Introduction
Caenogastropods include a vast number of species that play prominent roles in marine communities, enriching biodiversity and total abundance (Underwood, Reference Underwood1980; Fretter & Graham, Reference Fretter and Graham1994; Valentine et al., Reference Valentine, Roy and Jablonski2002). Among them, carnivorous moonsnails (family Naticidae) are distributed worldwide (Kabat, Reference Kabat1990), and play an important role in benthic communities by regulating abundance of prey molluscs (e.g. Sato et al., Reference Sato, Chiba and Hasegawa2012; Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019). The impact of predators on prey populations may be better predicted in the presence of information on recruitment. Recruitment is a complex process that depends on fecundity, mating and spawning frequency, duration of embryonic development, hatching rate and post-larval mortality (Underwood & Keough, Reference Underwood, Keough, Bertness, Gaines and Hay2001). Assessment of recruitment requires a solid understanding of early life history of the target species. Despite the important role that naticids play in marine benthic communities, the early life history of some of these predators is studied far less than their adult life.
Moonsnails lay egg masses known as egg or sand collars (Kang et al., Reference Kang, Tan and Liu2018). Morphology of the egg collars is often species-specific in naticids (Giglioli, Reference Giglioli1955; Kingsley-Smith et al., Reference Kingsley-Smith, Richardson and Seed2003; Kang et al., Reference Kang, Tan and Liu2018). In the majority of naticids, eggs develop within sand collars into planktonic larvae. There are only a few species with direct development that hatch as crawling juveniles (Pastorino et al., Reference Pastorino, Averbuj and Penchaszadeh2009), and in accordance with Thorson's rule (Reference Thorson1946) they are found in temperate and subpolar regions.
Because conspicuous and easy to collect, sand collars of moonsnails were studied for morphology (Giglioli, Reference Giglioli1955; Ziegelmeier, Reference Ziegelmeier1961; Murray, Reference Murray1969; Pastorino et al., Reference Pastorino, Averbuj and Penchaszadeh2009; Kang et al., Reference Kang, Tan and Liu2018), development of embryos and larvae (Ansell, Reference Ansell1981; Kingsley-Smith et al., Reference Kingsley-Smith, Richardson and Seed2003; Kulikova et al., Reference Kulikova, Kolbin and Kolotukhina2007; Pastorino et al., Reference Pastorino, Averbuj and Penchaszadeh2009), timing of spawning and duration of development (Ansell, Reference Ansell1981; Kingsley-Smith et al., Reference Kingsley-Smith, Richardson and Seed2003; Tomiyama, Reference Tomiyama2013). Works assessing density of naticid egg collars are extremely rare (Yoshida et al., Reference Yoshida, Sato, Narita and Tomiyama2017).
Adult naticids are generally regarded as drilling predators (see, for example, Carriker, Reference Carriker1981; Kabat, Reference Kabat1990) which sometimes show ontogenetic shift of diet and size-preferences (Clements & Rawlings, Reference Clements and Rawlings2014; Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019). Nonetheless, there is evidence that adult moonsnails can exploit non-molluscan food sources or consume some prey species without drilling (Thorson, Reference Thorson1935; Perry, Reference Perry1940; Jensen, Reference Jensen1951; Ansell, Reference Ansell1961; Paine, Reference Paine1963; Kabat, Reference Kabat1990; Huelsken, Reference Huelsken2011; Visaggi et al., Reference Visaggi, Dietl and Kelley2013). At the same time, the data about the early diet of juvenile moonsnails are controversial. While there are studies suggesting that some naticids are able to feed like adults immediately after metamorphosis (Berg, Reference Berg1976) there are many examples when the diet of juveniles differs from the adult one. For example, findings of drilled foraminiferal tests in soft sediments suggested that juvenile moonsnails could rely on such food items (Saidova & Beklemishev; Reference Saidova and Beklemishev1963; Culver & Lipps, Reference Culver, Lipps, Kelley, Kowalewski and Hansen2003). Predation by naticid juveniles on ostracods was observed in the laboratory (Page & Pedersen, Reference Page and Pedersen1998). Hatched juveniles of Polinices catena (da Costa, 1778) feed on bivalves, other gastropods, and by cannibalism in cultures (Ansell, Reference Ansell1982). Our previous data showed that small Amauropsis islandica (Gmelin, 1791) snails at the White Sea sandflats can feed on the minute mudsnail Peringia ulvae Pennant, 1777 (Aristov et al., Reference Aristov, Varfolomeeva and Puzachenko2015). On the other hand, Bernard (Reference Bernard1967) showed that newly settled Neverita [Euspira] lewisii (Gould, 1847) are able to feed on diatoms as well as on sea lettuces (Ulva sp.) under experimental conditions, although it was not confirmed by later observations (Pedersen & Page, Reference Pedersen and Page2000). Recently, stable isotope analysis suggested that the intertidal shark eye moonsnail Neverita duplicata (Say, 1822) at Long Island Sound could be omnivorous (Casey et al., Reference Casey, Fall and Dietl2016), but there were no direct observations on feeding. To our knowledge, for most species the information on early juvenile diet is lacking.
