INTRODUCTION
The small-spotted catshark, Scyliorhinus canicula (Linnaeus, 1758) (Elasmobranchii, Carcharhiniformes, Scyliorhinidae) is the most common among catsharks in European waters (Ellis & Shackley, Reference Ellis and Shackley1997) and inhabits many different types of soft bottom substrates, at depths mainly between 10–100 and up to 780 m in the Mediterranean Sea (Muus & Nielsen, Reference Muus and Nielsen1999; Mytilineou et al., Reference Mytilineou, Politou, Papaconstantinou, Kavadas, D'Onghia and Sion2005).
Their feeding repertoire includes a variety of benthic invertebrates, including molluscs, crustaceans, small cephalopods, polychaete worms and small bony fishes (Compagno, Reference Compagno1984; Thollot, Reference Thollot1996; Perari, Reference Perari2010). These prey items belong to a trophic level >2.8 (Gibson & Ezzi, Reference Gibson and Ezzi1987) and adult S. canicula is considered a predator belonging to a trophic level of 3.62–3.69 (Ellis et al., Reference Ellis, Pawson and Shackley1996).
The small-spotted catshark is considered a species of minor commercial importance and fisheries data from FAO's 2014 yearbook (FAO, 2016) indicate that catches in Europe (Atlantic coast, Mediterranean and Black Seas) remained relatively stable, ranging from 5793 to 7119 tons per year from 2005 up to 2014. According to IUCN (2012), this fish species is considered ‘Least Concern’ and harmless to humans. Scyliorhinus canicula is consumed fresh or after salting and drying and it is also utilized for the production of fish oil and fishmeal (Compagno, Reference Compagno1984).
Scyliorhinus canicula has been included in prey-predator relationship studies (Armstrong, Reference Armstrong1982), in studies on fish physiology (Butler & Taylor, Reference Butler and Taylor1975) and in numerous ichthyofaunal studies (Labropoulou & Papaconstantinou, Reference Labropoulou and Papaconstantinou2000; Bilecenoglu et al., Reference Bilecenoglu, Taskavak, Mater and Kaya2002; Ellis et al., Reference Ellis, Cruz-Martinez, Rackham and Rodgers2005; Mytilineou et al., Reference Mytilineou, Politou, Papaconstantinou, Kavadas, D'Onghia and Sion2005; Banon et al., Reference Banon, Villegas-Ríos, Serrano, Mucientes and Arronte2010).
According to the World Register of Marine Species (WoRMS, 2017) and Pollerspöck & Straube (Reference Pollerspöck and Straube2015) the small-spotted catshark has been reported as host for the following parasites: the cestodes Acanthobothrium coronatum (Rudolphi, 1819) (Brunel et al., Reference Brunel, Bosse and Lamarche1998), Ditrachybothridium macrocephalum (Rees, Reference Rees1959) (Tyler, Reference Tyler2006), Crossobothrium longicolle (Molin, 1858) (Moore, Reference Moore2001), Nybelinia lingualis (Cuvier, 1817) (Moore, Reference Moore2001; Palm, Reference Palm2004), Onchobothrium ganfini (Mola, 1934) (Bray, Reference Bray, Costello, Emblow and White2001), Hepatoxylon megacephalum (Rudolphi, 1819) and H. trichluri (Holten, 1802) (Palm, Reference Palm2004); the copepod ectoparasites Neoalbionella globosa (Leigh-Sharpe, Reference Leigh-Sharpe1918) (Raibaut et al., Reference Raibaut, Combes and Benoit1998; Ozdikmen, Reference Özdikmen2008), Lernaeopoda galei (Krøyer, 1837) (Henderson & Dunne, Reference Henderson and Dunne1998; Raibaut et al., Reference Raibaut, Combes and Benoit1998); the ectoparasitic monogeneans Hexabothrium appendiculatum (Kuhn, 1829), H. canicula (Cerfontaine, 1899) (Llewellyn et al., Reference Llewellyn, Green and Kearn1984; Henderson & Dunne, Reference Henderson and Dunne1998; Moore, Reference Moore2001) and Leptocotyle minor (Monticelli, 1888) (Henderson & Dunne, Reference Henderson and Dunne2001; Kearn et al., Reference Kearn, Evans-Gowing and Rees2001); the digenean trematode Diphtherostomum betencourti (Monticelli, 1893) (Bray & Moore, Reference Bray and Moore2000); the nematodes Anisakis simplex (Rudolphi, 1809) (Henderson & Dunne, Reference Henderson and Dunne1998), Proleptus obtusus (Dujardin, Reference Dujardin1845) (Henderson & Dunne, Reference Henderson and Dunne1998; Moore, Reference Moore2001) and Pseudoterranova decipiens (Krabbe, 1878) (Moore, Reference Moore2001).
Scyliorhinus canicula, in addition to its ecological importance, contributes to the income of coastal communities in the eastern Mediterranean Sea since it is not considered a by-catch and after processing (gutting and skinning) it is offered to local markets. Information regarding its role as a host of parasites in the north-eastern Mediterranean Sea is essentially lacking. Thus, the objective of the present study is to record parasites hosted by S. canicula in the north-eastern Mediterranean Sea and correlate them to the trophic and ontogenetic habits of this fish species at this locality.
