarities on log-transformed abundance data of each species within the ciliate community from the polyurethane foam enveloped slide system. I–VII, groups I–VII.

Fig. 7. Dendrogram of the species distribution using group-average clustering from Bray–Curtis similarities on log-transformed abundance data of each species within the ciliate community from the conventional slide system. I–VI, groups I–VI.
Analysis of similarities (ANOSIM) showed that no significant differences existed at the 60.37% dissimilarity level (P = 0.887) between the species compositions of the PFES and the CS samples with regard to the occurrence (presence/absence) of species. The species distribution, however, was significantly different at the 61.74% dissimilarity level (P = 0.007) as regards to both abundance and occurrence between the PFES and the CS samples. SIMPER analysis and paired t-test revealed the average Bray–Curtis dissimilarity and difference in abundance of the top nine ciliate taxa at 90% level of the cumulative contribution percentage between the PFES and the CS samples (Table 5). Furthermore, cluster analysis on either occurrence or log-transformed abundance revealed that the sessile ciliate Zoothamnium duplicatum was the most primary contributor to the dissimilarity of the two groups in abundance and/or occurrence. This was almost certainly attributable to much higher abundance in the CS samples than that in the PFES samples (P < 0.05) even though they predominated in both groups (Tables 1 & 5). The vagile ciliates Aspidisca steini and Aspidisca leptaspis presented higher contribution in high occurrence frequency to the samples of the PFES system, while heterotrichs Folliculinopsis producta and Protogastrostyla pulchra showed comparative lower contribution in both occurrence and abundance (Figure 8).

Fig. 8. Cluster analysis using the Bray–Curtis similarities on (A) occurrence and (B) log-transformed abundance data of the top 8 ciliate species at 90% level of the cumulative contribution percentage of the polyurethane foam enveloped slide (I) and the conventional slide (II) samples.
Table 5. Contribution to the average Bray–Curtis dissimilarity (61.74%) and abundance differences of the top nine ciliates between the samples from the PFES and CS system.

*, significant difference at the 0.05 level; Av. Diss., average dissimilarity; see Table 3 for other abbreviations.
Comparison of ciliate communities between the PFES and the CS system
A dendrogram and an MDS ordination of the 16 samples of both the PFES and the CS system were plotted respectively using the group-average clustering from Bray–Curtis similarities on log-transformed abundances (Figure 9). The cluster analysis resulted in the classification of 16 samples into 3 groups (Ia, Ib and II) at a 50% similarity level. Groups Ia and Ib represented the PFES samples due to the presence of 7 (88%) samples from this system, while group II included 7 (88%) samples from CS system (Figure 9). Furthermore, it should be noted that the PFES samples exhibited higher temporal variation than the CS samples: the former represented three temporal stages at 50% similarity level, while the latter showed a higher similarity (70%).

Fig. 9. Cluster analysis and multidimensional scaling (MDS) ordination of 16 samples of the polyurethane foam enveloped slide (I) and conventional slide (II) system using the Bray–Curtis similarities on log-transformed abundance data. (A) cluster dendrogram; (B) MDS ordination.
ANOSIM indicated that the community structure of ciliates of the PFES samples were significantly different from that of the CS samples (P < 0.05).
DISCUSSION
Efficiency of the glass slide method for bioassessments
So far previous investigations concerning the artificial substrate method have generally focused on freshwater ecosystems (Cairns & Yongue, Reference Cairns and Yongue1968; Bamforth, Reference Bamforth and Cairns1982; Cairns & Henebry, Reference Cairns, Henebry and Cairns1982; Railkin, Reference Railkin1995; Franco et al., Reference Franco, Esteban and Téllez1998; Strüder-Kypke, Reference Strüder-Kypke1999). Compared to the freshwater environments, tidal currents and strong circulations are considerably different factors in the marine ecosystems (Cairns & Yongue, Reference Cairns and Yongue1968; Xu et al., Reference Xu, Choi, Yang, Lee and Lei2002; Gong et al., Reference Gong, Song and Warren2005).
Glass slides have proven to be a robust, inexpensive and reliable method for collecting periphytic ciliates (Foissner, Reference Foissner, Lee and Soldo1992; Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999; Gong et al., Reference Gong, Song and Warren2005). Biomonitoring using periphytic ciliates is widely accepted and has many advantages: (1) they are easy to sample compared with other organisms; (2) the generation times are short and they are protected from the environment by only a delicate membrane, and thus their responses to pollution events are fast; (3) periphytic species in particular are relatively immobile, and the increasing availability of easily used taxonomic references; and (4) artificial substrates allowing colonization can be standardized for temporal and spatial comparisons (Cairns & Yongue, Reference Cairns and Yongue1968; Foissner, Reference Foissner, Lee and Soldo1992; Song et al., Reference Song, Xu, Shi, Hu, Lei, Wei, Chen, Shi, Wang and Song1999; Gong et al., Reference Gong, Song and Warren2005). The present study, however, showed that periphytic ciliates, especially the vagile forms, might be more or less broken away from the naked glass slides during the colonization process. Therefore, these indicators might be more or less affected by physical factors rather than chemical factors, thus limiting their use for bioassay in marine ecosystems.
