Introduction
The E2F transcription factor family is distributed widely in eukaryotes. Currently, this family comprises eight genes (E2F1, E2F2, E2F3a, E2F3b, E2F4, E2F5, E2F6, E2F7, and E2F8) (Attwooll et al., Reference Attwooll, Denchi and Helin2004; Iaquinta & Lees, Reference Iaquinta and Lees2007); however, this number might increase as new members are discovered in mammals and homologs are identified in other eukaryotes (Dyson, Reference Dyson1998; Nevins, Reference Nevins1998). On the basis of their structures and physiological functions, the E2F family members are classified into transcriptional activators (E2F1 to E2F3a) and transcriptional repressors (E2F3b to E2F8) (Iaquinta & Lees, Reference Iaquinta and Lees2007). They function in cell cycle progression and differentiation, as regulators of apoptosis, and in tumour suppression. The application of new technologies has expanded the number and nature of genes regulated by E2F. The functions of these genes range from cell growth regulators to genes involved in differentiation, development, DNA repair, recombination and apoptosis, mRNA processing, and includes genes with no identified function (DeGregori, Reference DeGregori2002; Cam & Dynlacht, Reference Cam and Dynlacht2003).
The Chinese oak silkworm (Antheraea pernyi (Guerin-Menebille)) is a wild insect species whose larvae feed on oak plant leaves. They are reared in several Asian countries, such as China, Korea, and India. The A. pernyi larvae are important producers of raw silk, and in China alone almost 70,000,000 kg of cocoons (pupae) are produced each year, which contribute 90% of the world's production (Chang et al., Reference Chang, McWatters, Williams, Gotter, Levine and Reppert2003; Wei et al., Reference Wei, Hong, Jiang, Tong and Lu2008; Liu et al., Reference Liu, Li, Li and Qin2010). Furthermore the larvae, pupae, and moths are edible and contain high quality proteins, which constitute all the amino acids required for human nutrition. They are also used to produce cosmetics. Their pupae contain 45–55% protein as dry matter, which could raise haemoglobin and serum total protein levels significantly when fed to rats, producing protective effects on the liver in a carbon tetrachloride-induced rat hepatic injury model (Yang et al., Reference Yang, Zhu and Lu2002; Zhou & Han, Reference Zhou and Han2006).
In the present study, we investigated E2F transcription factor 4 (E2F4) an important member of the E2F family in A. pernyi (Saturniidae: Lepidoptera). The E2F4 gene has been studied extensively in animals, particularly in mammals, and in plants (Müller & Helin, Reference Müller and Helin2000; Lammens et al., Reference Lammens, Li, Leone and De Veylder2009); however, there is a lack of literature on E2F4 in insects. Therefore, the present study aimed to characterize E2F4 in A. pernyi. We cloned the E2F4 gene and described its expression profile following challenge with viral, bacterial, and fungal pathogens. Moreover, we determined the expression of E2F4 in different developmental stages of A. pernyi. This basic knowledge will provide the foundation for further studies to explore the immune functions of E2F4.
Materials and methods
Experimental organism
The present study was conducted in the Silkworm Immunology laboratory, Department of Biochemistry and Molecular Biology, Anhui Agricultural University, Hefei, China. Pupae of A. pernyi were obtained from the Sericultural Research Institute of Liaoning, China, and raised to adults. Eggs were collected and incubated until hatching. The larvae were fed on fresh oak leaves and kept at room temperature, with a 10:14-h light:dark photoperiod, and 70% relative humidity until they transformed into pupae.
RNA extraction and cloning of E2F4 transcription factor
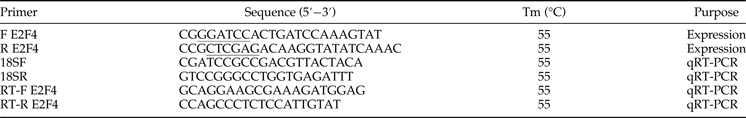
Total RNA was extracted from the fat bodies using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and first-strand cDNA was synthesized using TransScript Synthesis SuperMix (TransGen, Beijing, China). Oligonucleotide primers were designed using the Primer Premier 5.0 software package to amplify a fragment of E2F4 (table 1). Polymerase chain Reaction (PCR) was performed using the following amplification program: 5 min at 94°C; followed by 35 cycles at 94°C for 1 min, 55°C for 40 s and 72°C for 1 min; and a final elongation step at 72°C for 10 min. The PCR product was resolved by 1% agarose gel electrophoresis and analysed. Invitrogen than sequenced the obtained product.
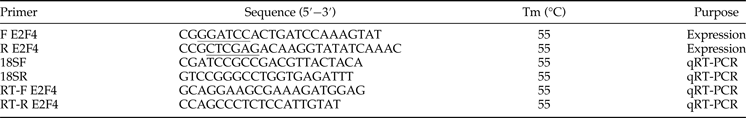
Table 1. Primers used in the present study.
Nucleotide sequence analysis
NCBI bioinformatics tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to determine conserved domains in the predicted E2F4 protein. Furthermore, the molecular weight of the E2F4 was calculated by ExPASy (http://web.expasy.org/compute_pi/). Multiple sequence alignments were performed using the ClustalX package with its default parameters (Livak & Schmittgen, Reference Livak and Schmittgen2001). A phylogenetic tree was constructed with MEGA 5.1 using the neighbour-joining algorithm method (Tamura et al., Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011), with a bootstrap test of 1000 replications.
Prokaryotic expression and protein purification
A pair of specific primers (F E2F4 and R E2F4) was designed to amplify the 803 bp DNA fragment comprising the entire open reading frame (ORF) of E2F4. The ORF was cloned into cloning vector pMD-19T, which was then digested with Bam HI and Xho I, and the fragment containing the ORF was ligated into the pET-30a(+) vector (Novagen, USA). The insertion of the correct fragment was confirmed in the recombinant plasmid, named pET-30a-E2F4, by DNA sequencing, and transformed into Escherichia coli Transetta (DE3) cells (Novagen, USA) for protein expression. The Transetta (DE3) cells were cultured in Luria-Bertani media for 4 h and then subjected to isopropyl-β-D-thiogalactopyranoside (IPTG) induction at a final concentration of 0.8 mM in the culture medium. After 12 h of culture, the cells were harvested by centrifugation at 8000 g for 5 min. The pellets (cells) were suspended in binding buffer (20 mM Tris–HCl, 500 mM NaCl, 5 mM imidazole, pH 7.9) and disrupted by sonication on ice. The disrupted cells were centrifuged at 12,000 g for 20 min at 4°C, and recombinant protein was purified using the QIAexpress® Ni-NTA Fast Start Kit (Qiagen, Germany), according to the manufacturer's protocol. The recombinant protein was analysed by 15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using antibodies prepared as detailed in the next section.
Antibody preparation
The antiserum was prepared according to a previously described method (Harlow & Lane, Reference Harlow and Lane1999). New Zealand white rabbits were immunized three times for 2-weeks with 100 µg eluted E2F4 protein homogenized in complete Freund's adjuvant. A booster injection was administered 1 week later. The rabbit serum was collected 7 days after the final immunization and stored at −80°C. A monoclonal anti-6-His antibody (Qiagen, Germany) was used to confirm protein expression and the molecular weight.
Expression analysis using quantitative real-time PCR (qRT-PCR)
The total RNA was extracted from fat bodies, haemocytes, midguts, silk glands, Malpighian tubules, and the epidermis of larvae using the TRIzol reagent (Invitrogen), according to the manufacturer's instructions. The extracted RNA was reverse transcribed into cDNA. Oligonucleotide primers specific for the E2F4 sequence and the endogenous control (18S rRNA, accession number: DQ347469) were designed using Primer 5.0, based on known sequences (table 1). Real-time PCR was performed in 25 µl reactions containing 12.5 µl of 2× SYBR Premix Ex TaqII (Takara), 1 µl each of the forward and reverse primers, 2 µl of the cDNA, and 8.5 µl of RNase-free H2O. The amplification program was performed as follows: 95°C for 30 s, followed by 39 cycles at 95°C for 15 s, 55°C for 30 s, and 72°C for 30 s. A melting curve was produced by monitoring the fluorescence continuously while slowly heating the sample from 65 to 95°C. The relative expression level of the E2F4 gene was calculated according to the 2−ΔΔCt method (Livak & Schmittgen, Reference Livak and Schmittgen2001). All qRT-PCR experiments were repeated five times. The expression of the E2F4 gene in the epidermis was arbitrarily set to 1 and used for normalization.