Amauropsis islandica and Euspira pallida (Broderip & G. B. Sowerby I, 1829) are direct-developing boreal arctic species distributed throughout the Arctic Ocean and the northern Atlantic. In the White Sea they can be found in the shallow subtidal and on intertidal sandflats. These species lay easily recognizable sand collars but the information about their morphology as well as the number and size of egg capsules is scarce. The spawning of A. islandica occurs at the White Sea in July when water temperature rises to 10°C (Golikov & Kusakin, Reference Golikov and Kusakin1978; Golikov, Reference Golikov, Starobogatov and Naumov1987), but there is no such information for E. pallida. The details of egg collar morphology, timing and developmental success of E. pallida at the White Sea as well as details of reproductive biology of A. islandica within its distribution range were totally unknown up to present. Here we report the first results on the characteristics, abundance of egg collars, development time and hatching success of the naticids A. islandica and E. pallida at the high latitude White Sea. We also briefly describe the feeding of A. islandica hatchlings.
Methods
Study area
The Kandalaksha Bay of the White Sea is a fjord-like basin with rugged coastal edges (Figure 1). The mean monthly temperature of the surface (0–5 m) seawater layer in this region varies from −0.4°C in February to 14.4°C in July (Leonov et al., Reference Leonov, Filatov, Zdorovennov and Zdorovennova2004). The tides are semi-diurnal with a range of ~1.8 m (Korsun et al., Reference Korsun, Hald, Golikova, Yudina, Kuznetsov, Mikhailov and Knyazeva2014). The small inlets of Kandalaksha Bay are usually covered by thick seasonal ice (up to 40–50 cm) from the beginning of November until late May (Naumov, Reference Naumov2013). We collected and counted egg collars from two locations: Youzhnaya (67°00′N 32°34′E) and Kluschikha (66°18′N 33°46′E) inlets. These small and shallow semi-enclosed inlets (5 and 7 m mean depth respectively) are located 95 km apart from each other. On the tidal sandflats, there are big boulders up to 1.5 m high, which could mitigate the negative effect of ploughing soft sediments by ice in winter (Kuznetsov, Reference Kuznetsov1960). In the subtidal zone, such boulders are absent, and sediments mainly consist of muddy sand. Adult Euspira pallida snails were found at both inlets in the subtidal zone, although Amauropsis islandica were observed only in Youzhnaya inlet. We did not find any naticids in the intertidal zone at Kluschikha inlet (Aristov, pers. obs.).

Fig. 1. Map of the sampling sites at the Kandalaksha Bay, the White Sea. Y – Youzhnaya inlet; K – Kluschikha inlet; WSBS – the White Sea Biological Station. Map data are from the Global Self-consistent, Hierarchical, High-resolution Geography Database (GSHHG version 2.3.3) (Wessel & Smith, Reference Wessel and Smith1996).
Abundance of egg collars
The egg collars of naticids were counted in the last 10 days of May 2018. In the subtidal zone, egg collars were counted along three transects 179 m in total length by a scuba diver. Each ~60 m long transect started from the same boulder at chart datum and went to different finish points at ~2 m depth. In the intertidal, nine transects 912 m in total length near the chart-datum level were surveyed during the same ebb tide. The length of each transect was measured by GPS positioning. The width of each transect was ~1 m. The total number of egg collars within each transect belt was recorded, and thus abundance of egg collars was calculated as units m−2.
