Introduction
The beech leaf mining weevil, Orchestes fagi (Linnaeus) (Coleoptera: Curculionidae), is a common pest of European beech, Fagus sylvatica Linnaeus (Fagaceae), trees in Europe (Mangels et al. Reference Mangels, Blüthgen, Frank, Grassein, Hilpert and Mody2015). Adult O. fagi feed on developing leaves at the time of budburst, causing shot holes as the leaves mature; larvae mine the leaves from the mid-rib outwards, forming blotch mines at the leaf margin. Infested leaves are often stunted with necrotic tips that appear scorched (Bale Reference Bale1984; Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012). The majority of leaf damage occurs in spring during leaf development (Bale Reference Bale1984). Although O. fagi is considered the most abundant herbivore on F. sylvatica in Europe (Bignucolo and Korner Reference Bignucolo and Korner2010) and occasional outbreaks may reduce tree growth and beechnut production, tree mortality is not reported (Verkaik et al. Reference Verkaik, Moraal and Nabuurs2009).
Orchestes fagi was discovered infesting American beech, Fagus grandifolia Ehrhart (Fagaceae), in Nova Scotia, Canada, in 2012, but was likely established at least five years prior to its discovery based on anecdotal reports of beech defoliation (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012). Infested American beech trees often respond to leaf damage by producing a second flush of leaves (J.T.L.G., personal observation). American beech in the Halifax, Nova Scotia area have sustained heavy O. fagi damage for six or more consecutive years and many mature trees have died in both natural stands and residential areas (Sweeney and Johns Reference Sweeney and Johns2016; J.T.L.G., personal observation).
The risk that O. fagi may be spread long distances by human movement of logs and firewood is high because adult O. fagi overwinter in large numbers on the trunks of trees, including beech, spruce (Picea Dietrich; Pinaceae), and maple (Acer Linnaeus; Sapindaceae) (Morrison et al. Reference Morrison, Sweeney, Hughes and Johns2017).
There are already several populations of O. fagi now established in areas of Nova Scotia hundreds of kilometres from Halifax, such as Cape Breton (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012). The relatively rapid rate of spread of O. fagi combined with significant tree mortality in infested stands indicates that O. fagi is a substantial threat to American beech, which is already in decline throughout its range in eastern North America due to beech bark disease, Nectria coccinea var. faginata Lohman, Watson, and Ayers (Nectriaceae) (Houston and O’Brien Reference Houston and O’Brien1983; Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012). We examined trap colour, trap height, and trap type preferences of O. fagi with the goal of developing an effective method of monitoring the spread of O. fagi in North America.
Historically, when developing trapping methods for phytophagous insects, visual cues have often been considered less important than olfactory cues for insect attraction (Reeves Reference Reeves2011), likely due to species specificity of olfactory cues. However, vision often plays a large role in host location by phytophagous insects (Brattli et al. Reference Brattli, Anderson and Nilssen1998). Preliminary laboratory bioassays employing a colour choice tube found that O. fagi was attracted to the blue, green, red, and white colours (Pawlowski Reference Pawlowski2017). To further explore trap colour preference in O. fagi, we compared six different colours of traps to determine which colours were most attractive.
Insects are often more sensitive to the shape of a visual stimulus than its colour (Reeves and Lorch Reference Reeves and Lorch2009; Machial et al. Reference Machial, Lindgren and Aukema2012). For example, in the Warren root collar weevil, Hylobius warreni Wood (Coleoptera: Curculionidae), host attraction was affected by the shape of the stimulus but not its colour (Machial et al. Reference Machial, Lindgren and Aukema2012). Previous trapping trials for O. fagi employed yellow sticky card traps baited with semiochemicals and deployed in the low canopy of beech trees (Pawlowski Reference Pawlowski2014). In this study, we compare three trap types to assess the efficacy of each trap type on attraction, as measured by trap catch: (1) nonsticky panel traps, (2) mini-prism sticky traps, and (3) traditional yellow sticky card traps. We predicted that mini-prism traps would outperform sticky card traps and panel traps would outperform mini-prism traps in mean trap capture due to greater trap surface area.
