Ventricular septal defects are the most common congenital cardiac malformations accounting for 30% of CHD.Reference Anderson, Becker and Tynan 1 Although surgery is the mainstay of therapy, transcatheter interventions have gained significant importance over the years, with increasing experience, improvement in skills and imaging, precise patient selection, and availability of lower-profile devices. Although transcatheter closure of a membranous ventricular septal defect may be potentially life-saving in selected patients, it is generally reserved for relatively older children. Specific challenges include haemodynamic instability during creation of an arteriovenous wire-loop, the size of the delivery system, and risk of conduction block.
In this article, we report the successful closure of a membranous ventricular septal defect in a 1.8-kg infant with bilateral femoral artery occlusion using Amplatzer Duct Occluder II additional size device via a 4-French delivery system.Reference Sungur, Karakurt, Ozbarias and Baspinar 2 The ventricular septal defect was crossed from the right ventricle without creating an arteriovenous loop.
Case report
A 3-day-old, term, female neonate with birth weight of 3.1 kg, delivered by a primigravida mother, with history of infertility treatment for 10 years, presented to the neonatal ICU with respiratory distress. Her clinical condition worsened, necessitating mechanical ventilation. Her chest X-ray showed cardiomegaly with pulmonary plethora. An echocardiogram showed a large patent ductus arteriosus (6 mm) and a membranous septal defect (2.5 mm). The ventricular septal defect’s margin was 2 mm away from the aortic annulus. Despite ventilation and optimal medical management, her clinical condition deteriorated over the ensuing days with onset of pneumonia, bloodstream sepsis, pleural effusion, and ascites. On the 13th day of life, emergency ductus arteriosus ligation was undertaken via sternotomy in view of haemodynamic instability.
After initial clinical improvement, respiratory distress worsened necessitating re-intubation. Despite optimised medical management, there were multiple failed extubation attempts and progressive weight loss to 1.8 kg. An echocardiogram revealed a residual ductus arteriosus (2 mm). The residual ductus together with the previously identified ventricular septal defect were identified as likely contributors. The risk of surgical re-intervention was considered prohibitive, given the clinical status and depleted nutritional reserves. We opted for a transcatheter approach for occluding both the defects using Amplatzer Duct Occluder II additional size devices. This device was specifically chosen because of the low profile of the delivery system and the relatively small size of retention discs. On the basis of the echocardiographic assessment, a 4-mm×2-mm device was chosen for the ductus and a 5-mm×2-mm device for the ventricular septal defect (Fig 1a).
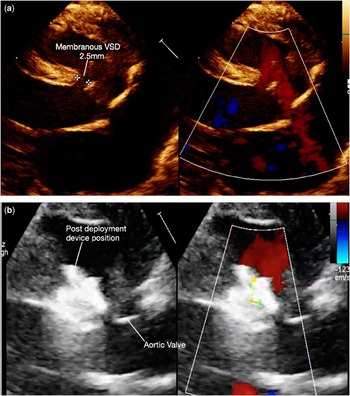
Figure 1 (a) Parasternal long axis view showing membranous VSD measuring 2.5 mm with left to right shunt. Note the left atrium, left ventricle enlargement. (b) Post device deployment echo, device well away from aortic valve and no residual flow.