Culture of juveniles
During counting, eight egg collars of Amauropsis islandica and 10 egg collars of Euspira pallida were collected on 28 May 2018 in Youzhnaya inlet and transferred to the laboratory at the White Sea Biological Station (66°20.230′N 33°38.972′E). Additionally, egg collars of E. pallida were collected from ~3 m depth in Kluschikha inlet on 2 April (six specimens) and 6 August (three specimens) 2019 by a scuba diver and transferred to the same laboratory. All egg collars were measured prior to cultivating using the ocular micrometer of a Leica binocular microscope. Amauropsis islandica egg collars were weighed using a digital scale to 0.01 g accuracy and photographed. We reduced the physical handling of egg collars as far as possible. Digital images of egg collars were examined for general morphology and number of egg capsules (ECN). To determine the density of egg capsules within each egg collar, the capsules were counted within three randomly positioned 1 cm2 frames, avoiding acapsular parts.
Egg collar culture conditions corresponded to the ambient conditions in the sea at the time of experiment: salinity 24.6 ± 0.3‰ and temperature 13 ± 1.3°C (Mean ± SE, N. Usov, pers. obs.). Each egg collar was individually placed in a separate 2 l container under constant aeration. The seawater in containers was changed every 2 days. Dates of the beginning and end of hatching were registered for each A. islandica egg collar. Identification of hatched juveniles was determined by the presence of empty capsules using a Leica binocular microscope. The opened empty capsule differs visually from the capsule with embryos. The number of hatchlings (HN) was counted, and hatching success per egg collar was calculated as HN/ECN. Hatching success of Euspira pallida was estimated as a proportion of empty capsules to their total number in randomly chosen parts of three different egg collars on 11 November 2019, at about 3 weeks after the start of hatching (the delay ensured that hatching was over by that time). The mean width of the juveniles was measured immediately after hatching using the ocular micrometer of a Leica binocular microscope.
Observations on feeding biology of Amauropsis islandica hatchlings
The juveniles of Amauropsis islandica were kept in Petri dishes for nine weeks. The water in the dishes was changed every 48 h. The water temperature and salinity corresponded to that in the sea at the time of the experiment (see above). Various food items were offered to the hatchlings 5–7 days after they hatched from the egg collars: newly settled blue mussels Mytilus edulis (450–600 μm), Limecola balthica (900–2500 μm), adult mudsnails Peringia ulvae (up to 3 mm), as well as periphyton and detritus from the intertidal sediments near the laboratory at the White Sea Biological Station. The food items were offered at random, one type at a time, and changed weekly. Juvenile moonsnails were measured every five days, and Petri dishes were searched for drilled shells of molluscs and A. islandica faeces. Naticid faeces differ in shape from Limecola balthica (L., 1758) (the description and photo are provided in Moffat, Reference Moffat1975), Peringia ulvae (see e.g. Rossignol et al., Reference Rossignol, Dupuy, Pascal and Debenay2007), and Mytilus edulis L., 1758 (Riisgård et al., Reference Riisgård, Egede and Barreiro Saavedra2011), as indicated by drawings of faeces of a closely related species Euspira nitida (Donovan, 1803) (formerly Natica alderi Forbes 1838) in Fretter & Graham (Reference Fretter and Graham1994).
Results
Euspira pallida egg collars were found only in the subtidal zone of Youzhnaya inlet, where their average density was 0.42 ± 0.07 units m−2 (3 transects, mean ± SE). At the same time, A. islandica egg collars were more abundant in the intertidal zone (0.04 ± 0.01 units m−2) than in subtidal, where only a single egg collar of this species was found (Table 1).
Table 1. Characteristics of egg collars, egg capsules and hatchlings of Amauropsis islandica and Euspira pallida, based on this study and published data
References: [1] – Thorson (Reference Thorson1935); [2] – Thorson (Reference Thorson1946); [3] – Giglioli (Reference Giglioli1955); [4] – Golikov & Kusakin (Reference Golikov and Kusakin1978).
a This value is for ~1/3 fragment of egg collar.
b These numbers of egg capsules are described by Thorson (Reference Thorson1935) for egg collar fragments.
