Published online by Cambridge University Press: 05 October 2004
Malaria is a major health concern particularly in Africa which has about 90% of the worldwide annual clinical cases. The increasing number of drug-resistant Plasmodium falciparum justifies the search for new drugs in this field. Antimalarial activity of 2-substituted 6-nitro- and 6-amino-benzothiazoles and their anthranilic acids has been tested. An in vitro study has been performed on W2 and 3D7 strains of P. falciparum and on clinical isolates from malaria-infected patients. Toxicity has been assessed on THP1 human monocytic cells. For the most active drug candidates, the in vitro study was followed by in vivo assays on P. berghei-infected mice and by in vitro assays in order to determine the stage-dependency and the mechanism of action. Of 39 derivatives tested in vitro, 2 had specific antimalarial properties. Each compound was active on all stages of the parasite, but one was markedly active on mature schizonts, while the other was more active on young schizont forms. Both drugs were also active on mitochondrial membrane potential. In vivo data confirmed efficiency with a sustained decrease of parasitaemia. Products A12 and C7 may be considered as potential antimalarial worthy of further chemical and biological research.
Increasing expansion of developmental projects such as irrigation-systems or deforestation, and drug resistance, have led to increased incidence of parasitic diseases in most developing countries. Malaria is due to an infection of erythrocytes by protozoa of the genus Plasmodium and is the world's most important tropical parasitic disease with 300 to 500 million people infected per year. The annual worldwide mortality is estimated to be between 1·1 and 2·7 million people, of whom 90% are in Africa south of the Sahara (World Health Organization, 2003). Malaria kills more people in Africa than any other communicable disease and is now frequently imported into developed countries. The expansion of drug-resistant malaria in endemic countries has led to an urgent need for new antimalarials.
Benzothiazoles are therapeutic compounds with a wide range of biological activities. Since the 1990s, pharmacological investigations involving the latest synthesized benzothiazole derivatives have demonstrated various pharmacological activities and led to the development of new medications for treating human diseases. Among the most efficient are riluzol (Bae et al. 2000; Kennel et al. 2000; Nogradi & Vrbova, 2001), sulphathiazole, mercapto-2-benzothiazole and 2-(phenylsulfonyl)-benzothiazole with neuroprotective (Bae et al. 2000; Kennel et al. 2000), anticonvulsive (Kennel et al. 2000), antiallergenic and antimicrobial activities (El-Shaaer et al. 1997; Trapani et al. 1994), respectively, while other derivatives such as 2-(4-aminophenyl)-benzothiazoles exhibited potent antitumoural activity, probably due to their capacity to bind to tumour-specific proteins (Bradshaw et al. 1998; Chua et al. 1999).
Compared to anticancer drugs such as acridines which have been extensively studied for their antileishmanial, trypanocidal and antimalarial activities (Cassileth & Gale, 1986; Gamage et al. 1997; Auparakkitanon & Wilairat, 2000), benzothiazoles have been poorly investigated for their antiparasitic activity. We synthesized 2-substituted 6-nitro-benzothiazoles and 2-substituted 6-amino-benzothiazoles and assessed the in vitro antiproliferative activity of each benzothiazole derivative on malaria parasites and also examined their toxicity on human monocytes. The most active drug candidates were tested in vivo on P. berghei in mice, and in vitro, in order to determine the stage-dependency. The mechanism of action was examined in the context of the main targets of antimalarial drugs: inhibition of haem crystallization, depolarization of mitochondrial membrane potential, free radical production and immune modulation by production of nitric oxide (NO). According to previously described activity of other benzothiazoles on DNA (Leong et al. 2003), we also performed in vitro assays to evaluate the action of our products on DNA.
2-Substituted nitro- and amino-benzothiazoles and their corresponding anthranilic acids (Fig. 1) were synthesized in the Laboratoire de Valorisation de la Chimie Fine, Université d'Aix-Marseille III, site de Saint Jérome, Marseilles, France. Purity (>99%) was determined by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. Chloroquine diphosphate (Sigma, St Louis, MO, USA) was used as a standard drug for positive controls. All compounds were dissolved in sterile dimethyl sulphoxide (DMSO, analytical grade, Sigma) and stored frozen at −80 °C until used.