SDS-PAGE and Western blotting
The different tissues, such as the fat bodies, haemocytes, and mid-gut, were ground in liquid nitrogen and dissolved in Radioimmunoprecipitation assay (RIPA) lysis buffer (Sangon, Shanghai, China). The concentrations of the extracted proteins were determined using the bicinchoninic acid (BCA) method. The proteins were subjected to SDS-PAGE and then transferred to a polyvinylidene difluoride (PVDF) membrane (Sigma, St. Louis, MO, USA) by electrophoretic transfer. Anti-beta-Actin antibodies (Transgen biotech, Beijing, China) were used as loading control primary antibodies. The membranes were blocked with 5% non-fat milk in PBST (PBS containing 0.1% Tween-20) overnight at 4°C, and then washed three times with PBST for 10 min each. The membranes were incubated with the anti-E2F4 antibodies (diluted 1:500 with 5% non-fat milk in PBST) for 2 h at room temperature, washed with PBST, and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (TransGen, Beijing, China; diluted 1:2000 with 5% non-fat milk in PBST) for 1 h at room temperature. Immunoreactive protein bands were detected using an HRP-diaminobenzidine (DAB) Detection Kit (Sangon, Shanghai, China).
Immune challenge assays
Fifth-instar larvae of A. pernyi were divided into five groups containing three larvae each. The larvae were injected in their abdomen with 5 µl of heat-treated E. coli (Migula) (DH5α, 1 × 106 cells), A. pernyi nucleopolyhedrovirus (Ap-NPV, 1 × 106 particles), Micrococcus luteus (Schroeter) (1 × 106 cells), Beauveria bassiana (Bals-Criv) (1 × 106 spores), or phosphate-buffered saline (PBS) as a control. The injections were performed using microliter syringes (Gaoge, Beijing, China), and the injection sites were sealed with Vaseline immediately after injection. The haemolymph and fat bodies were collected at 1.5, 3, 6, 12, 24, and 48 h post-injection. Three larvae were collected as one sample, and the biological sampling protocol was repeated three times. The transcript and protein expression analysis of E2F4 were performed using qRT-PCR or Western blotting analysis, respectively, as described above.
Results
Bioinformatic analysis of the E2F transcription factor 4 cDNA
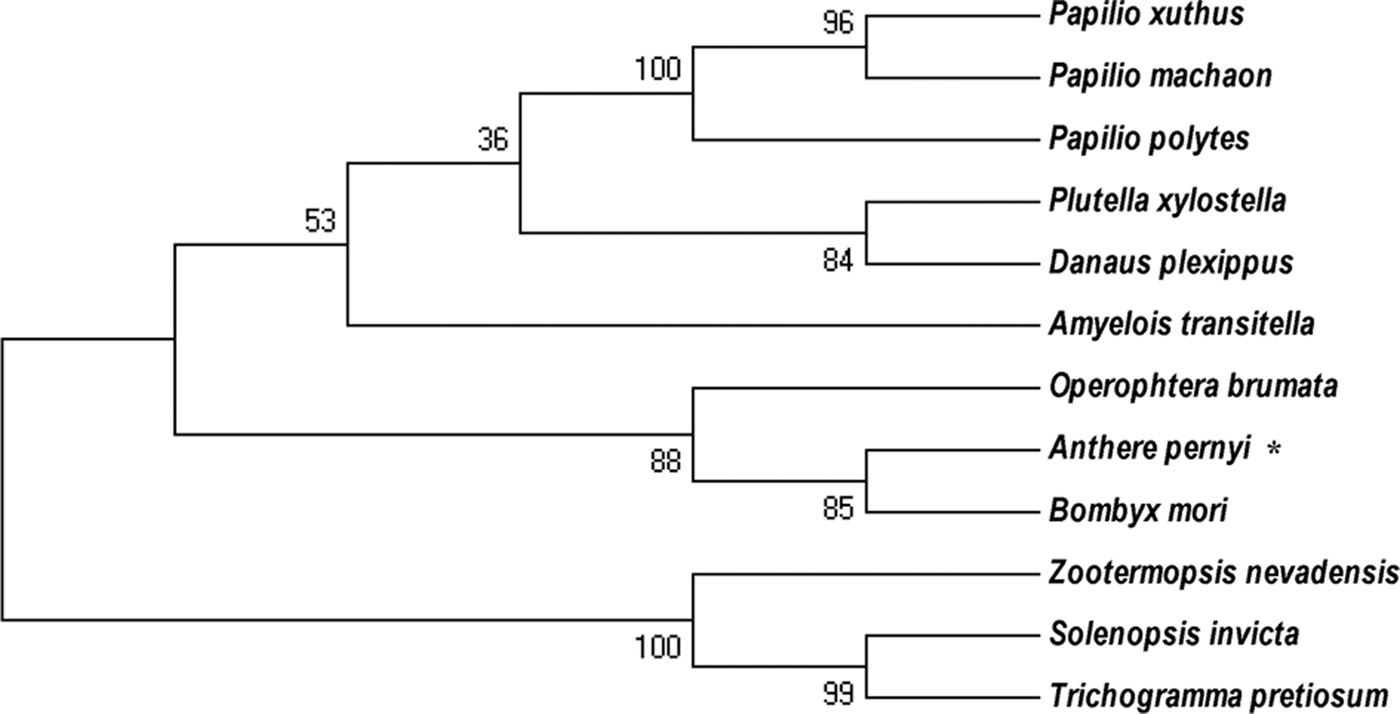
The isolated 967 bp cDNA fragment of E2F4 contained a 5′-untranslated region (UTR) of 64 bp, a 3′ UTR of 108 bp and an ORF of 795 bp, encoding a 264 amino acid residue protein, is provided in supplementary figure S1. No signal peptide was found in the deduced protein sequence. The deduced protein had a predicted molecular weight of 30.55 kDa and an isoelectric point of 5.27. BLAST analysis of the whole protein sequence revealed that E2F4 of A. pernyi shared high similarity to other insect E2F4 proteins, in particular to the Bombyx mori L (fig. 1). E2F transcription factor 4-like protein. The winged-helix DNA-binding (E2F_TDP), E2F_coiled coil-marked box (E2F_CC-MB), and E2F_Dimerization domain (E2F_DD) Putative conserved protein domains were detected in E2F4, is provided in supplementary figure S2. The E2F4 belongs to E2F and DP family. It has ability to bind DNA either as homodimer or as heterodimer in association with TDP1/2; however, the heterodimer increase its binding efficiency. The E2F_CC-MB forms a heterodimer with the corresponding domain of the DP transcription factor that binds the C-terminus of the retinoblastoma (Rb) protein. Furthermore, many authors reported that members containing the third domain (E2F_DD) are involved in several biological processes, such as DNA synthesis, cell cycle progression, proliferation, and apoptosis (Zheng et al., Reference Zheng, Fraenkel, Pabo and Pavletich1999; Rubin et al., Reference Rubin, Gall, Zheng and Pavletich2005; Korenjak et al., Reference Korenjak, Anderssen, Ramaswamy, Whetstine and Dyson2012). The phylogenetic analysis revealed that E2F4 is phylogenetically close to members from the Lepidoptera, but is more distantly related to the Isoptera and Hymenoptera (fig. 2).