MATERIALS AND METHODS
Collection of specimens
In total, 52 specimens of small-spotted catshark comprising 25 females and 27 males (48 and 52% of the sample, respectively) were collected from the north, north-east and south coastal areas of Lesvos Island (Figure 1), north-eastern Aegean Sea, Greece, during spring (last week of April and first week of May) (13 specimens), summer (last week of June and first week of August) (22 specimens) and autumn (last week of September and first week of October) (17 specimens) of 2015.
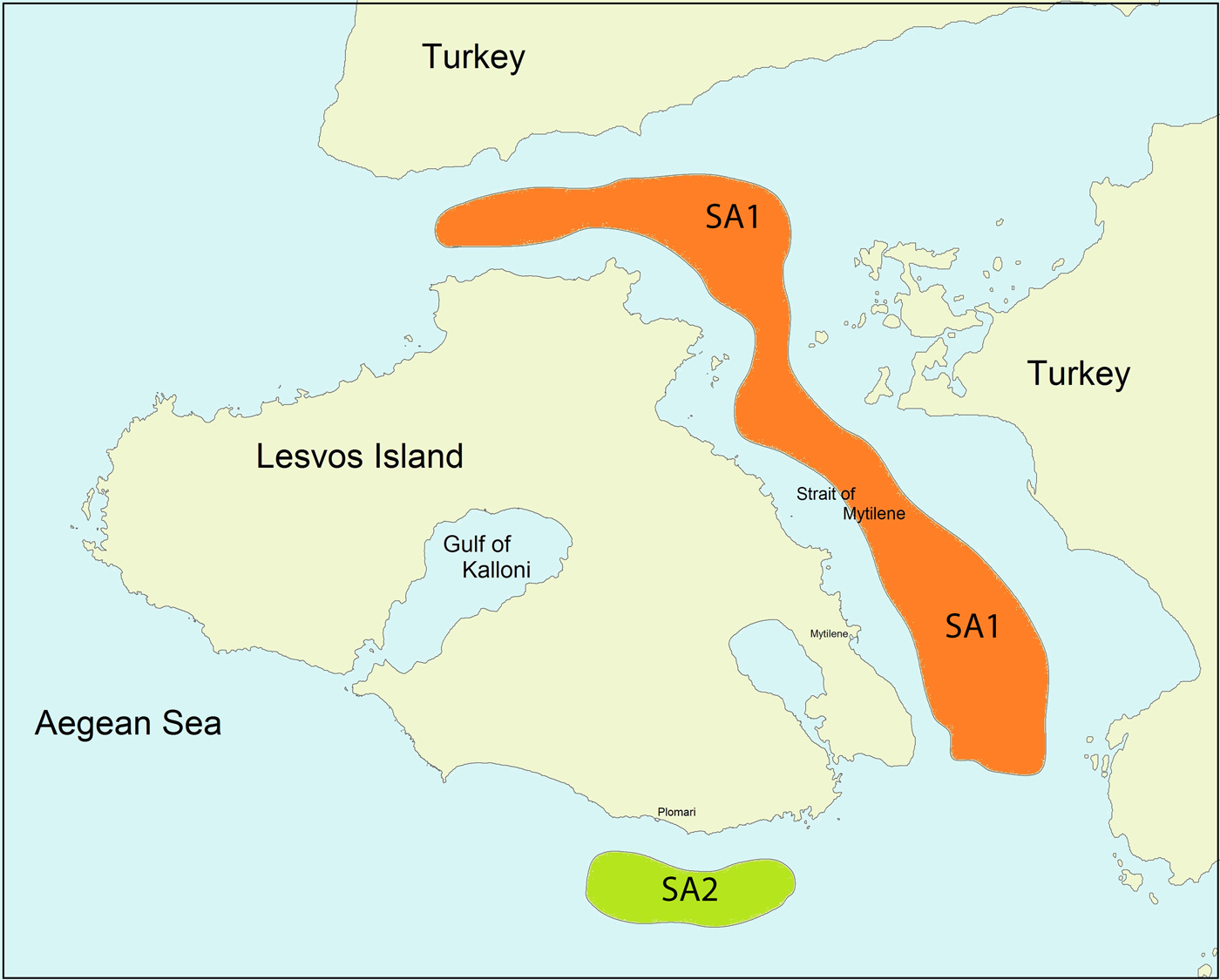
Fig. 1. Scyliorhinus canicula sampling areas (SA1 and SA2) around Lesvos Island, north-western Greece.
Fish were collected by commercial bottom trawlers at a distance of 3–6 nautical miles from the coastline and at depths from 40–100 m. Fish upon landing onto the deck were immediately placed on ice and stored at 0–2°C until the parasitological examination.
Examination of specimens
Species identification was performed according to the description of Compagno (Reference Compagno1984). All samples were sexed and their total body length (TL) was recorded (Figure 2).

Fig. 2. Total body lengths, median values and intervals for male and female S. canicula specimens during spring, summer and autumn.
The average (±SD) and median TL in cm for female and male specimens were: during spring 34.7 (±6.1), 34.4 and 34.7 (±7.4), 31.2, respectively; during summer, 37.9 (±8.5), 40.8 and 37.4 (±6.8), 38.9, respectively; and during autumn, 43.1 (±2.1), 43.6 and 44.7 (±4.4), 45.7, respectively.