Of all the other artificial substrates, polyurethane foam units (PFU) have been widely used to collect protist communities for bioassessments, but the conventional PFU method applied to marine waters was not efficient as in freshwater, mainly due to tidal currents and circulations (Xu et al., Reference Xu, Choi, Yang, Lee and Lei2002). Although a modified PFU method, named bottled PFU (BPFU) system which can effectively protect the substrate from tidal conditions, such a strategy is, however, not so well suited for studies that involve dynamics of community structures in long time scales or in large numbers of sampling sites, either due to enumeration or the demanding labour and time (Xu et al., Reference Xu, Choi, Yang, Lee and Lei2002; Gong et al., Reference Gong, Song and Warren2005). In addition, harsh handling methods such as squeezing PFUs may result in the failure to recover certain types of ciliates, e.g. sessile and highly thigmotactic species (Gong et al., Reference Gong, Song and Warren2005).
The PFES system incorporates the virtues of the CS system and ameliorates its weakness. With regard to its advantages, it might be summarized that: (1) the caved PFU, as a mechanical buffer, may protect periphyton colonization on the naked glass slides from being disturbed by tidal and rough conditions; (2) the caved PFU-barrier might prevent the sessile periphytic metazoan such as the coelenterates, annelids and barnacle from colonizing the enveloped slides and allows the periphytic ciliates to predominate in the periphyton communities; and (3) the PFU-filter, as sediment trap, may protect the naked slides from being covered with the particular sediments and make identification and enumeration of ciliates easily. The present study showed that the species diversity and evenness were higher in the PFES samples than in that on naked ones. This finding suggests the PFES system is effective to colonize periphytic ciliates with high species diversity, evenness and richness in marine ecosystems.
It should be noted that in the PFES system the caved PFUs enclosed the whole slide-frame and thus reduced the water exchange into the system. This may limit the availability of food particles which are transported inside. Therefore the feeding current of Zoothamnium spp. might not obtain much food supply available as in the CS system and thus result in a decrease of the peritrich numbers. Furthermore, the PFUs may act as sediment trap and/or a kind of biotic filters because of growing biofilm. This also makes the larvae of the sessile peritrichs difficult to pass through the 2-cm barrier, while the vagile forms Aspidisca spp. may be easily colonized on glass slides from the PFU biofilm (Zimmerman, Reference Zimmerman1961; Cairns & Yongue, Reference Cairns and Yongue1968). This may explain the differences that the periphytic ciliates (mainly peritrichs) represented higher abundance in the CS system while the vagile ciliates occurred in the PFES system with higher frequency.
Taxonomic composition
In the present study, 37 species of ciliates belonging to 9 orders and 27 genera were found by the PFES system. The results obtained were similar to the taxonomic composition that the 38 ciliates represented, 8 orders and 27 genera in samples of the CS system. Thirty-four ciliates belonging to 8 orders and 26 genera were found in both the PFES and the CS samples from August to November 2007 (Table 1).
Our results were also similar to other previously published reports. Persoone (Reference Persoone1968) reported 30 ciliate species that represented 9 orders and 27 genera in a polluted harbour at Ostend, Belgium, and Gong et al. (Reference Gong, Song and Warren2005) found 37 ciliate taxa belonging to 10 orders and 30 genera in the scallop-farming area of Jiaozhou Bay near Qingdao (China) using the glass slide method during a 1-year period respectively. Comparing the taxonomic compositions of Incheon and Qingdao communities, 17 species (e.g. Zoothamnium duplicatum, Aspidisca leptaspis, Aspidisca steini, Amphileptus litonotiformis, Holosticha bradburyae and Litonotus paracygnus) and 16 genera (accounting for 53.3% of genera recorded in the Qingdao study) were found in both locations. A comparison at the order level reveals even higher similarity between the two faunas: 8 out of 10 ciliate orders in Qingdao were also present in the Incheon samples. Over half of the species in the Qingdao samples were from the orders Hypotrichida (36%) and Cyrtophorida (23%). The same two orders accounted for similar proportions of the species composition in both the PFES (40.5% and 16.2%) and the CS samples (50.0% and 10.5%).
About 130 periphytic ciliates were found on a combination of submerged objects and glass slides in the Caspian Sea (Agameliev, Reference Agameliev1974). Species within the orders Hypotrichida (36.2%) and Peritrichida (18%) accounted for over half of the total community. However, it should be noted that the higher species richness of periphytic ciliates in the Caspian Sea compared to our study was almost certainly due to the larger number of samples (500) and the wider range of the locations sampled.