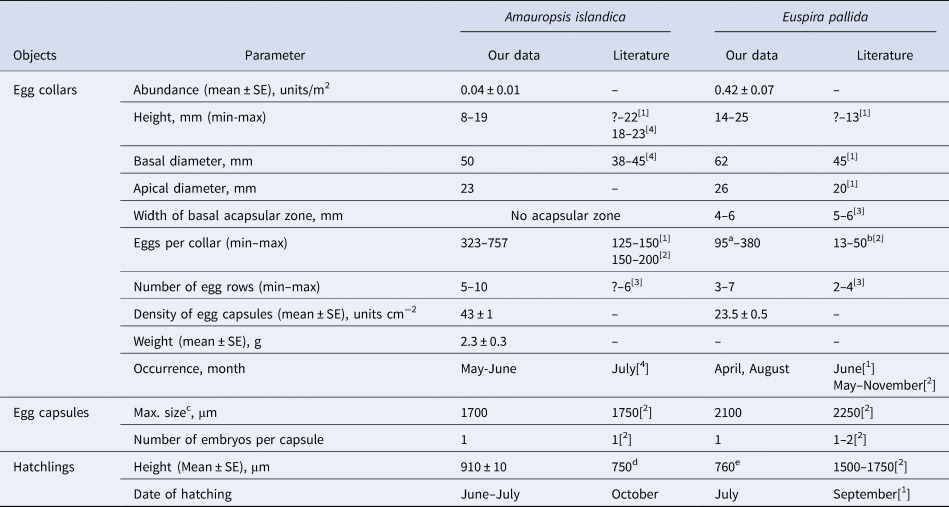
c Thorson (Reference Thorson1935) described oval egg capsules of Amauropsis, so the maximum diameter is provided here. We found that in our case egg capsules of Amauropsis were completely spherical (Figure 2D).
d Pastorino et al. (Reference Pastorino, Averbuj and Penchaszadeh2009) denote this size of larval shell with reference to Thorson (Reference Thorson1935), although there this size had corresponded to a developing embryo inside an egg capsule. The hatchling size in this species was not described.
e Only one measurement is available.
Description of egg collars
Collected egg collars of A. islandica and E. pallida can be easily distinguished visually. The egg collars of A. islandica resembled volcano craters with slightly concave and thick walls (Figure 2). The tightly bundled egg cases were surrounded by coarse sand particles and covered by smooth mud. The egg cases formed rows transversal to the egg ribbon that interlaced with each other. Egg capsules protruded into the crater so that the outer surfaces of the collars were smoother than the surfaces within the crater. Each egg capsule contained only one embryo. There was no distinctive acapsular part of ribbon at the apical and basal margins of egg collars, so both edges of egg ribbon were of irregular shape due to interlacing rows of egg capsules. The mean height of collected A. islandica egg collars was 13 mm, the mean weight was 2.3 ± 0.3 g and the mean number of embryos was 538.1 ± 53.8 (N = 8 egg collars, mean ± SE) (Table 1).

Fig. 2. Egg collars, juveniles and adult specimen of Euspira pallida (A–C) and Amauropsis islandica (D–F); egg collars (A, D), juveniles (B, E) and adults (C, F). A hatchling of Euspira pallida (B) on an empty egg capsule. Adult Euspira pallida reproduced with permission by Dr Pavel Luybin. Scale bars: A, C, D, F, 10 mm; B, E, 1 mm.
The egg collars of E. pallida at both sites were similar, in being thin egg-ribbons that were coiled to form craters with convex sloping edges (Figure 2). The walls of egg collars were encrusted with fine sand and mud. Both external and internal surfaces of E. pallida egg-ribbons were much smoother than those of A. islandica ones. There was only one embryo within each egg capsule of the E. pallida egg collar. There were distinctive acapsular strips at the basal margins of 4–6 mm width, that formed a plicated edge of a ribbon. The mean height of the E. pallida collars was 20 mm, and the mean number of embryos in each egg collar was 261.7 ± 27.1 (N = 10 egg collars, mean ± SE) (Table 1).
Amauropis islandica development and hatching
At the beginning of observations (the end of May 2018, Supplementary Figure S1) A. islandica embryos were on average at late veliger stage. Аfter 28 ± 2 (N = 8 egg collars, mean ± SE) days of incubation they started to hatch from egg capsules (Table 1). The hatching was asynchronous in each egg collar. Mean hatching duration (the interval between the first and the last hatch from an egg collar) was 10 ± 1 days (N = 8 egg collars, mean ± SE). Last hatchling appeared on 12 July 2018. The mean hatching success of A. islandica was 0.76 ± 0.03 (N = 8 egg collars, mean ± SE) (Table 1). We suggested that egg collars were incubated in good laboratory conditions, since there was no correlation between weight of the egg collar and hatching success (Welch's t-test, t = −0.04, df = 6, P = 0.97). Mean shell height of the A. islandica juveniles immediately after hatching was 910 ± 10 μm (N = 6 juveniles, mean ± SE, Figure 2).