Parasitological examination was performed according to Roberts (Reference Roberts1989) and Athanassopoulou (Reference Athanassopoulou1990) with modifications. All external (skin, gills, genital area and buccal cavity) and internal surfaces and organs (abdominal cavity, digestive tract and its contents, gall bladder, liver, spleen, heart, gonads and kidney) were inspected macroscopically for the presence of parasites and lesions. Material scrapped and/or dissected from external and internal surfaces along with parasites and lesions were further observed under a stereo microscope (Zeiss, West Germany, Axioscop) and a microscope (Olympus CH20) at 100× and 400× magnification. Samples and/or parasites were placed in an aqueous solution of 4% formalin for preservation.
Identification of parasites
The identification of parasites was based on their morphological and morphometric characteristics according to the keys and descriptions found in the scientific literature (Berland, Reference Berland1961; Hartwich, Reference Hartwich, Anderson, Chabaud and Wilmott1974; Williams & Jones, Reference Williams and Jones1994; Henderson & Dunne, Reference Henderson and Dunne1998; Moravec et al., Reference Moravec, Van As and Dykova2002; Ozdikmen, Reference Özdikmen2008; Rodríguez et al., Reference Rodríguez, Luque and Nascimento2010).
Descriptive statistics
Quantitative data of each parasitic infection were calculated according to Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) and statistically compared using the free Quantitative parasitology 3.0 software package (Reiczigel & Rózsa, Reference Reiczigel and Rózsa2005). Further statistical analysis was performed with the Wilcoxon and Kruskal–Wallis tests. Comparisons were considered significant when P ≤ 0.05. Correlations were investigated with the Pearson's test.
RESULTS
Twenty-one out of the 52 (prevalence 40.4%) specimens collected were found infected with parasites (Table 1); an arthropod (Neoalbionella sp.) and two nematode (Proleptus sp. and Anisakis sp.) genera.
Table 1. Quantitative data of the parasitism observed in Scyliorhinus canicula (N = 52).

a Confidence interval (95%).
The Anisakis sp. infection was the most prevalent, while the highest mean intensity and mean abundance of parasites were calculated for the nematode Proleptus obtusus. The mean intensity of infection by each parasite was statistically different.
The highest prevalence of infections was recorded for both sexes during summer (55.6 and 38.5% for female and male specimens, respectively) followed by autumn (33.3 and 37.5%, respectively). The highest mean intensity and mean abundance of infections were recorded during autumn for both sexes with the female S. canicula specimens having higher infection scores. Regarding the whole sampling period, the highest infection prevalence (36%), mean intensity (5.7) and mean abundance (2.16) were calculated for the female specimens (Table 2). Statistical analysis of nematode infections between males and females and seasons showed that mean intensity of nematode infection for male and female specimens in autumn and when the whole sampling period is considered were significantly different. Comparisons of mean abundance of infections were significant for all sampling seasons.
Table 2. Quantitative data of nematode infection observed in Scyliorhinus canicula according to sex and sampling season.

a Confidence interval (95%).
Statistical comparison of body TL of the 52 specimens used in this study (Figure 2) showed that TL of males or females per season was different. Furthermore, when TL of males and females in the same season was compared, no difference was found for spring and summer in contrast to autumn. Furthermore, the number of parasites found in each specimen (males or females and the whole sample) was not correlated to body TL.
Copepods
NEOALBIONELLA SP.
Eight adult female individuals, 5.8–6.0 mm long, belonging to the family Lernaepodidae, were observed on the skin of the peri-genital area and pterygiopodia (male intromittent organs) of seven specimens (prevalence 13.5%) (Table 1).
These adult female parasites were either carrying or not eggs externally (Figure 3A, B). The body of these copepods was covered by a semi-transparent white cover, the head was separated by the rest of the body and accounted for about 44.5% of the total length of the parasite (5.8–6.0 mm). Cephalothorax dorsoventrally flattened, separated from the trunk by a prominent constriction. Cephalothorax to trunk ratio, 1:2.3. At the first maxilla three rays were visible, bearing spine at the tip (Figure 4B). The second maxillae were cylindrical, measuring 2–2.2 mm in length and 0.35 mm in width, fused at their inner margins with a small bulla (Figure 3C). The trunk to second maxilla length ratio was 1.8:1. The tip of labrum was rounded with a rostrum bearing marginal setae (Figure 4C). The trunk was longer than wide, gradually wider posteriorly, with rounded postero-lateral endings. Two cylindrical uropods were placed at the posterior margin of the trunk with rounded posterior edges, bearing a tip point. The uropod to trunk length ratio measured 1:3.7. Two egg sacs were present at the posterior margin of the trunk, dorsally to uropods. According to the morphological and morphometric characteristics of the specimens these ectoparasites were identified as Neoalbionella sp. (Leigh-Sharpe, Reference Leigh-Sharpe1918) (Boxshall, Reference Boxshall1998; Raibaut et al., Reference Raibaut, Combes and Benoit1998; Rodríguez et al., Reference Rodríguez, Luque and Nascimento2010).

Fig. 3. Neoalbionella sp., female. (A) lateral view, (B) ventral view, (C) tip of second maxillae, (D) posterior end of trunk.