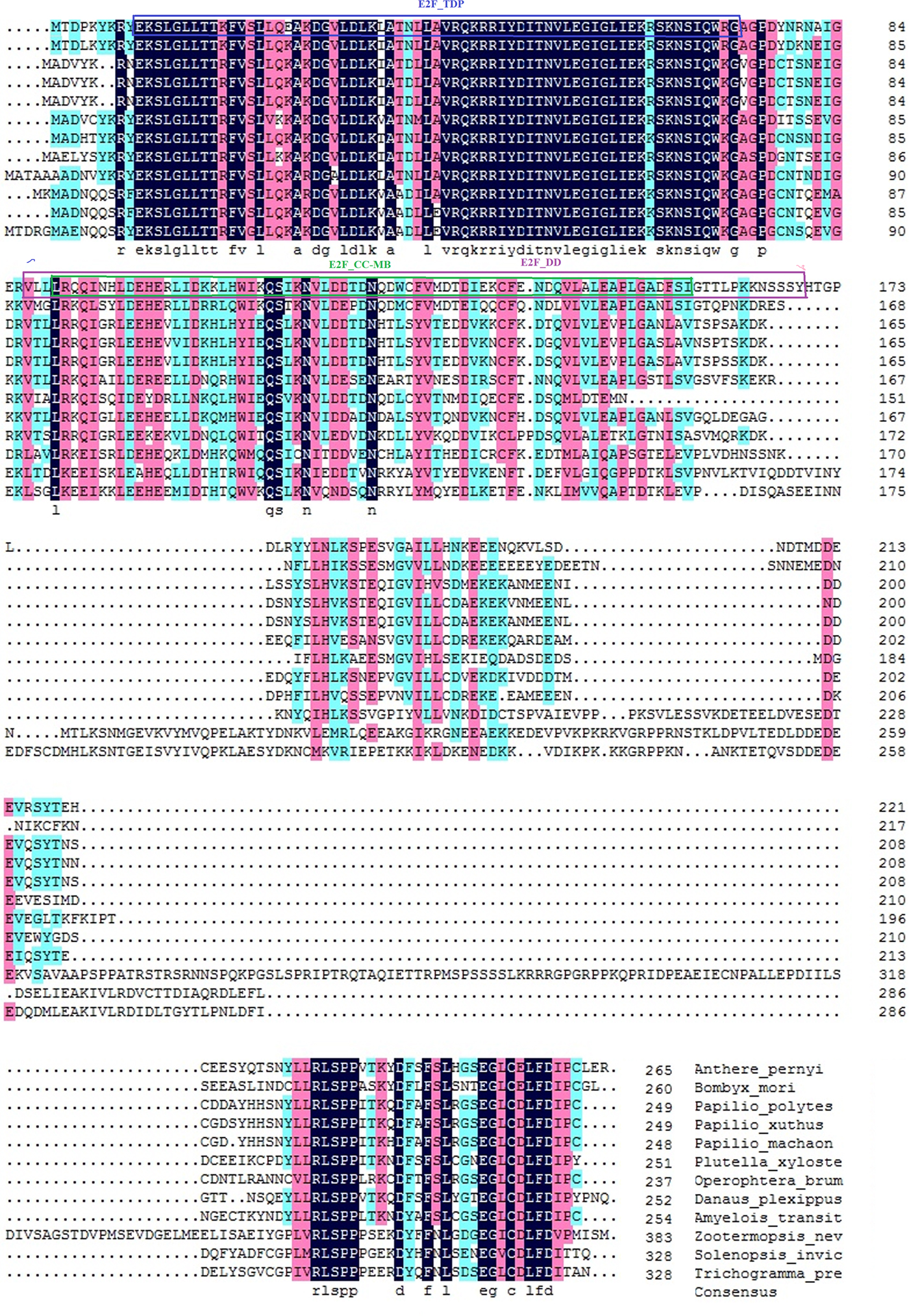
Fig. 1. Alignment of the E2F transcription factor 4 protein with its homologous proteins. The deduced amino acid sequence of E2F transcription factor 4 was aligned with the E2F transcription 4 proteins from Papilio polytes (XP_013146090), Papilio xuthus (XP_013182302), Plutella xylostella (XP_011561624), Papilio machaon (KPJ07879), Zootermopsis nevadensis (KDR18049), and the E2F transcription factor 4-like protein of Bombyx mori (NP_001040298), Papilio machaon (XP_014368162), Operophtera brumata (KOB76568), Danaus plexippus (EHJ67877), Amyelois transitella (XP_013190081), Solenopsis invicta (XP_011157894), and Trichogramma pretiosum (XP_014224938).
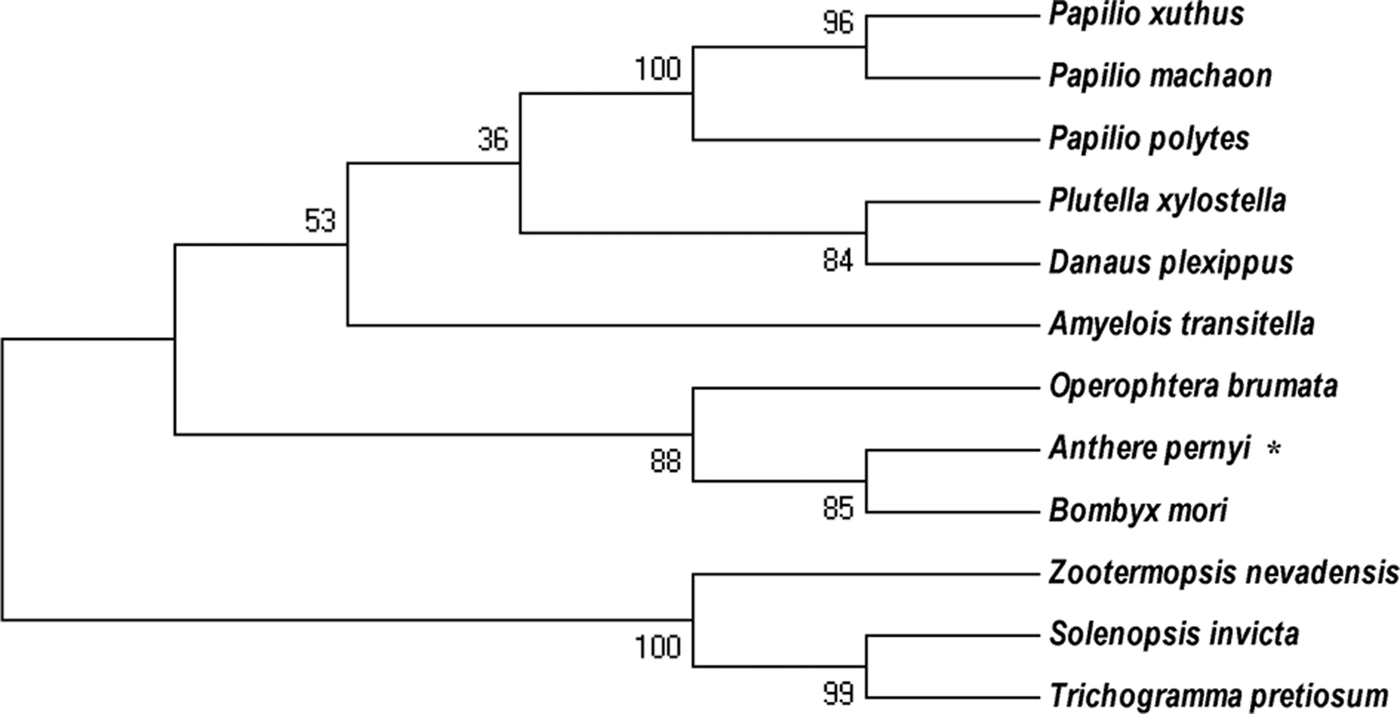
Fig. 2. Phylogenetic analysis of the A. pernyi Ap-E2F transcription factor 4 with other E2F4 E2F4-like protein from other species. The deduced amino acid sequences were aligned and a phylogenetic tree was constructed using MEGA (version 5.05) and the neighbour-joining method.
Protein expression, antibody preparation, and Western blot analysis
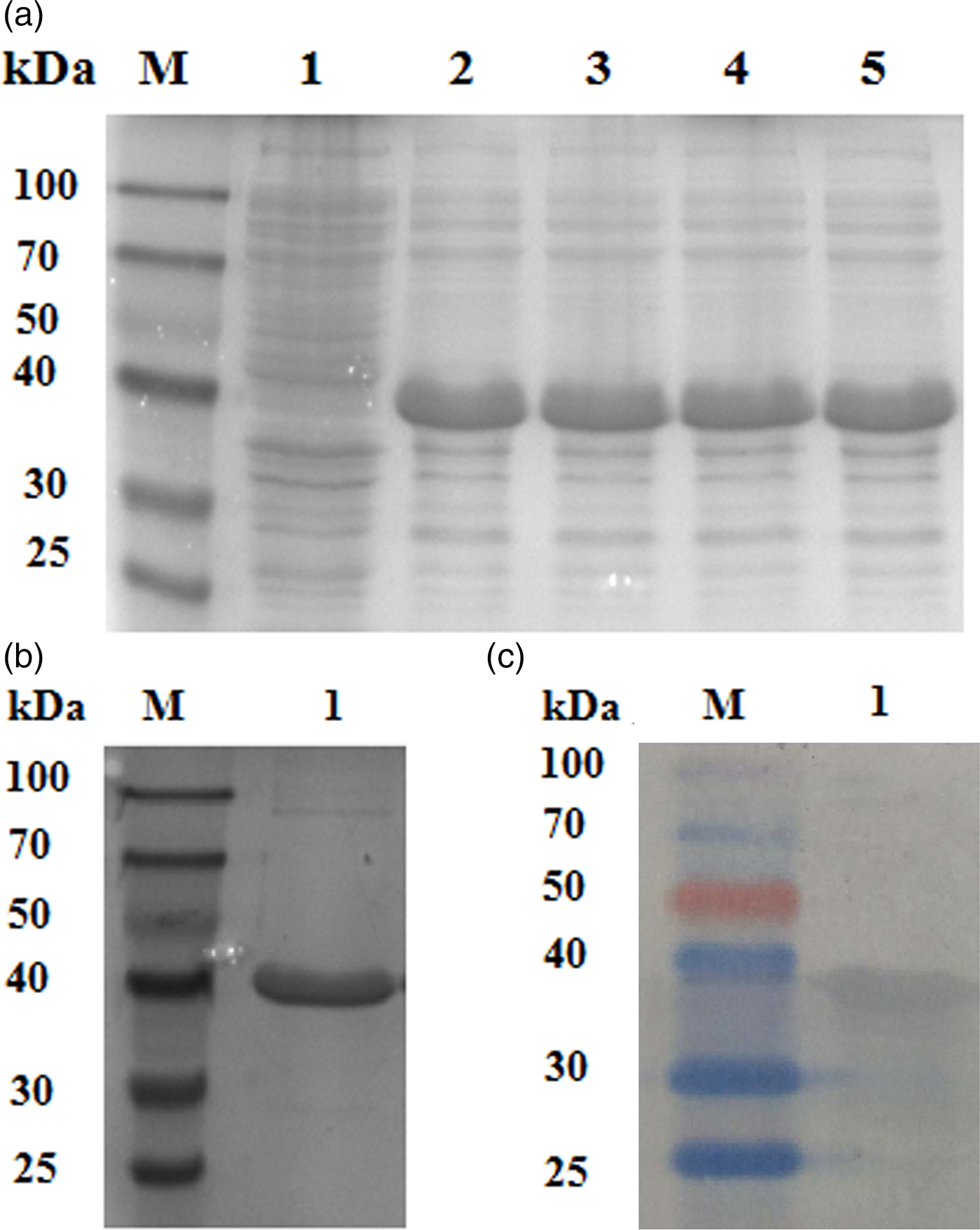
A protein of approximately 30 kDa was observed in protein extracts from E. coli induced by IPTG subjected to SDS-PAGE, which was consistent with the predicted size of E2F4 (fig. 3a). Different concentrations of IPTG did not influence the level of recombinant protein expression. A purified E2F × His fusion protein was obtained by affinity chromatography and a protein band corresponding to the predicted molecular weight of 30 kDa was observed (fig. 3b). Western blotting analysis of the recombinant proteins using an anti-His-tag antibody confirmed a consensus protein of 30 kDa (fig. 3c). The purified recombinant protein was then used to prepare rabbit anti-E2F4 antibodies, whose titre was approximately 1:10,000, as determined by an enzyme linked immunosorbent assay (ELISA).