Orchestes fagi females tend to oviposit in the lower canopy and feed in the upper canopy (Phillipson and Thompson Reference Phillipson and Thompson1983). Therefore, we predicted that traps in the upper canopy would catch more O. fagi than traps in the lower canopy.
Grimm (Reference Grimm1990) showed that O. fagi were attracted to bursting beech buds. This behaviour is adaptive as survival of first instars decreases as beech leaves mature and sclerotise (Bale Reference Bale1984). Silk et al. (Reference Silk, Mayo, LeClair, Brophy, Pawlowski and MacKay2017) found that 9-geranyl-p-cymene, a compound emitted in increased quantities from beech leaves at the time of budburst, increased catch on sticky card traps compared to unbaited controls and caused upwind movement in olfactometer studies. In the present study, we predicted that mini-prism traps baited with 9-geranyl-p-cymene lures would catch more O. fagi than unbaited controls.
Methods
Trap design
Mini triangular prism traps (hereafter referred to as prism traps) were constructed using 20-cm-wide and 40-cm-long sections cut from 122 cm × 244 cm sheets of corrugated polypropylene plastic. Except for light green (ultraviolet green-Pantone 360C, Laird Plastics, Moncton, New Brunswick, Canada), all colours of corrugated plastic (red-Pantone 186C, yellow-Pantone 123C, dark blue-Pantone 289C, light blue-Pantone 285C, and white-Pantone NA) were sourced from Sabic Polymershapes (Dartmouth, Nova Scotia, Canada), a distributer of Coroplast (Granby, Québec, Canada). Strips were bent to form hollow triangular prisms with sides measuring 12.5 cm wide and 20 cm tall (Fig. 1A). Prisms were wrapped with Alpha Scents adhesive roll tape (Alpha Scents Incorporated, Portland, Oregon, United States of America) to immobilise insects. The advantages of the adhesive roll tape over tangle-trap are: (1) it made for efficient trap maintenance and reuse of the corrugated plastic prism traps (by simply replacing a bug-covered sticky strip with a fresh one, as described below); and (2) unlike tangle-trap, the sticky material on the adhesive roll does not get stuck on one’s hands and fingers.
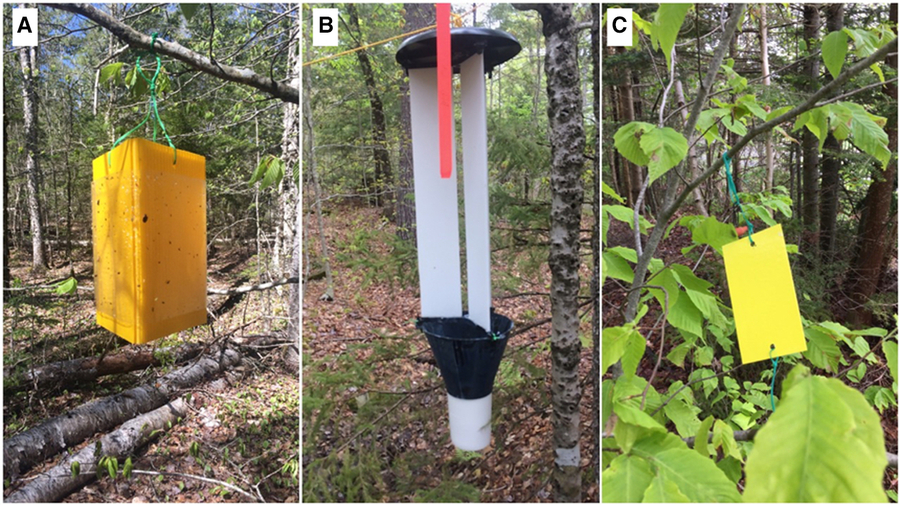
Fig. 1. Different traps used. A, Yellow triangular prism trap, June 2017, Oakfield Provincial Park, Oakfield, Nova Scotia; B, white panel trap, June 2017, Oakfield Provincial Park, Oakfield, Nova Scotia; C, yellow sticky card, June 2018, Wolfville, Nova Scotia. Photographs by J. Goodwin.