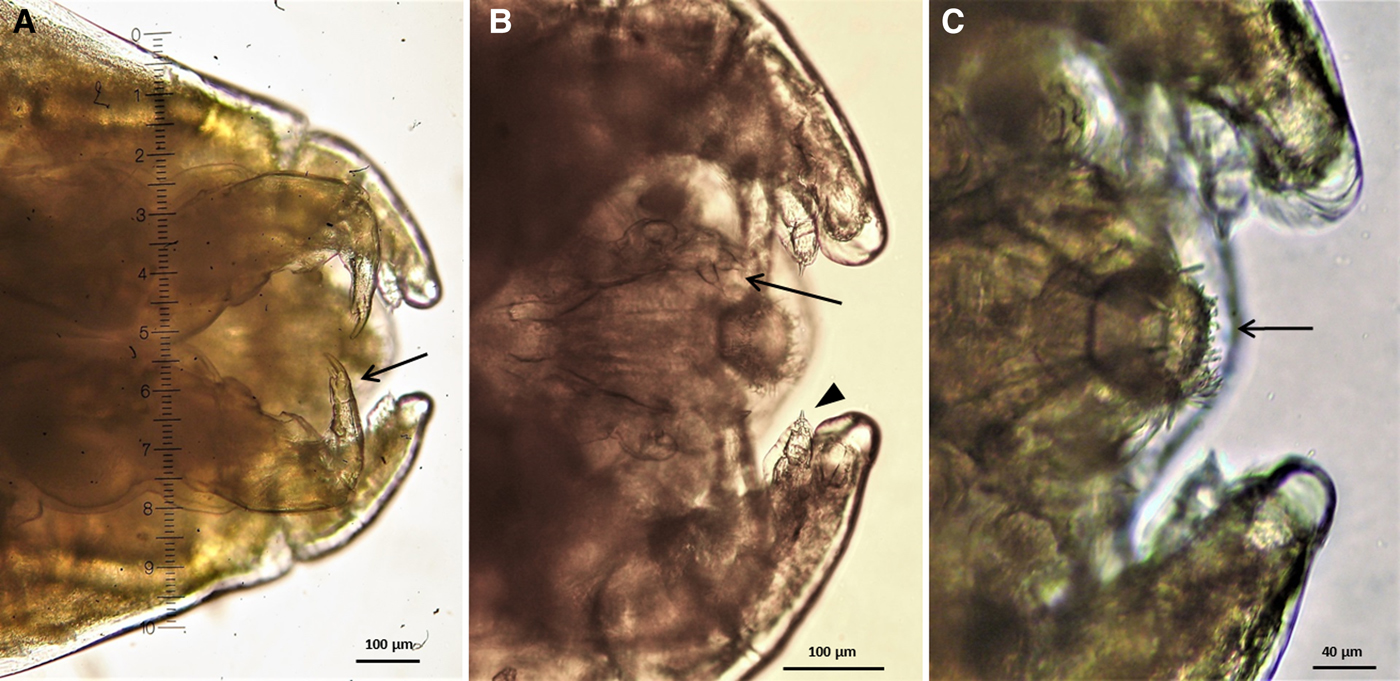
Fig. 4. Neoalbionella sp. anterior end, ventral view. (A) maxilliped (arrow), (B) first maxilla (arrow) and first antenna (arrowhead), (C) labrum.
Table 3 provides quantitative data for the parasitism by Neoalbionella sp. according to the sex of the specimen and season of collection. No Neoalbionella sp. infection was observed during spring for both genders and during autumn for female specimens. Prevalence of infections from this parasite was 22.2% and 30.8%, for female and male S. canicula specimens, respectively, during summer and 12.5% for male specimens, during autumn (Table 3).
Table 3. Quantitative data of Neoalbionella sp. infection observed in Scyliorhinus canicula according to sex and sampling season.
a Confidence interval (95%).
The highest mean intensity (2) and mean abundance (0.6) of infection were observed during summer for the male S. canicula specimens. Regarding the whole sampling period, the highest infection prevalence (18.5%) and mean abundance (0.19) of infection were calculated for the male specimens (Table 3).
Statistical analysis of data showed that the mean intensities of infection during summer between the two genders were different. There were also statistical differences in the prevalence of infection between spring/summer and in the mean abundances for both spring/summer and spring/autumn comparisons.
Nematodes
PROLEPTUS OBTUSUS
Sixty-seven adult nematode individuals (66 females and 1 male), belonging to the family Physalopteridae, were observed in the stomach and intestine walls of 10 specimens (prevalence 19.2%) (Table 1). These parasites caused a mild focal inflammation evidenced at examination as thickening and redness at the point of attachment, as previously described (Heupel & Bennett, Reference Heupel and Bennett1998).
These nematodes were identified as Proleptus obtusus (Dujardin, Reference Dujardin1845) according to the morphological characteristics described by Moravec et al. (Reference Moravec, Van As and Dykova2002). Females (Figure 5) are distinguished from males, except for the smaller size of the latter, from the upward lift of the tail of the former and the coiled shape of the tail of the latter, both adaptations used for the facilitation of copulation.