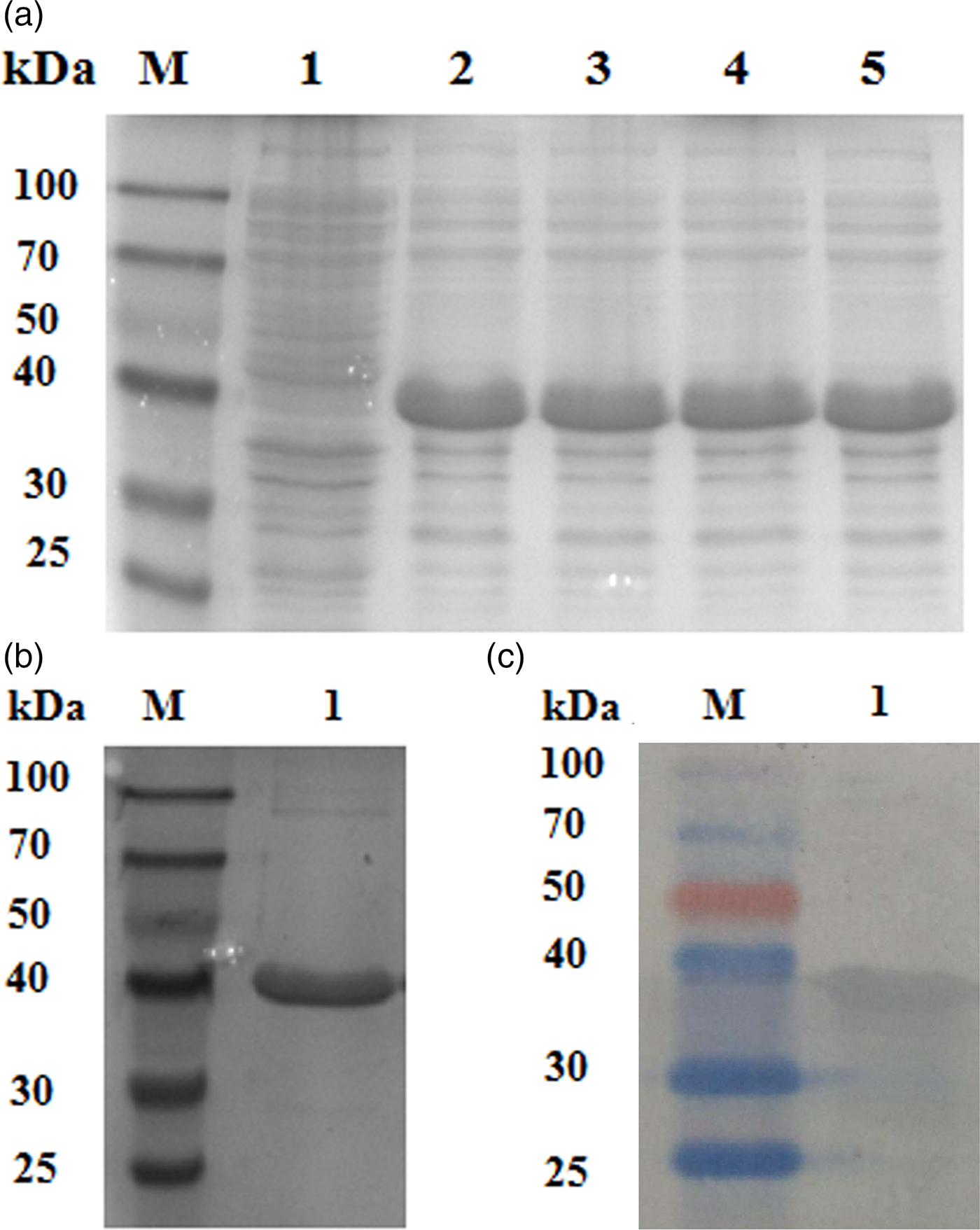
Fig. 3. Protein expression, antibody preparation, and Western blotting analysis. (a) SDS-PAGE of the E2F transcription factor 4 protein expressed in E. coli and induced by different isopropyl-β-D-thiogalactopyranoside (IPTG) concentrations. Lane 1: M: molecular marker; lane 2: non-induced expression; lanes 3, 4, 5, and 6: IPTG/Transetta DE3. (b) SDS-PAGE of expressed and purified E2F4 protein. (c) Western blotting analysis of the recombinant E2F4 protein visualized using with anti-His tag antibodies.
Expression of E2F transcription factor 4 in larval tissues
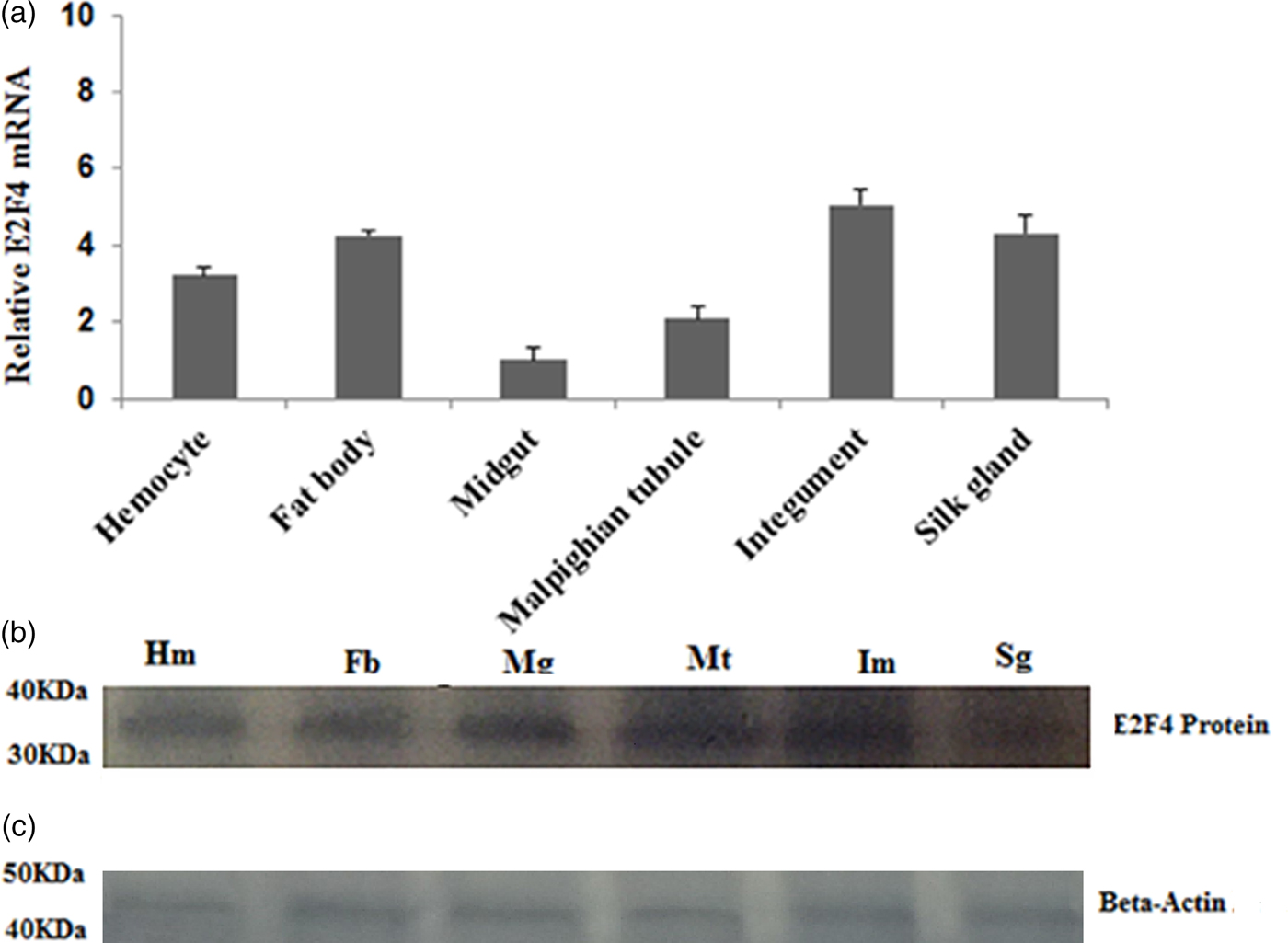
The E2F4 mRNA expression level in fat bodies, haemocytes, midguts, epidermis, silk glands, and Malpighian tubules was determined by qRT-PCR. E2F4 was expressed in all examined tissues; however, its levels in the fat bodies, haemocyte, and integument were higher compared with that in other tissues (fig. 4a). Western blotting analysis of proteins extracted from these tissues produced similar results to the qRT-PCR assays (fig. 4b).