Synergy Semiochemicals Multitrap Panel traps (Synergy Semiochemicals Corporation, Delta, British Columbia, Canada) (hereafter referred to as panel traps) had three corrugated plastic panels (each 11 cm × 61 cm) overtop of a funnel (22.5 cm top diameter 11.5 cm bottom diameter) and collecting cup (11.5 cm diameter). These traps allowed us to choose any available colour of corrugated plastic for the panels but we used a black trap top and funnel and a white collecting cup for all panel traps. The panels, and inside and outside surfaces of the funnels were coated in Fluon to reduce friction and increase catch (Graham et al. Reference Graham, Poland, McCullough and Millar2012; Allison and Redak Reference Allison and Redak2017). Collecting cups contained a saturated saltwater solution (Fig. 1B).
Yellow sticky cards (number 611; bright yellow; Contech Incorporated, Delta, British Columbia, Canada) (hereafter referred to as card traps) were 13 cm × 7 cm and were pretreated with Tanglefoot Tangle-Trap sticky coating on both sides (The Scotts Company, Marysville, Ohio, United States of America) (Fig. 1C). Holes were punched in the top and bottom centre of each card to allow for hanging.
Trap deployment and replacement
Unless stated otherwise, traps were deployed at shoulder height (approximately 1.5 m) from branches of American beech in a randomised block design with at least 5 m between traps and blocks. Traps were checked weekly in 2017 and biweekly in 2018. Prism traps and card traps were replaced when the sticky surface was compromised with bycatch or if the trap had caught at least one O. fagi, as follows: a new trap was put in place of the used trap and the wax paper strip was removed to expose the sticky surface; the wax paper strip was then used to cover the sticky surface of the used trap to transport back to the laboratory. Used traps were transported back to the laboratory. Captured O. fagi were removed from the sticky traps in the laboratory using Histo-Clear (HistoClear II; National Diagnostics, Atlanta, Georgia, United States of America). Any O. fagi caught in panel traps were removed using a strainer, and the saltwater in each trap cup was replenished weekly. From 2017 trap catch, the total number of males and females was recorded (beetles were sexed according to Pawlowski Reference Pawlowski2017), while in 2018 a subsample of six O. fagi from each trap catch was sexed. Beetles were identified according to Sweeney et al. (Reference Sweeney, Anderson, Webster and Neville2012). Voucher specimens have been deposited in the insect collection at the Atlantic Forestry Centre, Fredericton, New Brunswick, Canada.
Trap colour preference experiment
Trap colour preference was explored using prism traps of six different colours (yellow, green, white, dark blue, light blue, and red) (for colour spectra see Fig. 7) (Fig. 2) with eight replicates per treatment in Oakfield Provincial Park, Oakfield, Nova Scotia, Canada (44.9174°N, 63.5862°W), from 1 May through 28 July 2017.

Fig. 2. Various colours of triangular prism trap used in the study. Left to right: light blue, red, green, yellow, white, and dark blue. Acadia University, Wolfville, Nova Scotia. Photograph by J. Goodwin.
Prism trap versus panel trap experiment
Trap type preference was explored in a 2 × 3 factorial experiment with two types of traps (panel traps and prism traps) of three different colours (green, white, and light blue), and eight replicates per treatment in Oakfield Provincial Park, from 18 May through 28 July 2017.
Trap height preference experiment
Trap height preference was explored in a 2 × 2 factorial experiment with two colours of prism traps (green and light blue) placed either low (1.5 m) or high (≥ 3 m) in the canopy of trees in a beech stand at the K.C. Irving Centre gardens and Acadia University Woodland Trails in Wolfville, Nova Scotia (45.087489°N, 64.368563°W) from 31 May through 27 July 2017. We chose green and light blue traps for this experiment based on Pawlowski (Reference Pawlowski2017) who found green and blue attractive to O. fagi. For each colour, a low and high trap were placed in each of five trees, that is, five trees with green traps and five trees with blue traps.