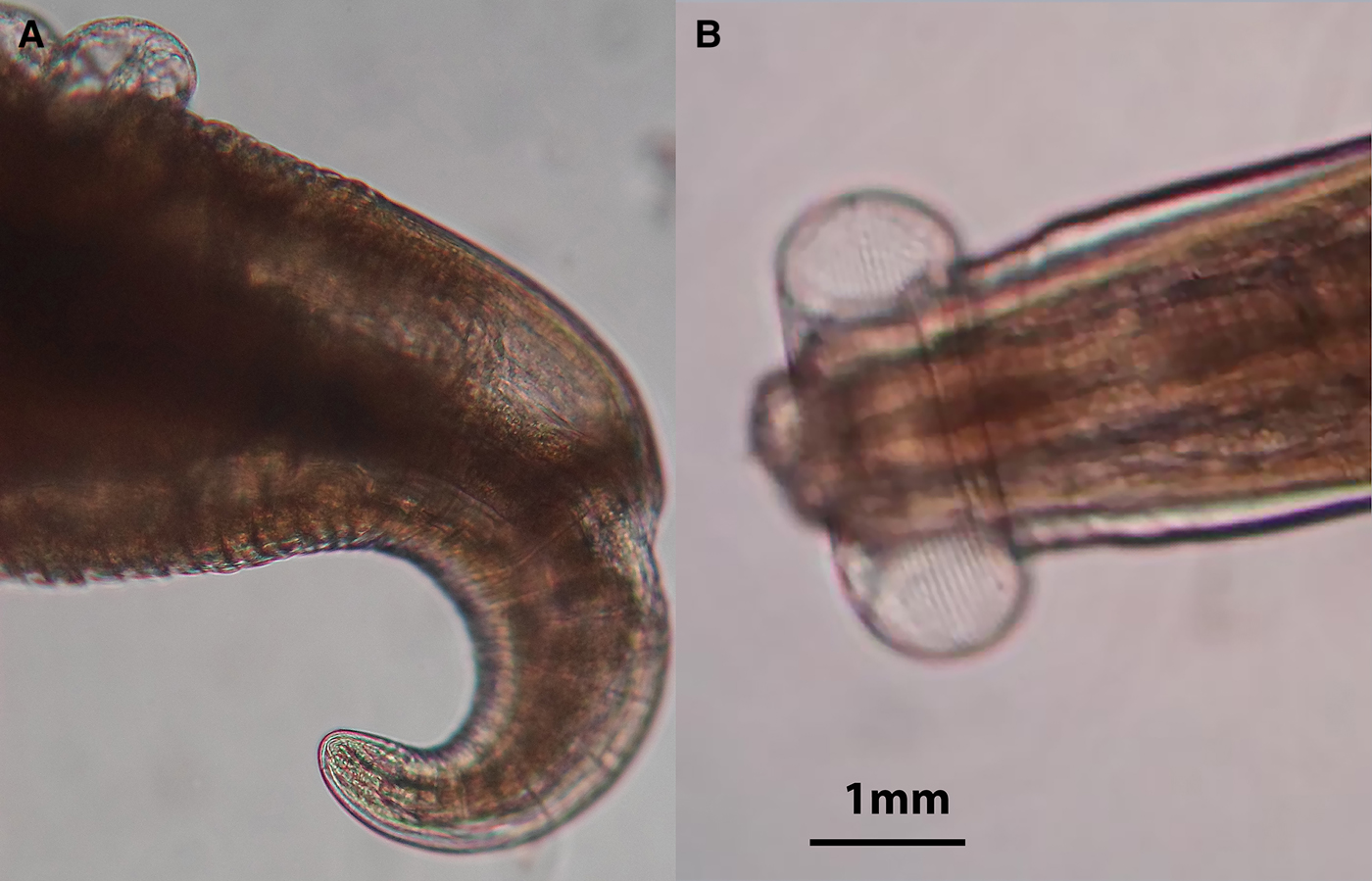
Fig. 5. Female Proleptus obtusus (Dujardin, Reference Dujardin1845) upward tail (A) and head (B).
Table 4 provides quantitative data for the parasitism by P. obtusus according to the sex of the specimen and season of collection. The lowest prevalence of infection by P. obtusus for all the specimens was noted during spring (7.7%) while prevalences of 22.7% and 23.5% were observed in summer and autumn, respectively. Although no P. obtusus infection was observed in males during spring, prevalence of infections recorded for male S. canicula specimens were higher in comparison to female specimens especially during summer and autumn reaching 30.8% and 25%, respectively (Table 4).
Table 4. Quantitative data of Proleptus obtusus infection observed in Scyliorhinus canicula according to sex and sampling season.

a Confidence interval (95%).
The highest mean intensity (17 and 11) and mean abundance (3.78 and 2.75) of infection were observed during summer for the female and male S. canicula specimens, respectively. Regarding the whole sampling period, the highest infection prevalence (22.2%) was calculated for male specimens while the highest mean abundance (1.6) of infection was calculated for the female specimens (Table 3).
Statistical analysis of data showed that there were statistical differences for the mean intensity of infection between genders in autumn and for the whole sampling period; the mean abundance of infection between spring/summer and in the mean intensity between autumn/summer comparisons.
ANISAKIS SP.
Twenty-two anisakid nematode parasites ranging in body length from 22–25 mm, were observed on the serosa of the liver, stomach and intestine of 14 specimens (prevalence 26.9%) (Table 1).
These nematodes were identified as Anisakis type I third stage larvae (sensu Berland, Reference Berland1961). Specimens displayed characteristic morphological features, i.e. a boring tooth in the anterior part, a cuticle protuberance like a spine (mucron) in the posterior part and absence of intestinal caecum at the ventriculus-intestinal connection (Berland, Reference Berland1961; Cannon, Reference Cannon1977).
Table 5 provides quantitative data for the parasitism by Anisakis sp. according to the sex of the specimen and season of collection. The lowest prevalence of infection by Anisakis sp. for all the specimens was noted during spring (7.7%) while prevalences of 36.4% and 29.4% were observed in summer and autumn, respectively. Although no Anisakis sp. infection was observed in females during spring, prevalence of infections recorded for the female S. canicula specimens were higher in comparison to male specimens especially during summer and autumn reaching 55.6% and 33.3%, respectively (Table 5).