Fig. 1. Synthesis of 2-substituted nitro- and amino-benzothiazoles and corresponding anthranilic acids. 2-chloro-6-nitrobenzothiazole (product A1) was the starting compound for the synthesis of the 3 chemical series. Products A2 to A13 were 6-nitro-benzothiazoles, products B1 to B13 were 6-amino-benzothiazoles, and products C1 to C13 were the corresponding anthranilic acids. Different radicals substituted all series in position 2.
Assays used 2 culture-adapted reference strains of P. falciparum provided by the Institute of Tropical Medicine of the Army Health Department (IMTSSA, Marseilles): type W2 from Vietnam, resistant to chloroquine, pyrimethamin and proguanil, and type 3D7 from Amsterdam airport, sensitive to chloroquine. Clones were maintained in continuous culture according to the methodology of Trager & Jensen (1976).
Parasites were cultured in 75 cm2 flasks containing RPMI 1640 medium (20 ml) supplemented with HEPES (25 mM; Gibco-BRL, Paisley, Scotland), NaHCO3 (25 mM), 10% non-parasitized A+human serum and 1 ml of parasitized blood (haematocrit 2·5%). Parasitaemia was maintained between 1 and 6%. Dilutions used non-infected A+erythrocytes. Cultures were incubated at 37 °C in 10% O2, 6% CO2 and 84% N2, with 90% humidity. Medium renewal and microscopic observation (×100) by blood smears fixed with methanol and stained with 10% Giemsa's stain were performed on a daily basis.
We also used fresh clinical isolates obtained from 2 travellers (CL1 and CL2, 17 and 65 years old, respectively) infected in Africa and treated in NORD Hospital in Marseilles. Patients were French residents for more than 10 years, and the duration of travel was 18 days and 3 months, respectively. Following hospitalization, a standard dose of mefloquine, used as routine therapy, led to parasite clearance in both patients. Drug resistance of each isolate was tested in triplicate in a 96-well tissue culture plate (Falcon microtest®, Becton Dickinson, Rutherford, NJ, USA) with increasing concentrations of chloroquine (range: 10 to 1000 nM). Before treatment, venous blood was collected in Vacutainer® ACD tubes (Becton Dickinson, Rutherford, NJ, USA) and transported at 4 °C to our laboratory. Thin blood smears were stained using May Grunwald Giemsa (MGG) and examined to determine percentage of P. falciparum-infected erythrocytes.
In vitro toxicity of benzothiazoles was assessed on human monocytic THP1 cells maintained at 37 °C in 6% CO2, in a medium of RPMI (Eurobio, Paris, France) supplemented with 10% foetal calf serum (Eurobio, Paris, France), 25 mM HEPES, 25 mM NaHCO3 and 1% of L-glutamine/penicillin-streptomycin mix. Cells were subcultured every 7 days (Ogunkolade et al. 1990).
We used hydroethidine, which is metabolized into ethidium bromide by the living parasite, as a fluorochrome (Interchim, Montluçon, France; Van Der Heyde et al. 1995). Parasite growth was assessed by flow cytometry according to the methodology previously described (Azas et al. 2002). For W2 and 3D7 P. falciparum clones, baseline parasitaemia was between 1 and 2% with a haematocrit of 2% using the medium and culture conditions previously described. Triplicate assays were performed in 96-well tissue culture plates (Nunc Brand products, Fisher, Paris, France) containing 200 μl of asynchronous parasite cultures and 5 μl of the appropriate benzothiazole dissolved in DMSO. After 48 h incubation without medium change, plates were centrifuged and the upper liquids were replaced with 200 μl hydroethidine solution (0·05 mg/ml in PBS). After a 20 min incubation in the dark at 37 °C and 3 washes in PBS (Sigma, St Louis, MO, USA), a final dilution in 1 ml of PBS allowed determination by flow cytometry of the number of cell events (around 300 per sec).
For the clinical isolates, blood samples were washed 3 times in RPMI 1640 medium (Gibco-BRL, Paisley, Scotland). After each centrifugation, upper liquid and leukocytes were discarded and replaced by RPMI. After the last centrifugation, the haematocrit was adjusted to 50% for preservation with culture medium. Chemosensitivity measurement was then performed as for the reference clones.
Parasitaemia readings were taken with a flow cytometer FACSort (Beckton Dickinson, Paris, France) equipped with an argon laser (power, 15 mWatt; wavelength 488 nm). Settings were: Forward Scatter (FSC-H), size: Voltage E-1, gain 1, mode Log; Side Scatter (SSC-H), granularity: Voltage 250, gain 1, mode Log; Fluorescence 2 (FL2), red fluorescence: Voltage 459, gain 1, mode Log. A negative control on each plate allowed determination of the non-specific fluorescence threshold of non-infected erythrocytes. This threshold value was subtracted from the value measured for test samples. Parasitaemia of controls and samples were evaluated by cytometer. Analysis used 2 cytograms. The first displayed cells by size and granularity and allowed selection of erythrocytes and elimination of cell debris. The second displayed erythrocytes by size and red fluorescence, and allowed identification of parasitized cells among the whole erythrocyte population. The data points representing erythrocytes selected in the first cytogram were divided in 2 distinct cell populations differentiated by the emitted intensity of fluorescence. Among parasitized erythrocytes, young forms of Plasmodium (ring) containing a smaller quantity of DNA emitted a weak fluorescence while older forms (schizont) emitted a stronger fluorescence.
The IC values represented the mean value calculated from 3 independent experiments. The concentration of drug capable of inducing a 50% reduction of infected erythrocytes (IC50-Plasmodium) was calculated by non-linear regression analysis processed on dose response curves, using the Table Curve Program (Sigma-plot scientific graph system, version 3.0, Jandel Scientific, Corte Madera, CA). The threshold IC50 for in vitro resistance to chloroquine was fixed at 100 nM (Basco & Ringwald, 2000; Pradines et al. 2002b).
In the cell toxicity assays, THP1 cells (105 cells/ml) were incubated with different concentrations of test products dissolved in DMSO or distilled water. A viability control free of drug and a control containing 5 μl DMSO were performed in parallel. After a 72 h incubation at 37 °C and 6% CO2 in complete RPMI medium, cell growth and viability were measured by flow cytometry after staining the monocytes with 5 μl of propidium iodide (1 mg/ml, Sigma; Azas et al. 2002). Antiproliferative activity was evaluated by counting the number of living cells in a 100 μl sample. Inhibitory concentration 50 (IC50-Human) was defined as the concentration of drug inducing a reduction of 50% in the THP1 cell proliferation compared to the standard. Lethal concentration 50 (LC50-human) was defined as the concentration of drug inducing a mortality of 50% of cells compared to the standard. A specificity index (SI) corresponding to the ratio of toxicities on human cells and on parasites was calculated according to the following formula:

For products having the best SI, stage- and time-dependence were evaluated on the different asexual stages of P. falciparum. (Whitehead & Peto, 1990). After 2 synchronizations by sorbitol lysis at 48 h intervals according to the technique of Lambros & Vanderberg (1979), infected erythrocytes were adjusted to a parasitaemia of 1·5% and were exposed to compounds A12 and C7 at concentrations of 5 fold IC50Plasmodium for 12 h at 0, 12, 24 and 36 h. Parasitized erythrocytes were washed 3 times with drug-free medium; the cell pellet was resuspended in complete drug-free medium and incubated for a further 48 h. Erythrocytes were then fixed with 0·025% glutaraldehyde in PBS, washed 3 times with PBS, and stained with propidium iodide 20 min before reading in the cytometer (Traore-Keita et al. 2000). Results were expressed in percentage of growth compared to controls at 48 h.
The DNA-methyl green assay is a simple microtiter assay for detection of compounds that bind DNA (Burres et al. 1992) and is used for the detection of agents that intercalate into DNA. After 24 h of contact with the products, the displacement of methyl green from DNA-methyl green complex (Sigma) was detected by an absorbency decrease using spectrophotometry at 620 nm. The assay solution was prepared by dissolution of DNA-methyl green (20 mg) in Tris buffer 0·05 M pH 7·5 (100 ml) containing MgSO4 (7·5 mM), at 37 °C with constant agitation for 24 h. Then 100 μl of solution were added to the wells of a tissue culture plate containing 1 μl of concentrations of the different products to be tested (from 1 to 100 μM). Optical density was measured at 620 nm before and after incubation (24 h, 37 °C), in order to evaluate the absorbency decrease (Jonckers et al. 2002). We used daunorubicine, an intercalating drug, as the positive standard.
The mitochondrial membrane potential (MMP) was assessed according to the protocol described by Srivastava, Rottenberg & Vaidya (1997) using DiOC6(3), a cationic lipophilic fluorescent probe. Erythrocytes infected with asynchronous parasites were concentrated by centrifugation over Percoll at 60% density gradient (Wahlgren et al. 1983). The parasitized cells at a concentration of 5×106/ml were incubated with 2 nM DiOC6(3) (VWR Prolabo) for 20 min at 37 °C. At the end of the incubation period the suspension was split into 250 μl aliquots in 5 ml tubes. Different concentrations of the compound to be tested were added (1 to 100 μM), and mixtures incubated for an additional 20 min. At the end of the incubation period, each sample was subjected to flow cytometry analysis using a FACScan (Becton Dickinson). The IC50-MMP was defined as the product concentration inducing a decrease of 50% in fluorescence intensity (corresponding to MMP decrease) compared to the control. Chloroquine and carbonyl cyanide m-chlorophenyl hydrazone (CCCP, Sigma) were used as negative and positive controls, respectively.
Haem crystallization can be reproduced from a solution of haemin (Sigma) at 37 °C forming β-haemin polymers having the chemical, spectroscopic, and biological properties of haemin (Slater et al. 1991). A microtitre-based method (Parapini et al. 2000) allowed the spectrophotometric measurement of in vitro haem polymerization. Data were expressed as the molar equivalents of test compounds relative to haemin required to inhibit haem polymerization by 50% (IC50-haemin). The dose-effect curve was established by non-linear regression with the Table curve software (Sigma-plot scientific graph system, version 3.0, Jandel Scientific, Corte Madera, CA), allowing determination of the IC50-haemin of the tested product.
We assessed the chemosensitivity of the W2 strain to combinations of products (either A12, C7, chloroquine or artesunate) and ascorbic acid (100 μM to 800 μM) according to a previously described technique (Pradines et al. 2002a). Ascorbic acid was used as an antioxidant and free radical scavenger to verify whether the mechanism of action of the tested products was due to free radical production. In this case, an increase of IC50Plasmodium was expected with the addition of ascorbic acid.
Maturation of human THP1 monocytic cells into macrophages was induced by treating exponentially growing monocytes (2×105 cells/ml) with 1 μM phorbol myristate acetate (Sigma). After a 48 h incubation period at 37 °C (6% CO2) in 24-well flat-bottom plates, cells were rinsed with fresh medium and suspended in complete RPMI medium containing various concentrations of A12 and C7 products (1 to 25 μM), with and without murine recombinant gamma interferon (rIFN-γ, 10 IU/ml). Compound C11 (2-({2-[(2-hydroxyethyl)amino]-benzothiazol-6-yl}amino)benzoic acid) (VWR Prolabo) was used as a positive control (Delmas et al. 2002). After 48 h of incubation, NO production was measured by assessment of the nitrite content in the culture supernatants, according to the method of Ding, Nathan & Stuehr (1988). Fresh Griess reagent (100 μl, Sigma) was added to an equal volume of culture supernatants. After 15 min incubation at ambient temperature the optical density was measured at 540 nm. Determination of nitrite concentration used a standard curve based on NaNO2 (Sigma) diluted in Dulbecco's Modified Eagle's Medium (DMEM) as standard solution (Delmas et al. 2002).
In vivo assays used 7-week-old BALB/c female mice (Laboratoire Janvier) and ANKA strain of P. berghei kept frozen in liquid nitrogen before use. Tap water and food were provided to mice ad libitum. Mice were infected by intra-peritoneal injection of P. berghei-infected mouse erythrocytes (106/50 μl) at day 0 and treated 3 h after infection by oral administration of products with the best SI, in a standard 4-day test (once daily administration for 4 consecutive days). Doses ranged from 10 to 50 mg/kg/day. This 4-day test was carried out as previously described by Peters et al. (1993) and was repeated 3 times on groups of 10 mice, including a standard group receiving drug-free food by force-feeding and a group receiving chloroquine 10 mg/kg/day.
In order to evaluate the putative toxicity of A12 and C7 products, a group of healthy mice was treated with A12 and C7 at 100 mg/kg/day for 4 days.
Parasitaemia percentage was determined microscopically at day 4 from mouse-tail blood smears fixed with methanol and stained with Giemsa's stain. Parasitaemia levels and survival of treated mice were compared to mice of control groups.
Nitro- and amino-benzothiazoles, along with their corresponding anthranilic acids, did not have marked toxicity on human THP1 cells since most of the LC50-human ranged between 100 and 400 μM (Table 1). Compounds A1, B6 and C6 had the highest toxicity on human cells (LC50-human<50 μM), indicating that chloro- and piperidino-groups could enhance derivative toxicity.
Table 1. Toxicity of benzothiazoles and corresponding anthranilic acids on human monocytic THP1 cells and in vitro antiparasitic activities on W2 strain of Plasmodium falciparum (*Mean value calculated from 3 independent experiments.)