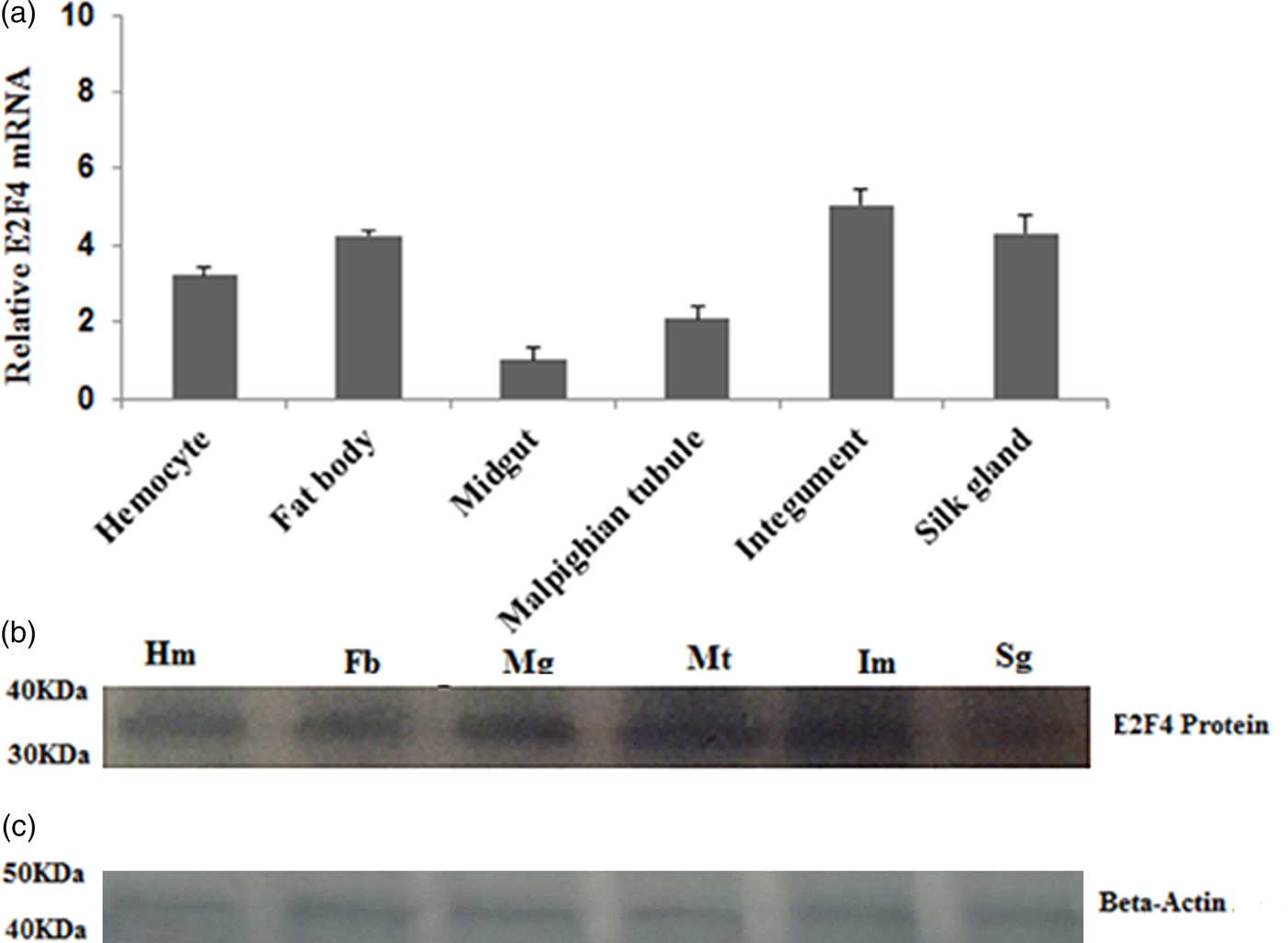
Fig. 4. Expression profile of the E2F4 protein in six different tissues of fifth-instar A. pernyi larvae. (a) Real-time PCR analysis of E2F4 mRNA in larval tissues. (b) Relative expression of E2F4 proteins in fifth-instar A. pernyi larval tissues, as analysed by Western blotting using antibodies specific to E2F4. (c) Beta-Actin antibodies were used as a loading control. Hm: Haemocyte; Fb: Fat body; Mg: Mid gut; Mt: Malpighian tubule; Im: Integument; Sg: Silk gland.