Table 5. Quantitative data of Anisakis sp. infection observed in Scyliorhinus canicula according to sex and sampling season.

a Confidence interval (95%).
The highest mean intensities were recorded during summer and autumn for both sexes. The highest mean abundance (1.11) of infection was calculated during summer for the female S. canicula specimens. Regarding the whole sampling period, the highest infection prevalence (32%), mean intensity (1.6) and mean abundance (0.64) were calculated for the female specimens (Table 5).
Statistical analysis of data showed that there were statistical differences between genders for the mean abundance in summer and mean intensity of infection in summer and autumn; prevalence and mean abundance of infection between spring/summer.
Infections from P. obtusus and Anisakis sp. were statistically compared. Comparisons between infections per sex of the specimen and season were statistically significant for the prevalence of infections during summer for female specimens (female specimens more often infected with Anisakis sp.), mean intensity of infections for male specimens, mean intensity of infections for both sexes during autumn and when both sexes were compared for the whole sampling period.
Comparisons of prevalence, mean abundance and mean intensity of arthropod and nematode infections per season and for the whole sampling period showed that there was no statistical difference between these infections during spring. On the contrary, mean abundance/mean intensity of infections for summer and prevalence/mean abundance of infection comparisons for autumn were significant. Furthermore, comparisons of prevalence, mean abundance and mean intensity of arthropod and nematode infections for the whole sampling period were also significant.
DISCUSSION
Copepods
NEOALBIONELLA SP.
The family Lernaeopodidae comprises parasitic copepods that infect elasmobranchs. Based on adult male morphology, Kabata (Reference Kabata1979) transferred four Lernaeopoda spp. to a new genus Albionella. Further morphological studies of female Albionella spp. indicated that the latter and former species could be separated (Kabata, Reference Kabata1986; Rubec & Hogans, Reference Rubec and Hogans1988; Benz & Izawa, Reference Benz and Izawa1990; Benz, Reference Benz1991a, Reference Benzb). To date, seven species have conformed to the generic diagnosis of Albionella spp. but more recently, Özdikmen (Reference Özdikmen2008) renamed Albionella spp. to Neoalbionella spp. Thus, these species according to their chronological discovery and description are: N. globosa (Leigh-Sharpe, Reference Leigh-Sharpe1918), N. centroscyllii (Hansen, 1923), N. longicaudata (Hansen, 1923), N. etmopteri (Yamaguti, 1939), N. fabricii (Rubec & Hogans, Reference Rubec and Hogans1988), N. kabatai (Benz & Izawa, Reference Benz and Izawa1990) and N. oviformis (Benz, Reference Benz1991a). More recently, another specimen that was isolated from the lanternshark (Etmopterus granulosus) in Chile, conformed to the generic diagnosis of Neoalbionella spp. but differed morphologically from the seven aforementioned species (Rodríguez et al., Reference Rodríguez, Luque and Nascimento2010). Based on the detailed morphological description of the Neoalbionella sp. recorded by the latter authors, the comparisons between the species they found with the other seven described Neoalbionella spp., the location on the S. canicula body where the parasites were found and their size, the specimens isolated in this study from S. canicula matched the Neoalbionella sp. described by Rodríguez et al. (Reference Rodríguez, Luque and Nascimento2010). This is the first record of a new Neoalbionella sp. in the north-eastern Mediterranean, since the only Lernaeopodidae copepods described from S. canicula are N. globosa (Leigh-Sharpe, Reference Leigh-Sharpe1918) and Lernaeopoda galei (Krøyer, 1837) in European–Mediterranean waters (Henderson & Dunne, Reference Henderson and Dunne1998; Raibaut et al., Reference Raibaut, Combes and Benoit1998).
Members of the family Lernaeopodidae, according to Boxshall (Reference Boxshall1998) have a wide range of hosts but they prefer hosts of deeper waters. This preference matches the habitat of the small-spotted catshark. According to Raibaut et al. (Reference Raibaut, Combes and Benoit1998) these copepods become parasitic when adults and in most species it is the female gender infecting the hosts.
Infection of S. canicula from Neoalbionella sp. in the north-eastern Mediterranean seems to manifests itself especially during the warmer seasons of the year, summer and autumn, with male S. canicula specimens exhibiting higher mean intensity of infections and parasite burden. Both prevalence and abundance of Neoalbionella sp. infection of S. canicula in this study differed slightly from those described by Rodríguez et al. (Reference Rodríguez, Luque and Nascimento2010) on a Neoalbionella sp. found on the lanternshark (Etmopterus granulosus) in Chile. These differences may be attributed to the geographic areas where sampling was performed and especially their different characteristics of depth and water temperatures which may influence parasite assemblages and Neoalbionella sp. populations and the different host species habitat preferences.
Nematodes
PROLEPTUS OBTUSUS
Early larval stages of Proleptus spp. infect decapod crustaceans being obligate intermediate hosts but sexual maturity is reached in the definitive elasmobranch host (Moravec, Reference Moravec2007).
Proleptus obtusus up to 2002 (Moravec et al., Reference Moravec, Van As and Dykova2002) was described from S. canicula only in the Atlantic Ocean (Henderson & Dunne, Reference Henderson and Dunne1998; Moore, Reference Moore2001). This nematode species has been found infecting members of the Pentachinae, Rajidae and Scyliorhinidae families, while other Proleptus spp. have been described from the Rhinobatidae, Hemiscyllidae, Narcinidae and Triakidae families (Pollerspöck & Straube, Reference Pollerspöck and Straube2015). The findings of this study represent the first record of P. obtusus infection of S. canicula in the north-eastern Mediterranean.