Euspira pallida development and hatching
The E. pallida embryos from six egg collars collected in Kluschikha inlet subtidal zone on 2 April 2019 were at the stage of blastula (Supplementary Figure S1). They were observed for 27 days and during that period they developed slowly up to the start of the larval shell formation. All egg collars of E. pallida that were collected from Youzhnaya subtidal on 28 May 2018 contained only abortive embryos. The third set of E. pallida egg collars that was collected on 6 August 2019 from Kluschikha subtidal contained embryos at the stage of gastrula. Unfortunately we were unable to obtain exact dates when hatching began, but on 24 October 2019 many juveniles were crawling in containers where egg collars were kept. Hatching did not start until 8 October 2019. The hatching success of E. pallida was 0.3 ± 0.02 (N = 3 measurements, mean ± SE). The height of the single measured newly hatched E. pallida was 760 μm (Figure 2).
Notes on early feeding of Amauropsis islandica
Observations on feeding of A. islandica hatchlings were started immediately after the beginning of hatching in June and continued for about 3 months until 24 August 2018 (Supplementary Figure S1). During this period the average height of juveniles increased from 910 to 1200 μm (Figure 3). While the various food items were being offered (see Methods), we did not find any drilled or empty shells of the potential prey until 3 August, some 7 weeks after they were introduced to the first hatchlings. However, many A. islandica faeces were found in Petri dishes. On 3 August, several empty shells of A. islandica juveniles were found with no characteristic drillholes. All these observations were made in Petri dishes with sand and live Peringia ulvae specimens (Figure 3).
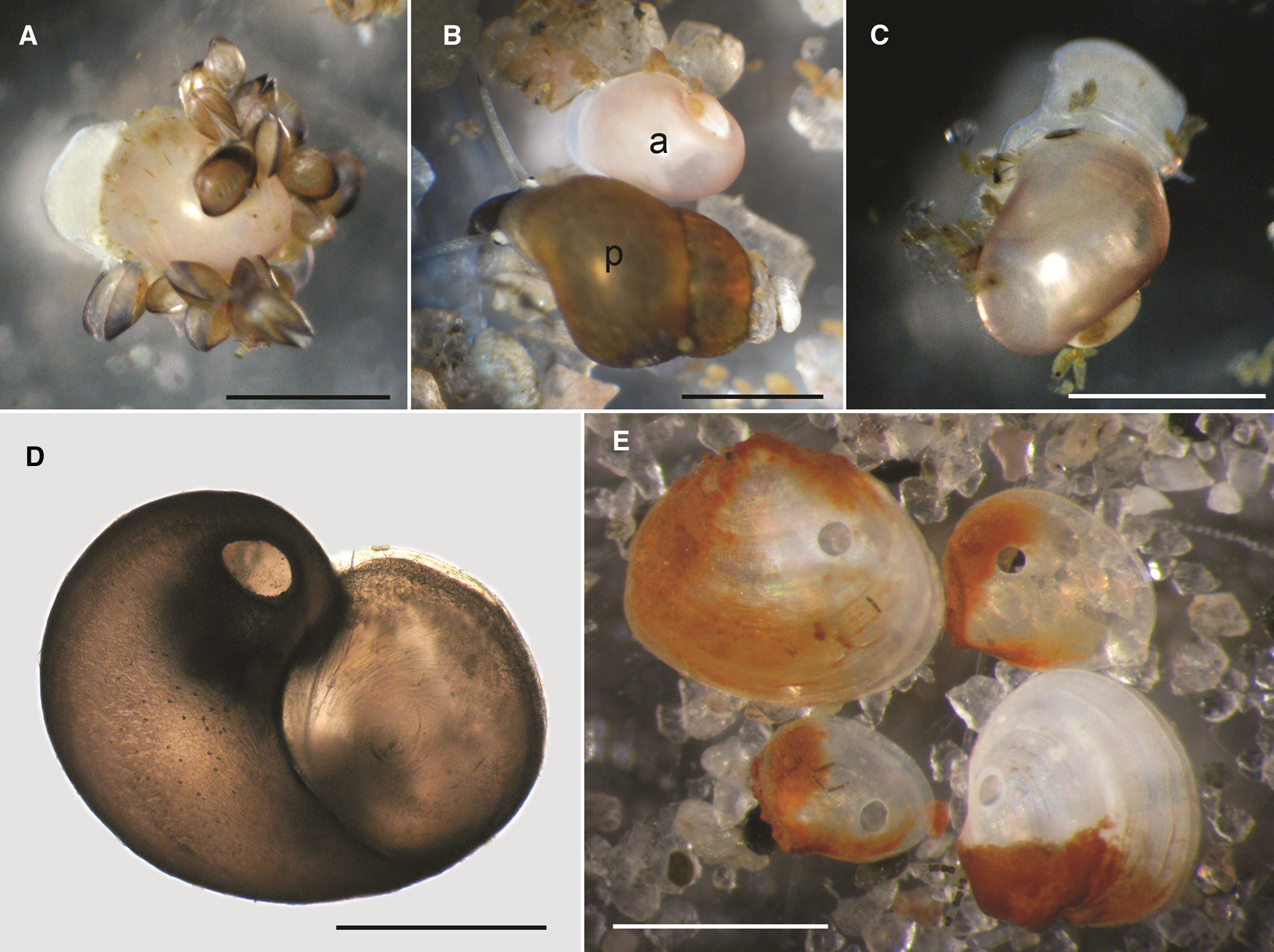
Fig. 3. Amauropsis islandica juveniles and prey items offered to them: (A) a hatchling with Mytilus edulis spat; (B) a hatchling (a) with adult Peringia ulvae (p); (C) juvenile at the size when it is able to drill a shell; (D) drilled shell of A. islandica juvenile; (E) drilled shells of Limecola balthica. Scale bars: A–C, E, 1 mm, D, 0.5 mm.