Trap colour and host volatile interaction experiment
In 2018, we tested the three most attractive colours (as determined in 2017: yellow, green, and white) with and without 9-geranyl-p-cymene in a 3 × 2 factorial experiment replicated eight times in Oakfield Provincial Park from 7 May to 2 August 2018. 9-geranyl-p-cymene was synthesised at the Canadian Forest service using the protocol developed by Silk et al. (Reference Silk, Mayo, LeClair, Brophy, Pawlowski and MacKay2017) and loaded on rubber septa (Septa – Raw Red; Scotts Canada, Delta, British Columbia, Canada) at 2 mg per lure, with a release rate of approximately 20 µg/day at 20 °C.
Prism trap versus sticky card experiment
In this experiment, we tested the most attractive colour (yellow) and most effective trap type (prism) from 2017 against commercially available yellow sticky cards to determine their efficacy at detecting O. fagi across a range of population densities. Twenty pairs of traps were deployed from 10 May to 2 August 2018. All traps were baited with 2 mg of 9-geranyl-p-cymene on rubber septa. Pairs of traps were deployed in three locations: Uniacke Estate Museum Park, Mount Uniacke, Nova Scotia (10 pairs) (44.896358°N, 63.834614°W); a residential property in New Ross, Nova Scotia (five pairs) (44.736965°N, 64.456596°W); and at the K.C. Irving Centre gardens and Acadia University Woodland Trails (five pairs). We did not measure the reflectance spectrum from the yellow sticky cards but based on published spectra they likely had a sigmoidal reflectance curve asymptotic at about 570 nm typical for bright yellow (Patt and Sétamou Reference Patt and Sétamou2010; Silva et al. Reference Silva, Salamanca, Kyryczenko-Roth, Alborn and Rodriguez-Saona2018).
Statistical analyses
All statistical analyses were completed using R (RStudio Team 2016), with total catch per trap over the entire trapping period as the response variable. If a trap was downed during a week of the study, we removed data from all traps from that block and week from analysis. Relationships among variables were assessed with generalised linear mixed models (GLMM; lme4 package) using the appropriate distribution. Negative binomial generalised linear mixed models were used to assess data from the trap colour preference and prism trap versus sticky card experiments, Gaussian generalised linear mixed models were used to assess data from the trap colour and host volatile interaction experiment, and the log(catch + 1)-transformed data from prism trap versus intercept trap experiment. Model fit was assessed using residual plots, Akaike information criterion values, and Hosmer–Lemeshow goodness-of-fit tests (ResourceSelection package) (Archer et al. Reference Archer, Lemeshow and Hosmer2007). Tukey’s post hoc tests (emmeans package) were used to determine whether differences in mean catch were significant among levels of each fixed effect (i.e., among different colours or trap types) (Ward et al. Reference Ward, Venette and Aukema2019).
To analyse data from the trap height preference experiment, we used a generalised linear model and the distribution with best fit (negative binomial) to assess differences in mean catch between trap height (colours pooled) and the Wilcoxon rank-sum test to assess differences in mean catch between colours. We used the Wilcoxon rank-sum test to assess differences in total catch for each colour. Type 1 error rate was controlled at 5% for all analyses (α = 0.05). A χ2 goodness-of-fit test was conducted to test for sex bias in overall trap catch.
Results
There was a significant sex bias in trap catch across all experiments, χ2 (1, n = 10886, P< 0.001), with females representing approximately 60–65% of O. fagi caught overall.
Trap colour preference experiment
We caught a total of 4893 O. fagi over the 13-week trapping period. Colour had an effect on trap catch (F = 18.2; df = 5, 35; P < 0.001). Total trap catch in each treatment was significantly greater on yellow traps, followed by white and green, with lowest catches on blue and red traps (Fig. 3).

Fig. 3. Mean ± standard error of the mean (SEM) number of Orchestes fagi adults caught per prism trap of each colour throughout the entire trapping season (1 May–28 July 2017) (n = 4893). Different letters denote significant differences between means (Tukey’s test, P < 0.05).
Prism trap versus intercept trap experiment
We caught 3206 O. fagi over the 10-week trapping period. Prism traps caught significantly more O. fagi than panel traps (F = 17.2; df = 1, 35; P < 0.001), but neither trap colour (F = 1.15; df = 2, 35; P = 0.328) nor the interaction between trap colour and trap type (F = 2.35; df = 2, 35; P = 0.110) was significant (Fig. 4).