E2F4 Expression profile under pathogenic stress conditions
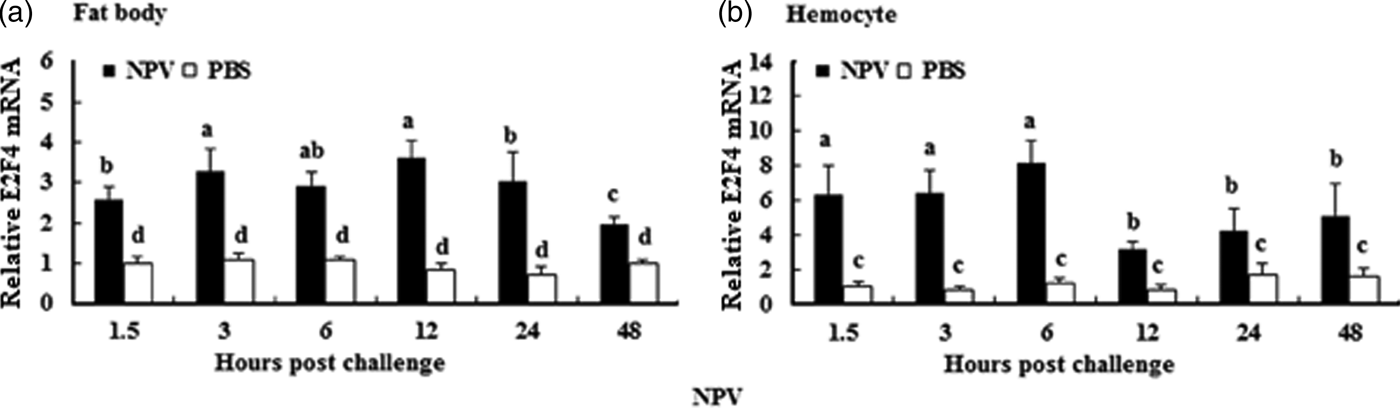
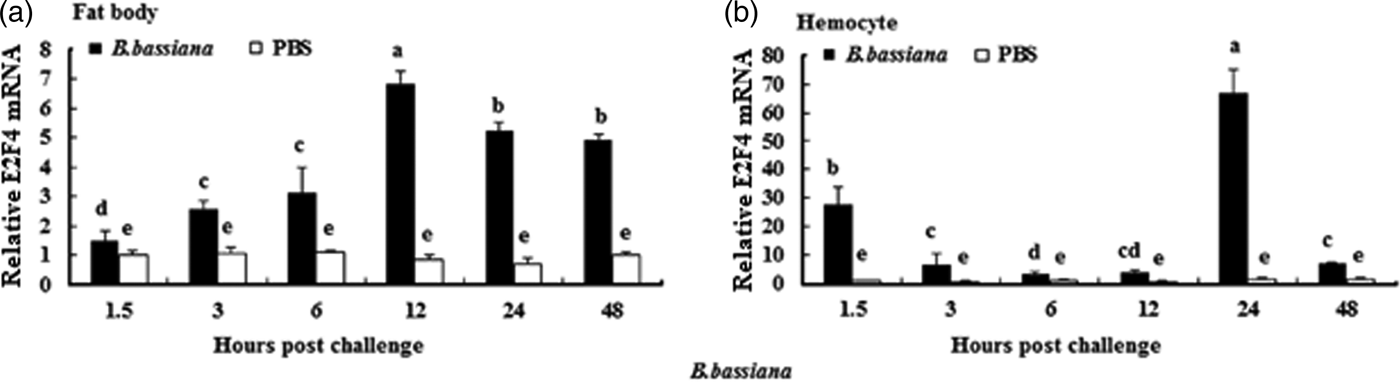
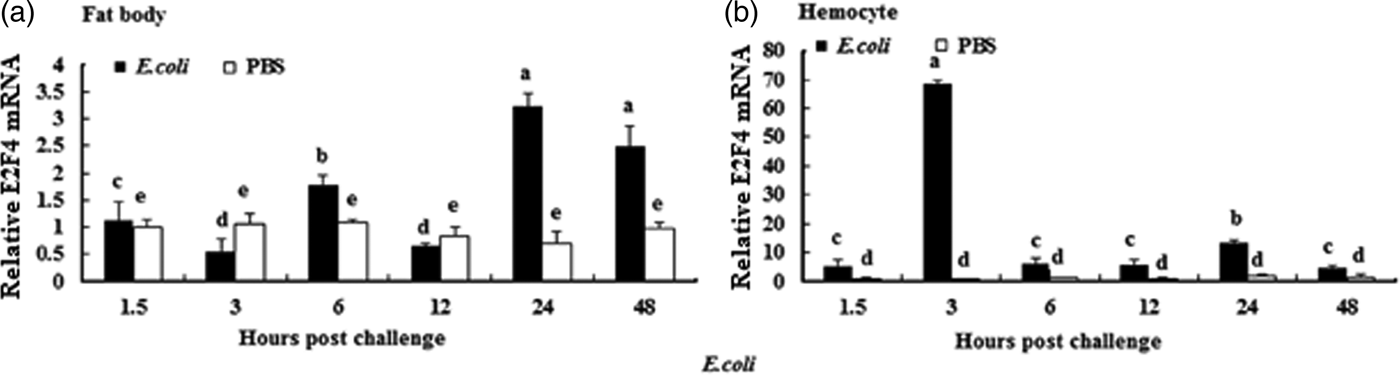
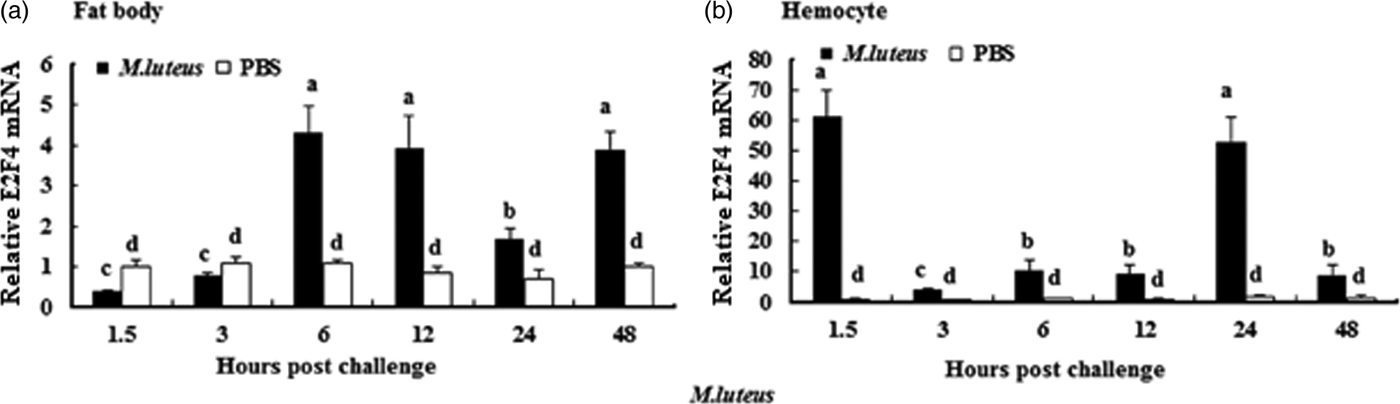
To understand whether E2F4 is involved in or influences the immune process of A. pernyi under biotic stress, we injected bacterial, fungal, and viral pathogens into fifth instar larvae. Total RNA were extracted from the fat body and haemocytes at different time intervals from 1.5 to 48 h, and were then investigated by real-time PCR. When A. pernyi larvae were treated with the pathogens, the abundance of E2F4 in the fat bodies and haemocyte varied considerably, the tissue expression varied with the type of pathogen. The viral (nuclear polyhedrosis virus), and fungal (Beauveria bassiana) pathogens induced E2F4 expression highly; however the expression level and time of maximum expression varied among them. In fat bodies, NPV produced two expression peaks at 3 and 6 h (fig. 5a), and the maximum expression in response to B. bassiana was recorded after 12 h of treatment of (fig. 6a). Meanwhile, two expression peaks were observed (at 24 and 48 h) after E. coli challenge (fig. 7a) and three were observed (6, 12, and 48 h) after M. luteus challenge (fig. 8a). Approximately similar trends were observed in haemocytes after treatment with the pathogens. The expression fluctuated greatly with the different pathogens and at different time intervals in the haemocytes compared with the fat bodies. NPV showed maximum expression level at 6 h (fig. 5b), whereas B. bassiana produced its maximum response at 5 h. The expression level of E2F4 was much higher in response to E. coli and B. bassiana: however, the maximum level attained by E. coli occurred at 3 h, while B. bassiana produced its maximum response at 24 h (figs 6b and 7b).
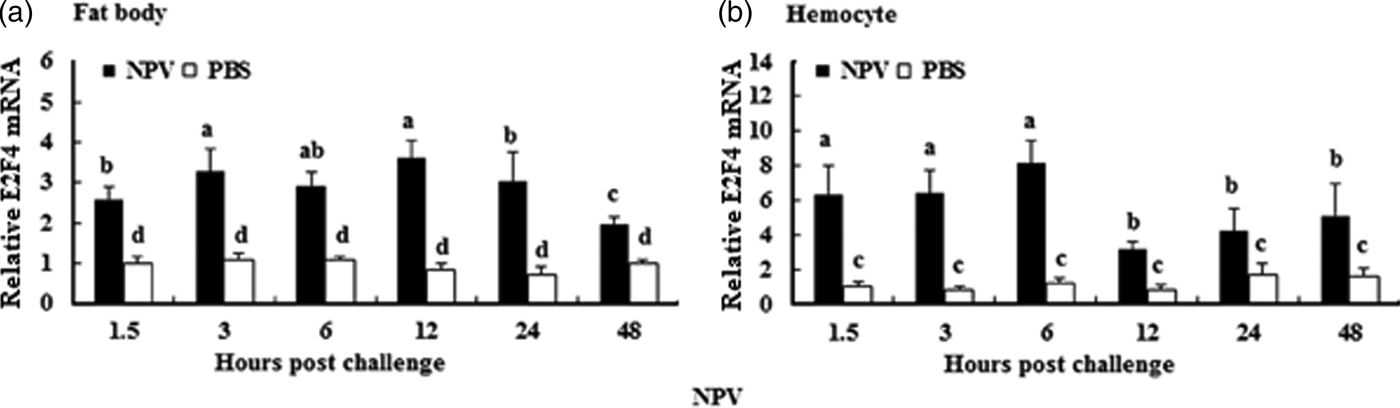
Fig. 5. Expression profiles of the E2F4 protein in haemocytes (a) and fat bodies (b) in the fifth-instar larval stage of A. pernyi from 1.5 to 48 h following nucleopolyhedrovirus challenge. The same letter indicates a non-significant difference, while values with different letters indicate a significant difference. The data were analysed using one-way analysis of variance. Data are represented as the means±standard error (SE). Differences were considered significant at P < 0.05.