Results concerning antiproliferative activity on human cells demonstrated that most of the chemical compounds could inhibit proliferation of human malignant cells. Nitro- and amino-benzothiazoles had higher antiproliferative properties than anthranilic acids. Compounds A6, B6 and C6 confirmed that the addition of a piperidino-group on these molecular structures could support significant antiproliferative activity on human cells. Compounds A9, A10 and A13 had little toxicity on THP1 cells and marked antiproliferative activity, suggesting that antiproliferative activity of nitro-benzothiazoles could be increased by 2-anilino, 2,3-hydroxy-anilino or 2-N-dimethyl-p-phenylenediamino groups. Compounds B3 and B4 showed that 2-amino and 2-N-dimethyl amino-groups could increase antiproliferative properties of amino-benzothiazoles. Compounds A12 (N-(3-methoxyphenyl methane sulphonamide)-6-nitro-benzothiazole) and C7 (2-[(2-{[3 (diethylamino) propyl]amino}-benzothiazol-6-yl)amino] benzoic acid) had the most interesting antiparasitic profiles according to their low IC50Plasmodium and selectivity index values. We focused on these compounds in the continuation of the study, since they were the best drug candidates.
IC50 values on travellers' isolates were very close to the IC50 of W2 and 3D7 reference strains (Table 2). Both clinical isolates were chloroquine-resistant (IC50>100 nM).
Table 2. In vitro antimalarial activity of compounds A12 and C7 on 3D7 and W2 strains and fresh clinical isolates (*Mean±S.D. calculated from 3 independent experiments.)