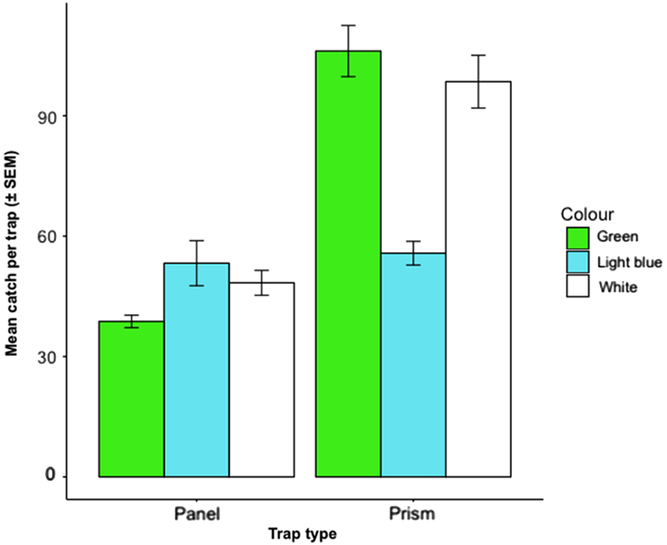
Fig. 4. Mean ± standard error of the mean (SEM) number of Orchestes fagi adults captured per trap of each colour and type throughout the entire trapping season (18 May–28 July 2017) (n = 3206). Prism traps caught more O. fagi than panel traps (F = 17.2; df = 1, 35; P < 0.001). No effect of colour or interaction between colour and trap type was observed.
Trap height preference experiment
We caught 1265 O. fagi over the nine-week trapping period (31 May–27 July 2017). Mean catch was greater in high traps than low traps (trap colours pooled) (F = 4.21; df = 1, 18; P = 0.055) but did not differ significantly between green and blue traps (heights pooled) (W(4) = 9, P = 0.548) (Fig. 5).
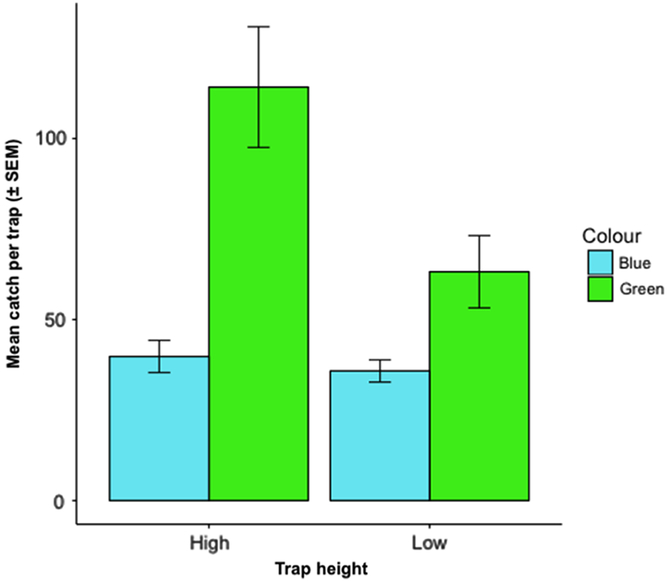
Fig. 5. Mean ± standard error of the mean (SEM) number of Orchestes fagi adults captured per prism trap of each colour and height throughout the entire trapping season (31 May–27 July 2017) (n = 1265). Mean catch was greater in high traps than low traps (heights pooled) (F = 4.21; df = 1, 18; P = 0.055) but did not differ between green and blue traps (heights pooled) (W(4) = 9, P = 0.548).
Trap colour and host volatile interaction experiment
We caught 3566 O. fagi through the course of this 12-week trapping experiment. Trap catch did not differ significantly with colour (F = 1.06; df = 2, 35; P = 0.356), lure (F = 0.428; df = 1, 35; P = 0.517), nor interaction between the two (F = 0.281; df = 2, 35; P = 0.757) (Fig. 6).