The infected specimens of S. canicula had a heavier parasitic burden during autumn and this was more pronounced for female specimens for the same season and when the whole sampling period was considered. Furthermore, prevalence of infection was lower in spring (with no male S. canicula being found infected) and higher during the warmer seasons of summer and autumn with male specimens exhibiting higher infection prevalence in comparison to females.
Henderson & Dunne (Reference Henderson and Dunne1998) observed a prevalence of 94% of P. obtusus in S. canicula in the west coast of Ireland. Sanmartin Duran et al. (Reference Sanmartín Durán, Quinteiro and Ubeira1989) in a previous study in the NW Spain reported a 91.2% prevalence. A more recent study (Casadevall et al., Reference Casadevall, Martinez and King2010), reported 96.9% and 100% prevalence of infections in specimens of S. canicula sampled in the north-western Mediterranean and in the south-western coast of Ireland, respectively and similarly high prevalence (94.8%) was recorded in specimens collected northern Portugal (Silva et al., Reference Silva, Verissimo, Cardoso, Cable and Xavier2017). The prevalence of infection by P. obtusus in S. canicula calculated in this study was by far lower (19.2%) compared with the aforementioned studies. Mean intensity of infection calculated in this study (6.7) was also much lower in comparison to the findings of Casadevall et al. (Reference Casadevall, Martinez and King2010) (21.6 and 30.2 in NW Mediterranean and SW Irish Coast, respectively) and the case is the same for mean abundance calculated in this study (1.35) and the latter study (20.7 and 30.2 in NW Mediterranean and SW Irish Coast, respectively) or the study of Silva et al. (Reference Silva, Verissimo, Cardoso, Cable and Xavier2017) (mean abundance 23.3).
ANISAKIS SP.
Larval forms of the zoonotic nematode of the genus Anisakis were found under the serosa layer of the liver, stomach and intestine of 14 S. canicula specimens caught in the N.E. Aegean Sea (prevalence 26.9%, the highest in comparison to the other parasite infections). Anisakis infection of S. canicula in the north-eastern Mediterranean is reported for the first time. Prevalence and mean abundance of infection tended to be lower during spring and higher during the warmer seasons of the year, especially in summer (P < 0.05) with female S. canicula specimens being found to be more frequently infected. Both mean abundance (summer) and mean intensity of infections (summer and autumn) of female specimens were significantly higher in comparison to male specimens.
Casadevall et al. (Reference Casadevall, Martinez and King2010) recorded quantitative data of anisakiasis in S. canicula in the north-western Mediterranean with the following characteristics: prevalence: 0.8%, mean abundance: 0.008 and mean intensity: 1. A previous study (Sanmartin Duran et al., Reference Sanmartín Durán, Quinteiro and Ubeira1989) reported a 3.5% prevalence of Anisakis simplex infection of S. canicula specimens caught in the N.W. Spain. In contrast to P. obtusus infection, prevalence of Anisakis (26.9%) as well as mean intensity (1.6) and mean abundance (0.54) of infections in the present study were much higher in comparison to these previous studies.
Further comparisons of the data showed that prevalence of infection during summer with Anisakis sp. for female specimens was significantly higher in comparison to P. obtusus prevalence for the same season. Both sexes were significantly more heavily infected with P. obtusus during autumn as well as during the whole experimental period. Thus, a trend has been identified, for heavier infections by P. obtusus of fewer specimens and lighter infections by Anisakis sp. of more individuals. When data on nematode infections were considered as a whole, female S. canicula specimens have been found to be significantly more heavily infected during autumn and during the whole sampling period. Nematode infection has been found to be significantly related to season, becoming progressively heavier from spring to autumn, reflecting probably increased prey availability and feeding activity. Finally, no correlation was found between TL and parasite burden for males or females in accordance to the study of Yeld (Reference Yeld2009) with shark species caught off South Africa, the study of Lloret et al. (Reference Lloret, Faliex, Shulman, Raga, Sasal, Munoz, Casadevall, Ahuir-Baraja, Montero, Repulles-Albelda, Cardinale, Ratz, Vila and Ferrer2012) on S. canicula in the Mediterranean or the study of Silva et al. (Reference Silva, Verissimo, Cardoso, Cable and Xavier2017) on S. canicula caught in northern Portugal.
The presence of parasites in a host is affected by the host's ecological preferences, such as habitat, daily migrations, depth preferences, geographic range, ecological traits including trophic level, diet and factors such as the size of the host, age, population size, etc. In fact, helminth parasites in the digestive tract can be used as indicators of trophic interactions and seasonal and developmental shifts in the diet of the host (Marcogliese & Cone, Reference Marcogliese and Cone1997; Marcogliese, Reference Marcogliese2004; Gracan et al., Reference Gracan, Culinovic, Mladineo, Lackovic and Lazar2016). To this end, Gracan et al. (Reference Gracan, Culinovic, Mladineo, Lackovic and Lazar2016) studying helminth fauna in the gastrointestinal tract of two shark species in the Adriatic Sea suggested that differences in infection parameters between the species studied could be attributed to different feeding habits and strategies and the availability, accessibility or preference of intermediate hosts which could be influenced by seasons.