The compounds inhibited all stages of P. falciparum; A12 had preferential action on mature schizonts while C7 and chloroquine were more active on young schizonts (Fig. 2).

Fig. 2. Activity of A12, C7 and chloroquine on Plasmodium falciparum stages. Mean±S.D. calculated from 3 independent experiments.
While daunorubicine markedly lowered the OD, there was no decrease of OD for A12 and C7 indicating an absence of displacement of methyl green from the DNA-methyl green complex (Fig. 3). Therefore, both A12 and C7 products did not directly interfere with DNA.

Fig. 3. Activity of A12 and C7 on DNA-methyl green (after 24 h contact). Mean±S.D. calculated from 3 independent experiments.
The change in flow cytometry profile indicated that A12, C7 and CCCP caused a depolarization of plasmodial mitochondria and dissipated the dye accumulation while chloroquine, a drug likely not to affect mitochondrial targets, had no effect on membrane potential (Fig. 4). Dose–effect curves showed that the fraction of membrane potential depolarization depended on A12 and C7 concentrations with an IC50-PMM of 6·64 μM and 8·78 μM for A12 and C7, respectively. Chloroquine had no effect on malarial MMP, even at millimolar concentrations.

Fig. 4. Fluorescence histogram of DiOC6(3) with (A) chloroquine 1 mM, (B) CCCP 40 μM, (C) A12 10 μM and (D) C7 10 μM.
The results reported in Table 3 indicated that haem crystallization might, in part, be responsible for the antimalarial activity of C7, but not of A12.
Table 3. Activity of A12, C7 and chloroquine on haem crystallization (*Mean±S.D. calculated from 3 independent experiments.)