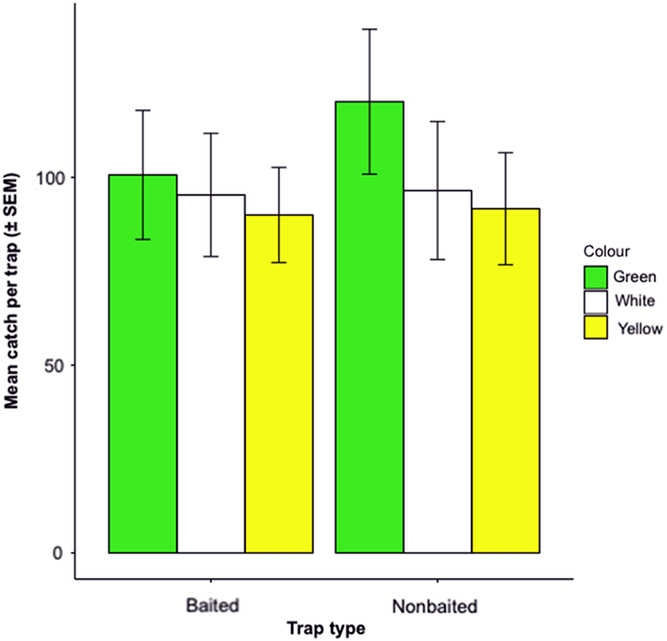
Fig. 6. Mean ± standard error of the mean (SEM) number of Orchestes fagi adults captured per green, yellow, or white sticky prism trap of each colour that were baited with a 2-mg 9-geranyl-p-cymene lure or unbaited (7 May–2 August 2018) (n = 3566). No significant differences were found between treatment groups.
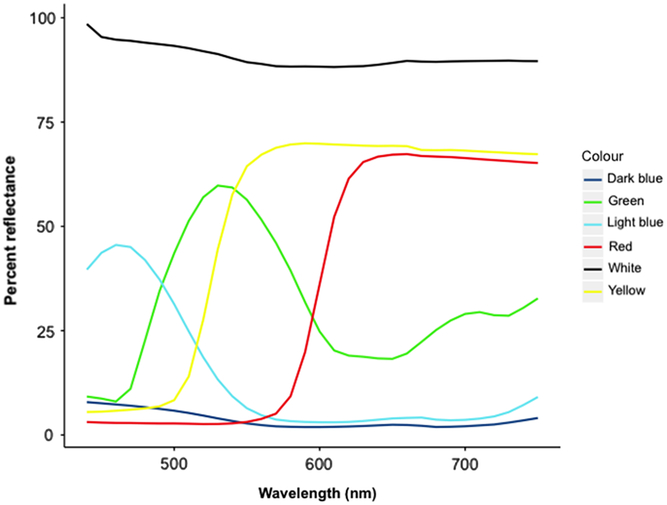
Fig. 7. Reflectance values from spectral analysis of coloured corrugated plastic used in trapping experiments. Measured using HunterLab UltraScan XE Reflected Color Spectrophotometer at Acadia University (Artisan Technology Group, Champaign, Illinois, Unites States of America).
Prism trap versus sticky card experiment
We caught 1377 O. fagi during this 12-week experiment. Mean catch (± standard error) on prism traps (56.1 ± 7.3) was significantly greater than mean catch on sticky cards (12.8 ± 2.3) (F = 92.6; df = 1, 38; P < 0.0001). However, after controlling for area of sticky surface (i.e., mean catch per square centimetre), there was no difference in trap catch between trap types (F = 0.15; df = 1, 38; P = 0.70).
Discussion
We found that yellow, green, or white traps captured more O. fagi adults than did red and blue traps. Our results differ somewhat from those from laboratory bioassays by Pawlowski (Reference Pawlowski2017) in which O. fagi was attracted to blue as well as green and white. However, it is difficult to directly compare our results with those of Pawlowski (Reference Pawlowski2017) due to differing conditions between laboratory and field bioassays. The laboratory bioassays used Creatology colour foam strips (Michaels, Irving, Texas, United States of America) placed directly adjacent to one another inside a 40-cm diameter cylinder with an overhead light-emitting diode full spectrum light source (Pawlowski Reference Pawlowski2017), whereas our corrugated plastic traps were spaced at least 5 m apart in natural sunlight. In terms of potential efficacy as survey tools, we have more confidence in results from field bioassays.