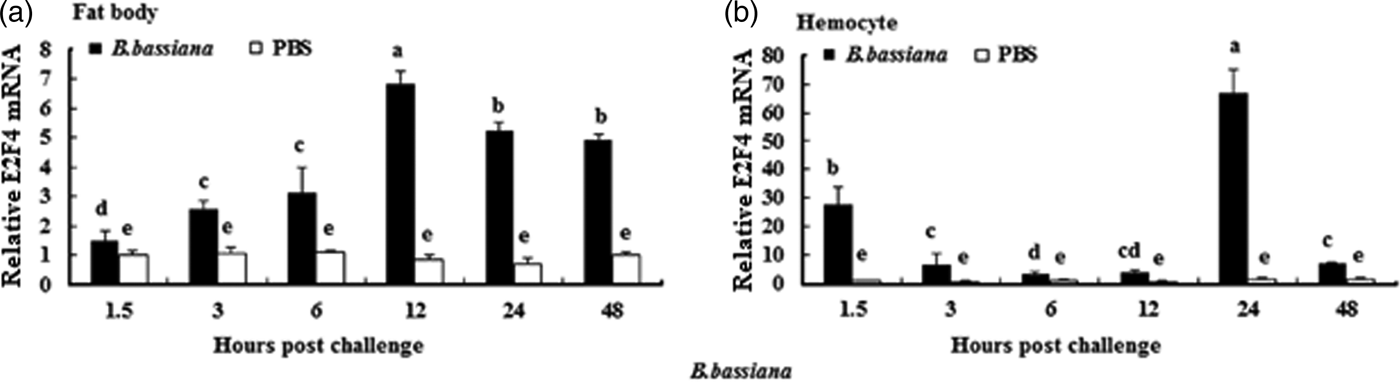
Fig. 6. Expression profiles of the E2F4 protein in (a) haemocytes and (b) fat bodies of A. pernyi fifth-instar larvae following B. bassiana challenge. The same letter indicates a non-significant difference while values with different superscript letters represent significant differences.
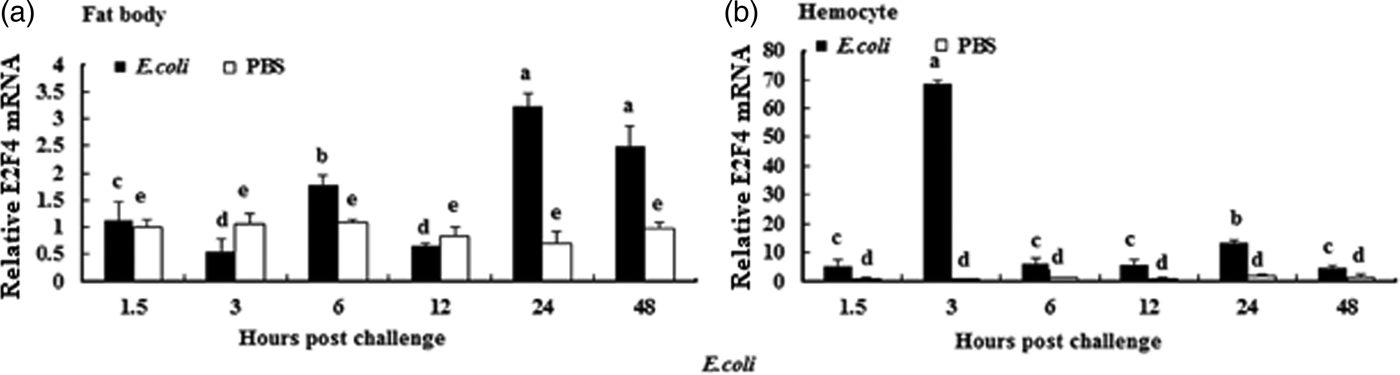
Fig. 7. Expression profiles of the E2F4 protein in (a) haemocytes and (b) fat bodies of A. pernyi fifth-instar larvae following E. coli challenge. The same letter indicates a non-significant difference while values with different superscript letters represent significant differences.
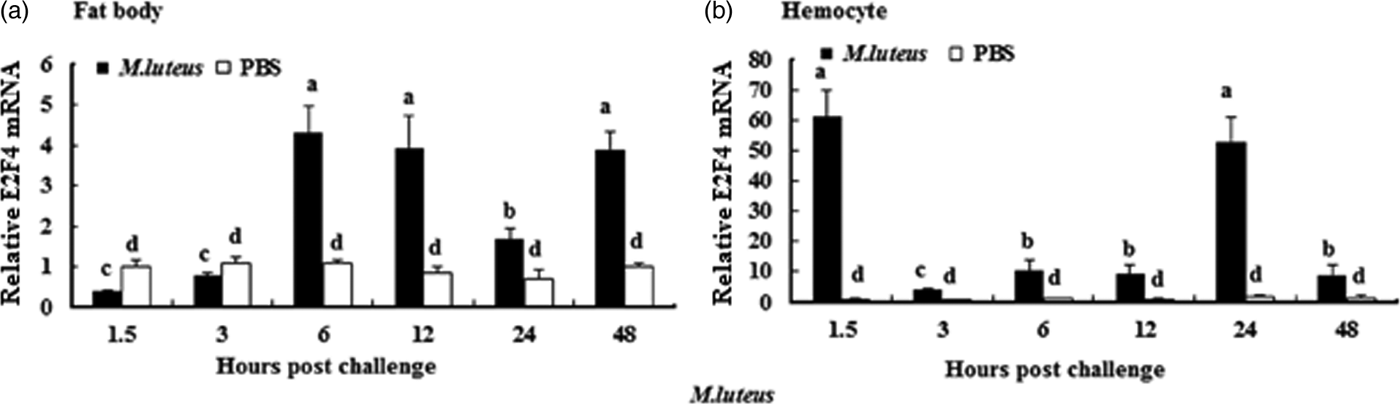
Fig. 8. Expression profiles of the E2F4 protein in (a) haemocytes and (b) fat bodies of A. pernyi fifth-instar larvae following Micrococcus luteus challenge. The same letter indicates a non-significant difference, while values with different superscript letters represent significant differences.
Developmental profile of E2F4 expression
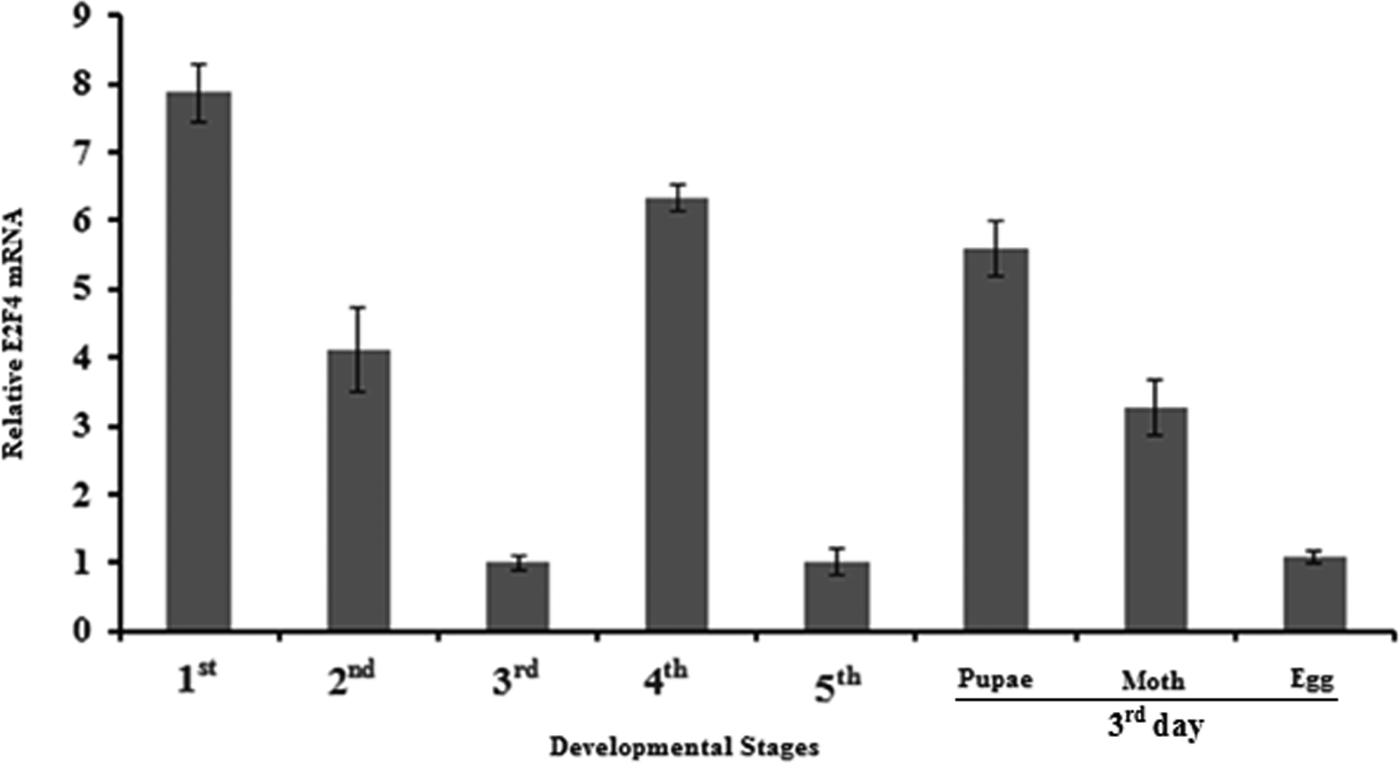
To determine the expression of the E2F4 gene in different developmental stages of A. pernyi, we extracted total RNA from egg, larvae (1st to 5th instar), pupae and moth stages and then synthesized cDNA to determine the relative expression of mRNA of E2F4 at these time points. As seen in fig. 9, E2F4 mRNA was expressed at high levels in the 1st instar larvae, while the lowest expression was recorded in the egg, and the 3rd and 5th instars.