On 6 August, the first drilled A. islandica shell was found with borehole diameter of 176 μm, and over the next 5 days, several more cases of cannibalism were registered (Figure 3). After Limecola balthica clams had been added to that container on 12 August, no more drilled A. islandica shells were detected; the first drilled L. balthica shells were found on 20 August. The borehole diameter in L. balthica valves varied from 150 to 200 μm. On 24 August, about 3 months after hatching, the shell height of A. islandica juveniles was 1200 ± 30 μm, and the shell apertural height was 900 ± 10 μm (N = 6 juveniles, mean ± SE, Figure 3).
Discussion
Abundance of egg collars
Data on the abundance of naticid egg collars in the field are scarce. Despite it being mentioned (Huelsken et al., Reference Huelsken, Marek, Schreiber, Schmidt and Hollmann2008) that naticid egg masses are ‘generally very abundant’, there is virtually no quantitative information about the density and distribution of egg collars of naticid species around the world. The density of egg collars of Laguncula pulchella, a species invasive to Japanese coasts in the sandflats of the Hiroshima Bay was on average 0.35 units m−2, while at 870 km to the north-east at Matsukawaura Lagoon it was much higher, comprising 1.74 units m−2 (Yoshida et al., Reference Yoshida, Sato, Narita and Tomiyama2017). It is difficult to compare these data with our results because of different counting methods (quadrat or transect sampling, total counting). We found that A. islandica egg collars were less abundant, while E. pallida egg collar density was comparable to the literature data. According to our data egg collars of E. pallida in the subtidal were 10 times more abundant than A. islandica in the intertidal.
Surprisingly, the density of adult moonsnails showed quite the opposite tendency. First, adult E. pallida in subtidal is much less abundant (1.9 ± 1.9 specimens m−2; D. Aristov, pers. obs.) than adult A. islandica (6.7 ± 3.6 specimens m−2 in subtidal, D. Aristov, pers. obs.; 10.8 ± 0.8 specimens m−2 in intertidal, Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019). Second, the density of adult A. islandica in the Youzhnaya intertidal was higher than the abundance of other naticid species in other localities (Aristov & Granovitch, Reference Aristov and Granovitch2011). This discrepancy could be explained by different counting methods, although we suggest that the difference in abundance is greater than the possible difference in accuracy. A more reasonable explanation is the interspecific variation in egg-collar production. We cannot ignore the possibility of occasional spawning success of E. pallida in the Youzhnaya subtidal due to the rarity of findings of this species there earlier (D. Aristov, pers. obs.). Amauropsis islandica was described at the White Sea intertidal as ‘mainly a summer immigrant’ from the subtidal (Golikov & Kusakin, Reference Golikov and Kusakin1978, p. 151). Our findings contradict this opinion. While the hydrological summer at the White Sea usually begins in mid-June, the presence of late veligers in A. islandica egg collars indicates that the spawning of this species in the intertidal begins at least a fortnight earlier. We suggest that A. islandica females start to produce egg collars under the ice, so there are no restrictions for the occurrence of this species in the intertidal all year round. The size structure of A. islandica snails in the intertidal confirms this suggestion (Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019). The simple method of counting the conspicuous egg collars along a transect using GPS positioning has accuracy limitations, but we feel it can still be used for a rough estimation of egg collar abundance.