A total of 45 species representing 34 genera were found using the CS method in the Lagoon of Venice, 13 (48%) of which were also found using the PFES and the CS system respectively in the Incheon study (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000). Like the ciliate faunas of Incheon, Qingdao, Ostend and the Caspian Sea, the Hypotrichida represented the largest proportion of species (33%) in the Venice samples, the second largest being the Peritrichida (17.8%). Cyrophorida accounted for only 2%. Moreover, the karyorelictids Trachelocerca lacrymariae, T. multinucleata, Tracheloraphis gracilis, Remanella multnucleata and Geleia swedmarki were also included in the species list although they are usually considered as benthic species (Fenchel, Reference Fenchel1969). The depth at which the artificial substrate was submerged might explain this finding since most samples of the Lagoon of Venice were recovered from just 60 cm above the bottom (Coppellotti & Matarazzo, Reference Coppellotti and Matarazzo2000). In our study, the benthic ciliate Tracheloraphis sp. was also detected. This may be explained by the similar reason that both our two systems were tied on a floating pier, which was adjacent (about 1 m) to the seashore.
Franco et al. (Reference Franco, Esteban and Téllez1998) classified various taxonomic orders of ciliates into feeding categories on the basis of three parameters namely, the structure and function of the oral apparatus, the manner in which the ciliate collects its food, and the size of the captured food particles. The taxonomic order itself, however, circumscribes certain aspects of the morphology of any given ciliate and thus, to an extent, provides a clue to its ecology. In the present study, the predominance of the dorsoventrally flattened hypotrich species is almost certainly due to this adaptation of protozoa that crawl on surfaces (Fenchel, Reference Fenchel1987). The bilaterally flattened cyrtophorids were the second largest group in the Ostend, Qingdao and Incheon surveys. These findings were also demonstrated by our modified slide method in the present study.
Univariate and multivariate analyses
Species diversity, evenness and richness indices are commonly employed in community studies and are amenable to simple statistical analysis (Magurran, Reference Magurran1991; Ismael & Dorgham, Reference Ismael and Dorgham2003). Generally, the higher these three indices were, the better the water conditions represented, excepted the cases in running waters with low organic pollution (Ismael & Dorgham, Reference Ismael and Dorgham2003). In our study, all three indices represented higher values in the PFES samples from those in the CS samples. This finding might be due to the more effective reduction of strong disturbances from tidal current and circulation in marine ecosystems by the PFES system. This may also explain why almost all three indices generally failed to show any significant correlation with environmental factors in some previous studies (Vaultonburg & Pederson, Reference Vaultonburg and Pederson1994; Gong et al., Reference Gong, Song and Warren2005).
Multivariate analyses were more sensitive than univariate ones in detecting changes in species distribution or community structure (Gong et al., Reference Gong, Song and Warren2005). In the present study, although the species composition showed similar patterns, both species distribution and community structure represented significant differences between the PFES and the CS samples. This may be attributed mainly to the sessile ciliates overly predominating the ciliate communities from the open glass slides. This finding is consistent with the ciliate faunas of Qingdao, Ostend and the Caspian Sea (Gong et al., Reference Gong, Song and Warren2005). Furthermore, analysis of MDS ordination indicated that the ciliate communities represented more sensitive temporal dynamics in the PFES system than in the CS system. It is therefore suggested that the PFES system is more effective to colonize periphytic ciliate communities with sensitive responses to the temporal changes of environmental conditions in marine ecosystems. It should be noted, however, that the present study was restricted to a single sampling site in Korean coastal waters. Thus, further studies on a range of sampling sites with different environmental conditions are needed, in order to conclude the temporal and spatial variations of the periphytic ciliate communities and their relationships to the changes of environmental parameters.
In conclusion, our studies have demonstrated that the periphytic ciliate communities in a modified glass slide system had similar taxonomic compositions but represented different species distribution and community structure with high species diversity and evenness in comparison to the conventional slide method. Furthermore, it was also found that the modified slide method might prove to be more efficient to detect the temporal dynamics of periphytic ciliate communities in marine ecosystems. However, further studies on a range of marine waters and over extended time periods are warranted in order to verify this conclusion.
ACKNOWLEDGEMENTS
Our special thanks are due to Dr P. Sun, Ocean University of China, Qingdao, China, for her help in identifying some ciliate species. This work was supported by the grants of the Korea Research Foundation (KRF-2007-COO265) and Ministry of Environment (The Eco-technopia 21 project of 2007), the KORDI's national fund (PM53400, K.J. Choi) and a post-doctoral fellowship from Inha University to H. Xu. The authors would like to thank a number of anonymous referees who offered valuable advice during the preparation of this manuscript.
