The colour yellow is considered to be universally attractive to all foliage-seeking insects (Prokopy and Owens Reference Prokopy and Owens1983). The wavelength of yellow ranges from 570–585 nm, while green ranges from 490–570 nm (Reusch Reference Reusch2013). The corrugated plastic we used had peak wavelengths of 540 and 590 nm for green and yellow, respectively (Fig. 7). Orchestes fagi may be attracted to colours in the 540–590 nm range (green–yellow) since they resemble the colour of healthy foliage. The peak reflectance frequency of white corrugated plastic was 440 nm, in the violet range of the visual spectrum (Reusch Reference Reusch2013), but also had high reflectance values in the green–yellow range of the spectrum (Fig. 7). Hausmann et al. (Reference Hausmann, Samietz and Dorn2004) suggested a trichromatic visual system in Anthonomus pomorum (Linnaeus) (Coleoptera: Curculionidae), with sensitivities to ultraviolet (350 nm), blue (455 nm), and green (550 nm) light. The most preferred colours by O. fagi, yellow, green, and white, closely relate to the area of the visible spectrum, which was attractive to A. pomorum (Reusch Reference Reusch2013), perhaps because both species are phytophagous and rely on similar host location mechanisms. The emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is sensitive to light in the ultraviolet (420–430 nm), violet (460 nm), and green (530–560 nm) range of the electromagnetic spectrum (Crook et al. Reference Crook, Francese, Zylstra, Fraser, Sawyer and Bartels2009). Tests of physiological sensitivity would need to be conducted to determine the retinal response of O. fagi to different wavelengths of light (i.e., electroretinography). Future studies should test the attraction of O. fagi to traps with peak reflectance wavelengths in the violet portion of the spectrum.
Our prediction that mean trap catch would be directly related to trap surface area was not supported. Prism traps significantly outperformed panel traps and card traps despite having a surface area that was intermediate between the other trap types. The success of this trap type over others could be attributed to its shape. Trap type or shape can affect the types of insects and numbers caught (Chénier and Philogène Reference Chénier and Philogène1989; Sweeney et al. Reference Sweeney, Gutowski, Price and De Groot2006; Dodds et al. Reference Dodds, Dubois and Hoebeke2010; Graham et al. Reference Graham, Poland, McCullough and Millar2012). However, catch was positively linked to surface area in our prism trap versus card trap experiment. Prism traps have approximately three times the surface area of card traps and when mean catch per surface area was compared between these two trap types, much of the difference in catch was accounted for by surface area. Therefore, the difference in trap catch between prism traps and panel traps must be due to a factor other than surface area. One explanation for the decreased effectiveness of panel traps compared with prism traps is the mechanism of action of the trap. When comparing sticky stovepipe traps with intercept traps, Chénier and Philogène (Reference Chénier and Philogène1989) describe larger trap catch of forest Coleoptera using sticky traps. A sticky trap surface may retain a greater proportion of attracted beetles than does a panel trap. However, we replaced traps every one or two weeks, reducing the chances of saturating trap surface area. Our results may have been affected if traps were replaced less frequently, for example, under seasonal deployment conditions.
We predicted that traps placed higher in the canopy would capture more O. fagi than those placed low in the canopy due to a preference of adult O. fagi to feed in the upper canopy (Phillipson and Thompson Reference Phillipson and Thompson1983) and our results supported our prediction, though significance was marginal (P = 0.055).