There was no increase of IC50 (Table 4) for both A12 and C7 associated with ascorbic acid, indicating that their antimalarial activity was probably not due to free radical production toxicity on P. falciparum. Chloroquine did not involve free radical production, in contrast to artesunate whose IC50 progressively increased with the ascorbic acid concentrations added.
Table 4. IC50Plasmodium values of A12, C7, chloroquine and artesunate when associated with increasing concentrations of ascorbic acid (*Mean±S.D. calculated from 3 independent experiments. P>0·05 for all IC50 values compared to value at 0 μM, except for artesunate 400/0 and 800/0 where P=0·001 and 0·002, respectively.)

After stimulation by interferon γ, A12 and C7 did not induce production of nitric oxide by macrophages (Fig. 4). The compound C11, used as positive control, induced a significant increase in nitrite concentration in the culture medium supernatant. There was no increase of nitrite concentration in the absence of stimulation by interferon, for any compound.

Fig. 5. Effect of A12, C7 and C11 on nitric oxide production by interferon-stimulated macrophages. Mean±S.D. calculated from 3 independent experiments.
There was no acute toxicity on healthy mice for compounds A12 and C7 at 100 mg/kg/day during the 4 days of the test. Parasitaemia decreased significantly at day 4 with administration of A12 and C7 at all doses compared to the control (Table 5). Mice treated with chloroquine at 10 mg/kg/day for 4 days did not develop disease. For treated mice, gain in mean survival time was 5 days and 15 days, for C7 and A12 at 10 mg/kg/day, respectively. For A12 at 50 mg/kg/day, survival time doubled with an increase of 20 days. There was no complete remission, however, since treated mice died after 15–20 additional days of survival.
Table 5. Oral treatmenta of Plasmodium berghei (ANKA)-infected BALB/c mice with products A12 and C7 (a, oral treatment in a 4-day test; b, values (%) are mean levels of parasitaemia (95% confidence intervals); c, compared to control (t test); d, number of dead mice/total number of mice; e, mean±S.D.)