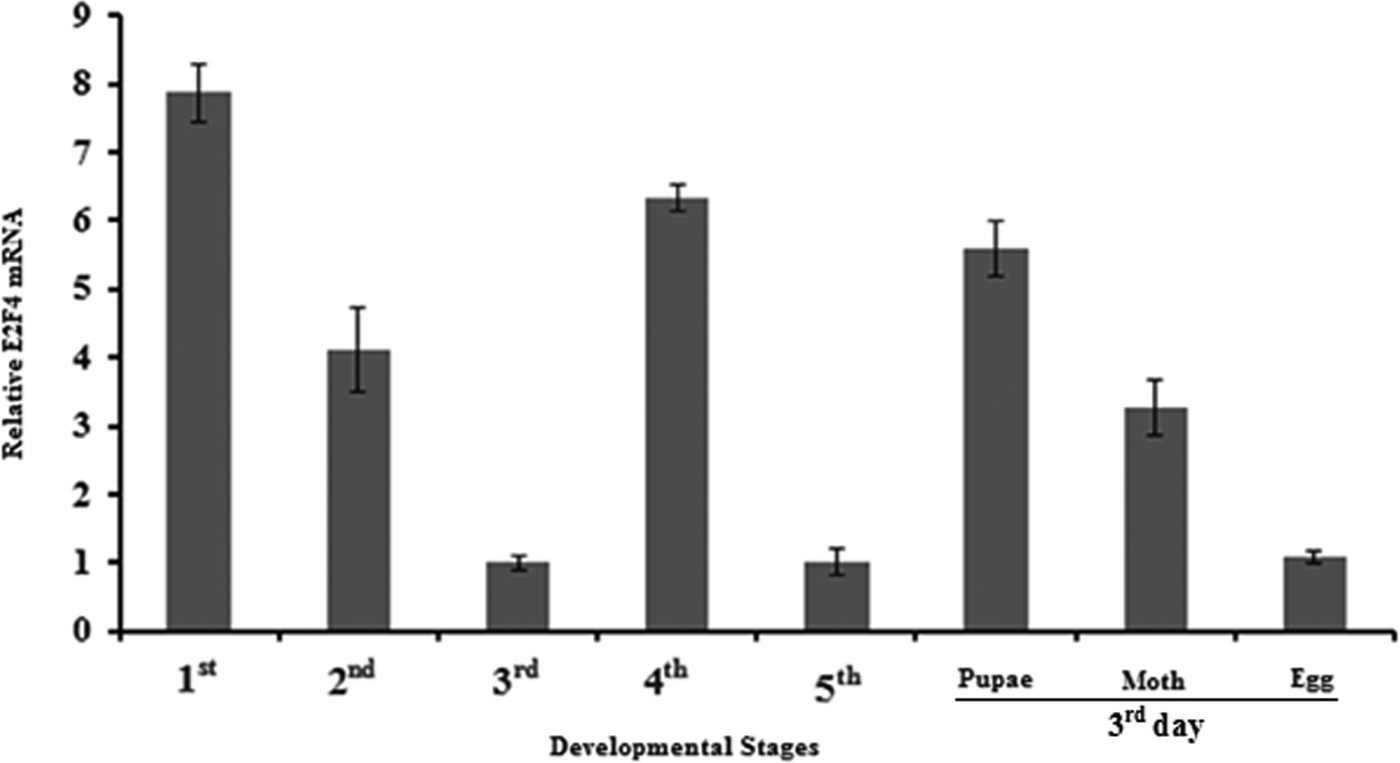
Fig. 9. Expression profiles of the E2F4 protein in different developmental stages of A. pernyi.
Discussion and conclusions
In the present study, we amplified the E2F4 cDNA from A. pernyi, and performed sequence analysis. The recombinant vector pET30a-E2F4 was constructed, which was transformed into E. coli and the recombinant protein was expressed successfully. Based on protein alignments and phylogenetic analysis of the deduced amino acid sequence, E2F4 is highly homologous to E2F4 proteins from other Lepidopterans, especially Bombyx mori (65%), but shows low homology to that from Zootermopsis nevadensis (53%), which suggested that that the E2F4 genes are species specific.
E2F4 is involved mainly in the synthesis of DNA in undifferentiated cells (van Amerongen et al., Reference van Amerongen, Diehl, Novoyatleva, Patra and Engel2010; Ren et al., Reference Ren, Cam, Takahashi, Volkert, Terragni, Young and Dynlacht2012). However, recent studies revealed that E2F4 plays a variety of roles in differentiated cells, e.g. as a regulator of apoptosis and in tumour suppression, and as technology advances, further functions might be revealed (Zheng et al., Reference Zheng, Fraenkel, Pabo and Pavletich1999; Cam & Dynlacht, Reference Cam and Dynlacht2003; Dingar et al., Reference Dingar, Konecny, Zou, Sun and von Harsdorf2012). In the present study, we characterized E2F4 by evaluating its developmental expression profile, its expression in different tissues, and its response in immune tissue (fat body and haemocyte) to viral, fungal and bacterial challenge. Interestingly, E2F4 expression was high in the integument, fat body, and haemocytes compared with that in the other tissues examined. This indicated that the level of E2F4 mRNA is tissue specific and might change depending on its function. Kusek et al. (Reference Kusek, Greene, Nugent and Pisano2000) also documented variable patterns of E2F4 mRNA expression in different tissues in both the embryonic and adult stages of mice.
To explore the role of E2F4 in response to microbial infection, the expression levels of E2F4 were determined after challenge by four types of pathogen antigens at different time intervals. The results revealed that, although the E2F4 gene can be induced by all four pathogens, the maximum transcript level achieved in response to each pathogen appeared at different times. The response of the E2F4 gene to the different pathogens in the fat bodies and haemocyte varied. In the fat bodies, NPV, B. bassiana, and E. coli treatment elicited high expression at 12 h, whereas M. luteus elicited high expression at 6 h after treatment. However in haemocytes, the expression of the transcript fluctuates largely with time and pathogen type. NPV induced maximum expression at 6 h, whereas B. bassiana induced it at 5 h. The transcript expression was much higher in response to E. coli and M. luetus; however, the maximum level was achieved at different times: at 3 h for E. coli and at 24 h for M. luteus. Our data suggested that E2F4 can be induced by microbial infection in A. pernyi, and exhibited time-dependent increases during infection. However, the expression pattern of E2F4 was different when challenged with the different pathogens. These observations were consistent with the study of Isomoto et al. (Reference Isomoto, Furusu, Shin, Ohnita, Miyazaki, Omagari, Mizuta, Murase, Inoue, Murata, Koji and Kohno2002), who reported that the expression of E2F transcription factors is enhanced following biotic stress (Helicobacter pylori challenge) in mucosal cells. In addition, abiotic stress (Infra Red Radiation) can also increase the expression of E2F4 (DuPree et al., Reference DuPree, Mazumder and Almasan2004). Furthermore, Bernales et al. (Reference Bernales, Fullaondo, Marin-Vidalled, Ucar, Martinez-Taboada, Lopez-Hoyos and Zubiaga2008) observed differential expression of genes that are regulated by E2F, and suggested that an altered RB/E2F pathway could account for this differential expression of the target genes in primary anti-phospholipid syndrome. Further studies are required to determine the mechanism by which the expression of E2F4 changes following microbial infection. Furthermore, whether the immune system is controlled by pathways related to E2F4 or E2F4 is controlled by pathways related to the immune system should be determined in future research.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485317000426
Acknowledgements
We are grateful to Sericultural Research Institute of Liaoning, China for kindly providing the A. pernyi specimens in this study. This work was supported by the Biology Key Subjects of Anhui Province, the National Natural Science Foundation of China [grant numbers 31301715, 31472147, 31402018], the Sericulture Biotechnology Innovation Team [grant number 2013xkdt-05], the PhD. Programs in Biochemistry and Molecular Biology [grant number xk2013042], and the Graduate student innovation fund of AHAU [grant number 2015-34].