Egg collar morphology
The egg collars of A. islandica and E. pallida differed not only from each other but also from known descriptions (Figure 2; Giglioli, Reference Giglioli1955). Thorson (Reference Thorson1935) described a fragment of A. islandica egg collar from Eastern Greenland and noted that it possessed several tightly bundled egg capsules. Later he claimed that A. islandica egg collars are typical for the genus Natica (Thorson, Reference Thorson1946). Meanwhile the egg collars we found in Youzhnaya inlet had unique features that were not typical of descriptions we found for Natica genus. Specifically, the basal margins of Natica egg collars can be either smooth or plicated (Giglioli, Reference Giglioli1955), whereas the basal margins of A. islandica egg collars from the White Sea were of irregular shape due to the position of basal egg-capsules from the transversal rows. These dissimilarities in descriptions could be due to age of egg collars as it was shown that the smooth gelatinous pellicle of a collar diminished in time (Giglioli, Reference Giglioli1955). Since we found late veligers in the A. islandica egg collars in Youzhnaya inlet we assume that the time span from the spawning to the sampling date was at least 15 days, so this suggestion could be plausible. Another reason for the dissimilarity could be the differences in environmental conditions between the Øresund subtidal and the intertidal zone in Youzhnaya inlet (Thorson, Reference Thorson1946). It should be mentioned that the egg collars of E. pallida we found at both locations had plicated basal margins, while Giglioli (Reference Giglioli1955) categorized egg collars of this species to Division I, Subdivision A group which is for egg collars with thick and rigid walls and smooth basal margins (Giglioli, Reference Giglioli1955). We are quite sure of identification of egg collars since the juveniles from these collars resembled adult E. pallida (Figure 2). So this discrepancy between our observations and literature data deserves an explanation from further studies.
The walls of A. islandica egg collars were thicker and more rigid than those of E. pallida. We link this fact to differences in environmental conditions where these egg collars occur. It was earlier shown that the structure of naticid sand collars is related to the surrounding sediments (Ziegelmeier, Reference Ziegelmeier1961). In the study area, the grain size structure is different in the intertidal and subtidal, in general with a coarser sand in the intertidal (D. Aristov, pers. obs.). On the other hand, the interspecific differences of egg collar morphology may reflect adaptations to the intertidal habitat where tidal currents are quite strong. Unfortunately, not enough A. islandica egg collars were found in the subtidal or in the location with different sediment grain size distribution to prove or refute this suggestion.
Our data confirm that the density of eggs in E. pallida egg collars is nearly two times lower than that of A. islandica (Table 1; Giglioli, Reference Giglioli1955). We suggest that fecundity of A. islandica in the Kandalaksha Bay of the White Sea is higher, which could compensate for lower density of egg collars of this species in the intertidal of Youzhnaya inlet.
Hatching success
Observed hatching success was twice as high for Amauropsis islandica in Youzhnaya inlet than for Euspira pallida from Kluschikha inlet. Although our method of hatching success determination may not be precise for E. pallida, we assume that the difference between the estimates for both species is not biased due to similarity in cultivating conditions (see Methods). We presume that the morphology of A. islandica egg collars allows them to endure the harsher intertidal environment than egg collars of E. pallida, which have never been seen at the intertidal zone. The large number of eggs and high hatching success lead to a high number of offspring, increasing the odds of survival in the intertidal of subpolar seas. The number of hatchings we obtained for both of the naticid species should be treated as potential hatching success since various abiotic and biotic factors, such as water flow, changing temperature and salinity, microbial fouling, and predation may reduce the number of hatchlings per brood under natural conditions (Przeslawski, Reference Przeslawski2004). Nevertheless, the combined effect of high hatching success and high number of eggs per collar in A. islandica could not only compensate for the low abundance of egg collars of these species in the intertidal, but can also explain the high abundance of adult A. islandica snails in particular years in some localities at the White Sea (Aristov & Granovitch, Reference Aristov and Granovitch2011).