Our finding that baiting traps with 9-geranyl-p-cymene lures did not affect mean trap catch conflicts with results determined by Silk et al. (Reference Silk, Mayo, LeClair, Brophy, Pawlowski and MacKay2017) even though lures were loaded with the same initial dosage of compound in both studies. Discrepancies between our findings and those by Silk et al. (Reference Silk, Mayo, LeClair, Brophy, Pawlowski and MacKay2017) may be due to different conditions in the vicinity of traps in the two studies. Trap placement can greatly affect the success of a trap (Dodds et al. Reference Dodds, Dubois and Hoebeke2010); baited traps in more open areas may attract more target insects than those placed in more wooded areas due to decreased obscuring of lure emissions. Alternatively, an interaction between host volatile sensation and trap type (shape) or trap colour could account for the discrepancy we see between these results (Campbell and Borden Reference Campbell and Borden2006). Orchestes fagi may exhibit a sensory hierarchy in which reactions to olfactory cues are decreased in the presence of certain visual stimuli (see Otálora-Luna et al. Reference Otálora-Luna, Lapointe and Dickens2013). Perhaps septa placement in our prism traps affected our results. Septa were placed inside the mini-prism traps used in our study, making them less open to air movement than the lures on yellow sticky cards used by Silk et al. (Reference Silk, Mayo, LeClair, Brophy, Pawlowski and MacKay2017), which could have decreased the release rate of the compound.
Every trap type used in our study captured large numbers of adult O. fagi in areas where O. fagi populations were previously recorded. Future studies should examine how beetle density affects trap performance. Additionally, practicality of each trap design is also an important point to consider. Panel traps are large and heavy relative to sticky traps; in areas with small trees, these traps were strung between two trees using a piece of rope. The salt water in the collection cup is prone to evaporation, so lengthy unaided deployments of this trap design are not feasible. However, the latter problem could be reduced using a different preservative such as recreational vehicle antifreeze (50% propylene glycol in water) or dry cup traps with insecticide strips (Miller and Duerr Reference Miller and Duerr2008). Additionally, about 10% of clips provided by the manufacturer to hold the pieces of the trap together broke after a few weeks of use, requiring twist tie wraps to tie the trap together. On the contrary, card traps are small, lightweight, portable, and disposable. They are easily deployed in the field and are durable enough to last an entire trapping season. The only downside to card traps is their small surface area. The surface area of this trap type limits its longevity in the field, since once the surface is obscured by catch it is no longer effective. Like card traps, prism traps are lightweight, durable, and can be deployed in trees of any size; prism traps have the added advantage of being reusable, requiring only the sticky tape to be replaced. Despite the similarities between prism traps and card traps, prism traps have a larger surface area (approximately three times larger) than card traps. This increased surface area theoretically increases the practical longevity of prism traps in the field.
This study sought to determine factors that enhanced trap performance by comparing the effects of trap colour, type, placement, and presence of a host volatile on trap catch. We were primarily interested in trap efficacy and, therefore, ignored any potential effects of pheromones, sounds, or visual cues from trapped insects on trap catches. In theory, chemical, auditory, and visual cues released by trapped O. fagi may have repelled or attracted subsequent O. fagi (Graham et al. Reference Graham, Poland, McCullough and Millar2012; Domingue et al. Reference Domingue, Imrei, Lelito, Muskovits, Janik and Csóka2013). However, when monitoring traps are deployed in the field, trapped insects would produce these cues and so controlling for them in trap efficacy experiments would not help us understand the true efficacy of a trap in field conditions.
Overall, results from our experiments suggest that yellow prism traps are the best combination of trap type and colour used. Further research is necessary to determine whether yellow prism traps would have greater catch in the upper versus lower canopy. We found no increase in trap catch by baiting traps with 9-geranyl-p-cymene under the conditions of our study, but further studies to discern additional attractive host volatiles may lead to a more effective monitoring tool for O. fagi.
Acknowledgements
We thank Cory Hughes (Canadian Forest Service) for his assistance with site location, trap design, and experiment set-up, Sara Edwards for advice and guidance on statistics and use of R, Jeremy Allison (Canadian Forest Service) for comments on an earlier version of this manuscript, Thomas Davis-Moore for his assistance with field work, Michael Robertson (Acadia University Department of Physics) for his help in measuring the reflectance spectra of corrugated plastic, and Stefan Richard (Sylvar) for providing leftover pieces of green corrugated plastic that we used to make our green mini-prism traps. We thank the Atlantic Canada Opportunities Agency Atlantic Innovation Fund (197853), Canada Foundation for Innovation (22087), Natural Sciences and Engineering Research Council of Canada (RGPIN-2017-04319), Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Canada Graduate Scholarship, and Nova Scotia Research and Innovation Graduate Scholarship for funding this research.