The results observed in the present study confirmed that the 2-subtituted 6-amino benzothiazoles, the 2-substituted 6-nitro benzothiazoles and their corresponding anthranilic acids had promising antimalarial activities. Among the 39 products tested, only A12 and C7 had in vitro antimalarial potential. This potential observed on W2 and 3D7 strains and on clinical isolates was confirmed in vivo in P. berghei-infected mice.
The most interesting antiparasitic property was obtained with A12, which inhibited P. falciparum growth in human erythrocytes. The methoxyphenyl methane sulphonamide radical is known to exert antitumoural activity (Cassileth & Gale, 1986; Bradshaw et al. 1998; Chua et al. 1999) and induction of DNA cleavage (Auparakkitanon & Wilairat, 2000) when fixed on 9-anilino-acridine. Our results suggested that these radicals could have an antimalarial potential when fixed in position 2 on the 6-nitrobenzothiazole. Reduction of the nitro group into an amino group (B12) removes this activity. The second promising molecule, C7, demonstrated that a consistent antimalarial activity could be obtained by addition of a diethylamino propylamino group on a parent anthranilic acid and confirmed the hypothesis that anthranilic acids could be used as a support for antiparasitic radicals.
The P. berghei-infected mouse model has been widely used in previous studies as a preliminary test for the in vivo activity of potential antimalarial agents, since it provides a preclinical indication of any in vivo potential activity and of a possible toxicity of the tested sample as well (Ager, 1984; Heiffer, Davidson & Korte, 1984; Cox, 1988). Poor bioavailability or metabolic processing could explain the discordance between in vitro and in vivo effects.
We performed the assay at high drug dose on mice in order to determine the maximum potential gain in survival time. Rane & Kinnamon (1979) evaluated antimalarial activity according to survival time, indicating that a compound was considered to be active if treated mice had a 2-fold increase in survival time compared to controls, and curative if treated mice survived more than 60 days. According to these authors, any product capable of doubling survival of treated animals compared to standard would be of interest in antimalarial research.
Mortality occurring during the first 48 h was attributed to compound toxicity. The compounds tested had no acute toxicity since no deaths occurred during this early period. The results strongly indicated that compounds A12 and C7 were active on the asexual erythrocyte stages of P. berghei. The marked, although incomplete, parasitaemia decrease at day 4 in the treated mice revealed antimalarial activity (Peters et al. 1993). No cure occurred despite increasing the dosage of A12, which had the best study parameters (IC50Plasmodium, SI, and survival time). This preliminary in vivo study confirmed the antimalarial activity observed in vitro.
Knowledge of any stage dependent-effect provides information on a drug's mechanism of action (Whitehead & Peto, 1990). Determination of the mechanism of action of antimalarial drugs is particularly complex, due to the close relationships between parasite and erythrocyte metabolisms and difficulty in isolation of the parasite and its components (enzymes, cell contents) in sufficient quantities for analysis. Plasmodium matures over 48 h in successive forms: early ring (0–12 h), late ring and early trophozoite (12–24 h), late trophozoite and young schizont (24–36 h) and mature schizont (36–48 h) (Yayon et al. 1983; Whitehead & Peto, 1990). Drugs inhibiting synthesis of proteins and nucleic acids or haem crystallization act on the mature parasite, while drugs targeting digestive vacuoles (e.g. ammonium chloride) act principally on young forms at the beginning of the asexual erythrocyte cycle (Whitehead & Peto, 1990). The products did not seem to have any stage dependent-effect since inhibition was observed at all parasite stages. However, A12 had a preferential action on mature schizont forms while C7 was more active on young schizont forms. Chloroquine inhibits young and mature schizonts by accumulation in digestive vacuoles and blockade of haemoglobin degradation and haem crystallization (Parapini et al. 2000).
The DNA-methyl green assay cannot predict efficacy of tested products on enzymatic systems but allows selection of molecules acting directly on DNA structure. We verified this hypothesis for our products since generation of DNA adducts has been reported for some benzothiazole derivatives (Leong et al. 2003). Daunorubicine was used as positive control since it is an inserting agent directly interfering with DNA.
Mitochondria play a major role in the parasite's pyrimidic nucleotide synthesis and energy metabolism. They also intervene in the metabolism of amino acids, lipids, haem, and homeostasis of intracellular calcium (Alberts et al. 1994). Mitochondria isolated from P. falciparum contain the aa3, b, c and c1 cytochromes of a classic respiratory chain (Fry & Beesley, 1991), and can oxidize glycerophosphate, succinate, proline and dihydro-orotate that participate in the classic electron transport system. They are the only ones oxidizing glutamate and provide a NADH-fumarate reductase responsible for the re-oxidation of NADH (Fry, Webb & Pudney, 1990). Antimalarial drugs inhibiting the bc1 cytochrome complex, such as hydroxynaphtoquinone (atovaquone), can target this respiratory chain leading to parasite death (Fry & Pudney, 1992). A previous study (Srivastava et al. 1997) reported a good correlation between the DiOC6(3) assay of mitochondrial membrane potential and respiration of intact infected erythrocytes. Carbonyl cyanide m-chlorophenyl hydrazone protonophore (CCCP), used as positive standard, allowed depolarization of the mitochondrial membrane. Measurement of membrane potential is a good indicator of electric equilibrium around cellular membranes since any alteration of this membrane leads to a modification of the membrane potential. Compounds A12 and C7 altered the mitochondrial membrane, causing a drop of potential, and could alter the respiratory chain leading to the parasite's death, as atovaquone does (Srivastava et al. 1997). This value of IC50 indicated that these products had a marked effect on mitochondrial membrane potential.
Haem crystallization is the mechanism of action of numerous antimalarial drugs belonging to the amino-4-quinoline group, such as chloroquine, and to the amino-alcohol group, such as quinine and mefloquine (Parapini et al. 2000). Using chloroquine as reference, compound A12 did not have an action on haem crystallization. In contrast, compound C7 could partly alter this mechanism.
Free radical production was shown to be the mechanism of action of artemisinin (Meshnick et al. 1993), by which antimalarial activity is inhibited by free radical captors and increased by free radical generators (Krungkrai & Yuthavong, 1987). Within the range of concentrations used, ascorbic acid is an antioxidant and a free radicals captor and was used in order to evaluate indirectly the inhibition of free radical production (Pradines et al. 2002). Unlike artemisinin, free radical production did not seem to be the mechanism of action of A12 and C7, since there was no change in IC50Plasmodium for these products.
The absence of production of nitric oxide by interferon-stimulated-macrophages indicates that the antimalarial activity of both A12 and C7 on P. falciparum was probably not due to this host's defence mechanisms. Products were tested in order to examine their action on immune modulation of NO production since close immunological relations between parasite and host have been described (Ogunkolade et al. 1990; Goldring & Nemaorani, 1999). The technique employed did not evaluate a direct action on the parasite but on a host defence mechanism. There was no significant result although this mechanism has been described for compound C11 on Leishmania infantum (Delmas et al. 2002). To date, the only mechanism of action reported for these compounds was that of atovaquone. The products acted on mitochondrial membrane potential and did not interfere with haem crystallization, free radical production or DNA. This mechanism of action differs from that of most antimalarial drugs which act on haem crystallization. The associations of A12 and C7 with antimalarial drugs having a mechanism of action other than mitochondrial membrane potential would act on several targets rather than a single one. This would avoid competition between drugs and allow increasing efficacy or reducing resistance. Performing in vitro isobolograms would confirm the interest of an association of A12 and C7 with other antimalarial drugs. Further in vivo studies using other administration routes and murine models would determine their actions on neuropaludism.
Thus, these compounds have an interesting antimalarial potential that needs to be further explored and improved. A Quantitative Structure Activity Relationship (QSAR) methodology, attempting to find consistent relationships between variations in the values of molecular properties and the biological activity, can be utilized to help guide chemical synthesis. Compound optimization by QSAR, structural modelling and directed chemical synthesis starting from these structures would allow the provision of more active and less toxic products.
We thank Pr. A. Penaud and the laboratory staff of the hospital Nord in Marseille for kindly providing the two clinical isolates. We thank Sébastien Hutter and Jeanine Guerri for their technical cooperation.