Scyliorhinus canicula is a broad generalist in its diet and habitat requirements. It is also an opportunistic feeder, cannibalism is frequently recorded, and S. canicula has been reported to eat discarded viscera of their own and other fish species (Lyle, Reference Lyle1983; McClelland et al., Reference McClelland, Misra, Martell and Bowen1990; Olaso et al., Reference Olaso, Velasco and Pérez1998; Abollo et al., Reference Abollo, Gestal and Pascual2001).
It feeds mostly on crustaceans and pelagic bony fishes, but differences in feeding behaviour have been described with age. Various studies have reported that adults consume more teleosts than juveniles (Olaso et al., Reference Olaso, Velasco, Sanchez, Serrano, Rodriguez-Cabello and Cendrero2005; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Martinho et al., Reference Martinho, Sa, Falcao, Cabral and Pardal2012; Santic et al., Reference Santic, Rada and Pallaoro2012). Furthermore, Olaso et al. (Reference Olaso, Velasco, Sanchez, Serrano, Rodriguez-Cabello and Cendrero2005) reported that adults were found in deeper waters than juveniles in the Cantabrian Sea. Differences in feeding habits have been reported between sexes, with adult males and juveniles of both sexes feeding more during the warmer seasons of the year when prey is more available, in contrast to adult females (Martinho et al., Reference Martinho, Sa, Falcao, Cabral and Pardal2012). Sims et al. (Reference Sims, Nash and Morritt2001) reported the occupation of different depth-related habitats of males and females in south-west Ireland. Depth can exert an important effect on the diet of this species, with individuals retrieved from deeper waters feeding mostly on euphausiids and individuals caught in shallower habitats feeding on reptantians, polychaetes and teleosts (Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011).
To the best of our knowledge, only two studies attempted to correlate infection parameters of S. canicula to ontogenetic feeding behaviour and seasonal prey availability. Lloret et al. (Reference Lloret, Faliex, Shulman, Raga, Sasal, Munoz, Casadevall, Ahuir-Baraja, Montero, Repulles-Albelda, Cardinale, Ratz, Vila and Ferrer2012) found no relationship between host TL and intensity of infection in the north-western Mediterranean and suggested that this parameter was better related to feeding frequency and prey availability. Silva et al. (Reference Silva, Verissimo, Cardoso, Cable and Xavier2017) in northern Portugal suggested that P. obtusus burden of S. canicula could be explained by differences in the ontogenetic and foraging behaviour of this species and seasonal differences in prey availability.
According to the findings of Silva et al. (Reference Silva, Verissimo, Cardoso, Cable and Xavier2017) the specimens of S. canicula used in this study belonged to the subadult group of ontogenetic development. The higher prevalence of P. obtusus infection of male specimens during the warmer seasons of the year recorded in this study could be explained by the increased feeding activity of male S. canicula during these seasons, in contrast to females (Martinho et al., Reference Martinho, Sa, Falcao, Cabral and Pardal2012).
The lower prevalence of P. obtusus and the higher prevalence of Anisakis sp. infections in this locality lead to the hypothesis that the S. canicula specimens used in this study either behaved as adult specimens regarding their feeding preference, feeding more on teleosts than on crustaceans (Olaso et al., Reference Olaso, Velasco, Sanchez, Serrano, Rodriguez-Cabello and Cendrero2005; Valls et al., Reference Valls, Quetglas, Ordines and Moranta2011; Martinho et al., Reference Martinho, Sa, Falcao, Cabral and Pardal2012; Santic et al., Reference Santic, Rada and Pallaoro2012), hence becoming more infected with Anisakis sp. or that the higher prevalence of Anisakis infection was due to prey availability and preference. As a matter of fact Perari (Reference Perari2010) in the same locality recorded differences in the stomach contents of S. canicula specimens with larger female individuals consuming more teleosts during the spring of 2005 and 2006. In the same study, year to year differences were also noted in the stomach contents of S. canicula for the same season, strongly suggesting a shift in food items consumption according to their availability. Furthermore, the higher presence of Anisakis sp. in S. canicula caught in the area of Lesvos may be associated with the presence of permanent populations of dolphins in this locality, which act as definitive hosts for this nematode (Pozio, Reference Pozio2013), and the higher prevalence of Anisakis sp. infection during summer and autumn coincides with the existence of large populations of sardines and anchovies which act as paratenic hosts (Abollo et al., Reference Abollo, Gestal and Pascual2001; McClelland & Martell, Reference McClelland, Martell, Desportes and McClelland2001; Mattiucci et al., Reference Mattiucci, Abaunza, Ramadori and Nascetti2004, Reference Mattiucci, Farina, Campbell, MacKenzie, Ramos, Pinto, Abaunza and Nascetti2008; Rello et al., Reference Rello, Adroher, Benitez and Valero2009; Mladineo & Poljak, Reference Mladineo and Poljak2014) and represent an important fishery in this locality.
Concluding, the present study provided a first record of Neoalbionella sp., P. obtusus and Anisakis sp. infections of S. canicula in the north-eastern Mediterranean. The higher prevalence of P. obtusus infection of male specimens during the warmer seasons of the year could be explained by the increased feeding activity of male S. canicula during these seasons, in contrast to females. The lower prevalence of P. obtusus and the higher prevalence of Anisakis sp. infections in this locality are probably due to prey availability and preference by S. canicula and parasite populations in this area.
FINANCIAL SUPPORT
This research was funded by institutional funds of the Department of Marine Sciences, University of The Aegean.