Early feeding of Amauropsis islandica hatchlings
The hatchlings of several species of naticids with direct development are able to feed in the same way as adult ones by boring bivalve shells or their own kind (Giglioli, Reference Giglioli1955; Ansell, Reference Ansell1982; Yakovlev & Kolotukhina, Reference Yakovlev and Kolotukhina1996) in cultures or could drill foraminiferans in the field (Saidova & Beklemishev, 1963). On the other hand, there is evidence that a newly settled naticid Neverita lewisii (Gould, 1847) (formerly Polinices lewisii) can feed on diatoms and other algae (Bernard, Reference Bernard1967; but see Pedersen & Page, Reference Pedersen and Page2000). In our study, the newly hatched A. islandica juveniles did not drill the prey when it was offered to them. But the observed growth of juveniles (Figure 3) and egestion of faeces suggested that they were feeding in that period. Although it is possible that the juveniles used the remaining yolk, it is unlikely to be the only source of energy, given that they grew by a third, and crawled in Petri dishes for 7 weeks (counting from the start of hatching). We can only speculate that A. islandica juveniles could rely on external food sources (dissolved organic matter, detritus or periphyton) without drilling, similar to N. lewisii, as shown by Bernard (Reference Bernard1967). Recently it was discovered that the adult naticid Neverita duplicata is omnivorous based on CN-analysis (Casey et al., Reference Casey, Fall and Dietl2016). Similarly, our results suggest that other species of naticids could rely not only on carnivory during the first month after hatching.
Within 25–53 days after hatching, A. islandica seemed to have switched to the juvenile mode of feeding, and they began to prey on conspecifics as well as Limecola balthica clams provided later on. The literature data suggest that naticid juveniles consume their siblings when other prey items are absent or scarce (Giglioli, Reference Giglioli1955; Ansell, Reference Ansell1982); this explains the observed cases of cannibalism in A. islandica. Meanwhile, Mytilus edulis mussels have never been drilled by A. islandica juveniles whenever they have been offered to them. These results are consistent with our previous observations on the feeding preferences of the adult A. islandica snails, where the drilled shells of M. edulis occur much more rarely than those of L. balthica and Mya arenaria L., 1758 in the sediments (Aristov & Granovitch, Reference Aristov and Granovitch2011). We showed that small A. islandica snails can feed on the mudsnail Peringia ulvae in the experimental cages and in the intertidal, when L. balthica abundance is low (Aristov et al., Reference Aristov, Varfolomeeva and Puzachenko2015). In this study we did not find any P. ulvae drilled by A. islandica juveniles probably because juvenile predators were too small to wrest down a gastropod of equal or larger size (Figure 3). The largest borehole (which is correlated to the naticid size (Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019)) we found on L. balthica clams was 200 μm, whereas the smallest drillings on P. ulvae were 300 μm in diameter (D. Aristov, pers. obs.). We suggest that A. islandica can prey on P. ulvae of up to 5 mm height, as happened in the caging experiment (Aristov et al., Reference Aristov, Varfolomeeva and Puzachenko2015).
Conclusion
Our findings fill the gap in the studies of reproductive biology of naticids at the high-latitude White Sea. The abundance of naticid egg collars in the North Atlantic is provided for the first time with a new simple method of counting offered. We added some details to Thorson's (Reference Thorson1935) description of the morphology of Amauropsis islandica egg collars and called into question that the egg collars of Euspira pallida always have a smooth basal margin (Giglioli, Reference Giglioli1955). We suggest that in the White Sea the high density of eggs in the egg collar and high hatching success allow A. islandica to colonize the tidal flats protected by boulders from ice ploughing. In such habitats the abundance of A. islandica can be high, so this predator could affect the population structure of its prey, the Limecola balthica clams (Aristov & Varfolomeeva, Reference Aristov and Varfolomeeva2019). Although uncovering specific mechanisms of early juvenile feeding in these naticids will require suitably designed experiments, the evidence of non-drilling feeding of naticid juveniles that we provided could enrich our knowledge of feeding mode switching during ontogeny as has been shown for other gastropod species (Montiel et al., Reference Montiel, Chaparro and Segura2005).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315420001083.
Acknowledgements
We give thanks to the Kandalaksha State Nature Reserve authorities for granting the access to the protected area during sampling and counting egg collars. We thank Prof. Kirill Galaktionov, Dr Vadim Khaitov, Dr Igor Bakhmet and Dmitriy Kurtov for helping in fieldwork, Dr Peter Lezin for helping with cultivation of egg collars, and Dr Nikolay Usov for providing seawater temperature and salinity data. We appreciate the suggestions made by three anonymous reviewers, which helped us to improve the manuscript.
Financial support
The project was partly supported by the Russian Fund of Basic Research grant 18-34-00405, and by the ongoing Program of the Russian Academy of Sciences ‘Dynamics of the structure and functioning of the ecosystems of the White Sea and adjacent Arctic seas’ (AAAA-A19-119022690122-5).