Cardiac catheterisation was performed via the left femoral venous approach (4-French). Bilateral femoral arteries were occluded, presumably during previous ICU stay. A 4-Fr right Judkins catheter/0.025 Terumo wire combination was used to cross the ductus into the descending aorta. The mean pulmonary artery pressure was 24 mmHg (mean aortic pressure: 54 mmHg). The catheter was exchanged for a 25-cm, 4-Fr sheath (Cook Inc., Bloomington, IN, United States of America), which was positioned in the descending aorta. The ductus was angiographically profiled with wire in situ using a previously described technique.Reference Francis, Singhi, Srinivas and Kumar 3 The device (4-mm diameter×2-mm length) was introduced through the sheath and deployed across the ductus under fluoroscopic guidance. The device was released after confirming device position echocardiographically. A 4-Fr Judkins right catheter/0.025 Terumo wire combination was introduced through the sheath into the main pulmonary artery; the sheath was then withdrawn into the right atrium (Fig 2a). The catheter was gently withdrawn in the posterior–anterior view and placed in the right ventricular outflow just below the pulmonary valve. In lateral projection, the catheter was rotated posteriorly, and the wire was gently advanced across the ventricular septal defect into the left ventricle (Fig 2b and c). Its position was confirmed by the posteriorly located wire-loop in the left ventricle. The catheter was then advanced into the left ventricular cavity followed by advancement of the sheath over the catheter. After removing the catheter and wire, a 5-mm diameter×2-mm length device was loaded and introduced through the sheath into the left ventricle. The aortic retention disc was exposed under flouroscopy guidance (Fig 2d). Using echocardiographic guidance, the assembly was withdrawn until the device was at the left margin of the septal defect (Fig 2e). After confirming the absence of impingement of the aortic valve, the device was deployed across the septal defect. The device was released after confirming satisfactory position.

Figure 2 Steps of crossing VSD from RV side and device deployment. (a) Diagramatic representation of catheter course in AP projection with heart anatomy. (b) Lateral projection diagramatic representation of Judkin’s right catheter pulled back below pulmonary valve and turned to face posteriorily and terumo wire inserted into posterior placed LV. (c) Lateral angio image of VSD crossing with terumo wire into LV. (d) 4 French long sheath tracked into LV and LV disc of device deployed under flouoro guidance. (e) Under TTE guidance, device being adjusted to LV side of VSD. LV = left ventricle; MPA = main pulmonary artery; RV = right ventricle; SVC = superior vena cavae; IVC = inferior vena cavae; TTE = transthoracic echo; VSD = ventricular septal defect.
The child was successfully extubated 2 days after the procedure. Steady clinical improvement and weight gain ensued. She was discharged on the 68th day of life weighing 2.3 kg. Serial post-procedure echocardiograms showed optimal device positions with no residual flow and no aortic regurgitation (Fig 1b). Her electrocardiogram was normal. The child was discharged without any cardiac medications.
Follow-ups at 1 month and 6 months after the procedure showed good device positions, no residual shunts or aortic regurgitation, sinus rhythm, and good weight gain (6.6 kg at the 6-month follow-up).
Discussion
To the best of our knowledge, this is the smallest and youngest patient to have undergone catheter closure for a membranous septal defect. This case highlights the technique of crossing a ventricular septal defect from the right ventricle into the left ventricle cavity, thereby avoiding an arteriovenous loop and the use of low-profile Amplatzer Duct Occluder II additional size device that can be delivered via 4-Fr introducer sheath.
Alghamdi et alReference Alghamdi, Al-Habshan and AL-Mutairia 4 reported transcatheter ventricular septal defect closure in a 9.5-month-old infant with Down syndrome weighing 4.8 kg. Device closure has been successfully accomplished in infants <1 year of age weighing as low as 3 kg for muscular septal defects using the perventricular approach.Reference Saurav, Kaushik, Mahesh Alla, White and DelCore 5 Arteriovenous loop creation is associated with haemodynamic instability and risk of arterial thrombosis due to arterial sheath. With this technique, we are able to cross the ventricular septal defect consistently from the right ventricular side in most cases. This technique has an added advantage of introducing the sheath directly into the left ventricular cavity, which allows us to deploy the device without entrapment of aortic cusps. The Amplatzer Duct Occluder II additional size device has the advantage of being a low-profile device. Although there was no heart block in this infant until 6 months, strict follow-up is necessary over the next several years to be absolutely sure.
Conclusion
In carefully selected cases, transcatheter closure of a ventricular septal defect is feasible and safe in small infants. The venous approach avoids the arteriovenous loop, which is associated with haemodynamic instability. Low-profile duct occlusion devices may be safe for ventricular septal defect closure in infants.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interests
None.
Ethical Standards
This case report was approved by the institutional review board registered under Central Drugs Standard Control Organisation, Government of India.