Fig. 1. Synthesis of 2-substituted nitro- and amino-benzothiazoles and corresponding anthranilic acids. 2-chloro-6-nitrobenzothiazole (product A1) was the starting compound for the synthesis of the 3 chemical series. Products A2 to A13 were 6-nitro-benzothiazoles, products B1 to B13 were 6-amino-benzothiazoles, and products C1 to C13 were the corresponding anthranilic acids. Different radicals substituted all series in position 2.

Table 1. Toxicity of benzothiazoles and corresponding anthranilic acids on human monocytic THP1 cells and in vitro antiparasitic activities on W2 strain of Plasmodium falciparum

Table 2. In vitro antimalarial activity of compounds A12 and C7 on 3D7 and W2 strains and fresh clinical isolates
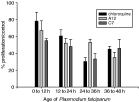
Fig. 2. Activity of A12, C7 and chloroquine on Plasmodium falciparum stages. Mean±S.D. calculated from 3 independent experiments.
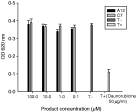
Fig. 3. Activity of A12 and C7 on DNA-methyl green (after 24 h contact). Mean±S.D. calculated from 3 independent experiments.

Fig. 4. Fluorescence histogram of DiOC6(3) with (A) chloroquine 1 mM, (B) CCCP 40 μM, (C) A12 10 μM and (D) C7 10 μM.

Table 3. Activity of A12, C7 and chloroquine on haem crystallization

Table 4. IC50Plasmodium values of A12, C7, chloroquine and artesunate when associated with increasing concentrations of ascorbic acid
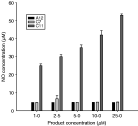
Fig. 5. Effect of A12, C7 and C11 on nitric oxide production by interferon-stimulated macrophages. Mean±S.D. calculated from 3 independent experiments.
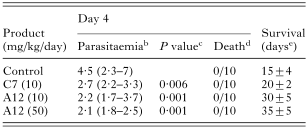
Table 5. Oral treatmenta of Plasmodium berghei (ANKA)-infected BALB/c mice with products A12 and C7