Introduction
A parasite's fitness is often tied closely to the behaviour of its host, especially when the transition to another specific host or environment is required in its life cycle (Poulin, Reference Poulin2010). Thus, natural selection has driven many parasites to control their host's behaviour to varying extents. For example, parasitoid wasps can remove their insect host's ‘motivation to move’, allowing the wasps to lead hosts to locations optimal for completing their own life cycle (Libersat et al., Reference Libersat, Delago and Gal2009). Toxoplasma gondii can lure its host (rat) into close proximity of its feline predators (the parasite's next host) via increasing aggression, risk-taking and altering attraction to cat urine (Webster, Reference Webster2007; Kaushik et al., Reference Kaushik, Knowles and Webster2014). The extent of control over host behaviour can appear so absolute that in some systems, parasitologists consider the hosts as extended phenotypes of the parasite genome (Adamo, Reference Adamo2013; Hughes, Reference Hughes2013; Poulin and Maure, Reference Poulin and Maure2015).
One of the most profound demonstrations of behavioural alteration comes from hairworm and mermithid endoparasites (from the phyla Nematomorpha and Nematoda, respectively). Both taxa have convergently evolved similar life cycles, in which juvenile worms develop as endoparasites of terrestrial arthropods, before emerging from their host as free-living adult worms. In both hairworms and mermithids, adult worms are usually aquatic, i.e. they are prone to rapid desiccation and must lay their eggs in water or a water-saturated substrate. Therefore, inducing the host to allow the parasite to emerge in water would be highly adaptive to the parasite, and there is evidence that this occurs in both hairworm- and mermithid-infected hosts (Christie, Reference Christie1937; Baylis, Reference Baylis1947; Nickle, Reference Nickle1972; Capinera, Reference Capinera1987; Schmidt-Rhaesa, Reference Schmidt-Rhaesa2001; Thomas et al., Reference Thomas, Schmidt-Rhaesa, Martin, Manu, Durand and Renaud2002, Poinar et al., Reference Poinar, Latham and Poulin2002, Sanchez et al., Reference Sanchez, Ponton, Schmidt-Rhaesa, Hughes, Misse and Thomas2008). The onset of what appears to be hydrophilia, or some other behavioural alteration causing a host to visit water, is well-documented in hairworm-infected hosts (Schmidt-Rhaesa, Reference Schmidt-Rhaesa2001; Thomas et al., Reference Thomas, Schmidt-Rhaesa, Martin, Manu, Durand and Renaud2002; Biron et al., Reference Biron, Marché, Ponton, Loxdale, Galéotti, Renault and Thomas2005; Biron et al., Reference Biron, Ponton, Marché, Galeotti, Renault, Demey-Thomas and Thomas2006, Poinar et al., Reference Poinar, Latham and Poulin2008, Sanchez et al., Reference Sanchez, Ponton, Schmidt-Rhaesa, Hughes, Misse and Thomas2008). However, in mermithid-host systems, very few examples of this type of manipulation have been reported relative to the diverse range of arthropods they infect (Maeyama et al., Reference Maeyama, Terayama and Matsumoto1994; Vance, Reference Vance1996; Poulin and Latham, Reference Poulin and Latham2002). Given that mermithid parasites lack an obligate intermediate aquatic host (unlike hairworms), their egression habitat is not restricted to bodies of water. For example, mermithid-infected sandhoppers burrow more deeply into the sand to reach higher moisture contents for the worm (Poulin and Latham, Reference Poulin and Latham2002). Yet, in infected ants and mayflies, mermithids drive them into water (Maeyama et al., Reference Maeyama, Terayama and Matsumoto1994; Vance, Reference Vance1996). This may suggest that a non-specific attraction to water is being induced in infected hosts, allowing mermithids to emerge in either water or water-saturated microhabitats.
The mermithid Mermis nigrescens (Nematoda: Mermithidae) infecting the European earwig Forficula auricularia (Dermaptera: Forficulidae) readily egresses into water-saturated soil (Christie, Reference Christie1937; Baylis, Reference Baylis1944, Reference Baylis1947). We hypothesize that infected earwigs will display non-specific positive hydrotaxis. Therefore, we predict that if infected earwigs are presented with a strong water stimulus, i.e. an open pool of water, they will enter the water at a higher frequency than control, uninfected earwigs. Using a simple experiment in a laboratory setting, this prediction was tested to provide the first experimental evidence of earwig manipulation by mermithids, and demonstrate that the behavioural change induced in mermithid-infected hosts results in a higher probability of emergence in water for the parasite.
Furthermore, our experiment allows us to assess at what size mermithids are capable of inducing water entry. Manipulation studies often predict that only large/mature parasites, ready for transmission, induce manipulation, as the host provides valuable resources and exiting prematurely would preclude maturation of the parasite. Therefore, we further hypothesize that only large, mature mermithids will induce hydrotaxis in their hosts.
Methods
Field sampling of earwigs
Earwig sampling began on 5 February 2018 at the Botanical Gardens and Mercy Hospital gardens (Dunedin, New Zealand), from flower heads (Dahlia spp.) over a 100 m2 area at both sites. Four batches of 100 earwigs each were collected, two from each locality. Sampling was biased towards larger individuals, assuming that older earwigs are more likely to be infected. Earwigs were transferred to 20-L buckets, with a different bucket used for each batch. Each bucket was lined with soil and Dahlia heads from the sampling site to reduce the stress of transfer. In addition, more soil and Dahlia heads were taken from the gardens to generate environmentally realistic arenas in the laboratory.
Experimental conditions
The earwigs were kept in a temperature-controlled room (cycling from 15 to 12 °C, day/night) with a photoperiod of LD 16:8 in the animal control facilities at the Department of Zoology, University of Otago. In this room, four identical behavioural choice arenas were set up (Fig. 1). Each arena provided the earwigs with ample space, cover (flower heads) and a clear water stimulus in the form of an open pool. One hundred earwigs were added to each arena from the respective buckets (one batch per arena). Earwigs were left in the arenas for 48 h to acclimate and reduce transfer/handling stress. Importantly, during this time the pool area was empty and closed off in all the arenas. To ensure that the earwigs remained hydrated, the arenas were sprayed with very fine water vapour (5 mL total) at 9:00 am and 5:00 pm on 5 and 6 February 2018.
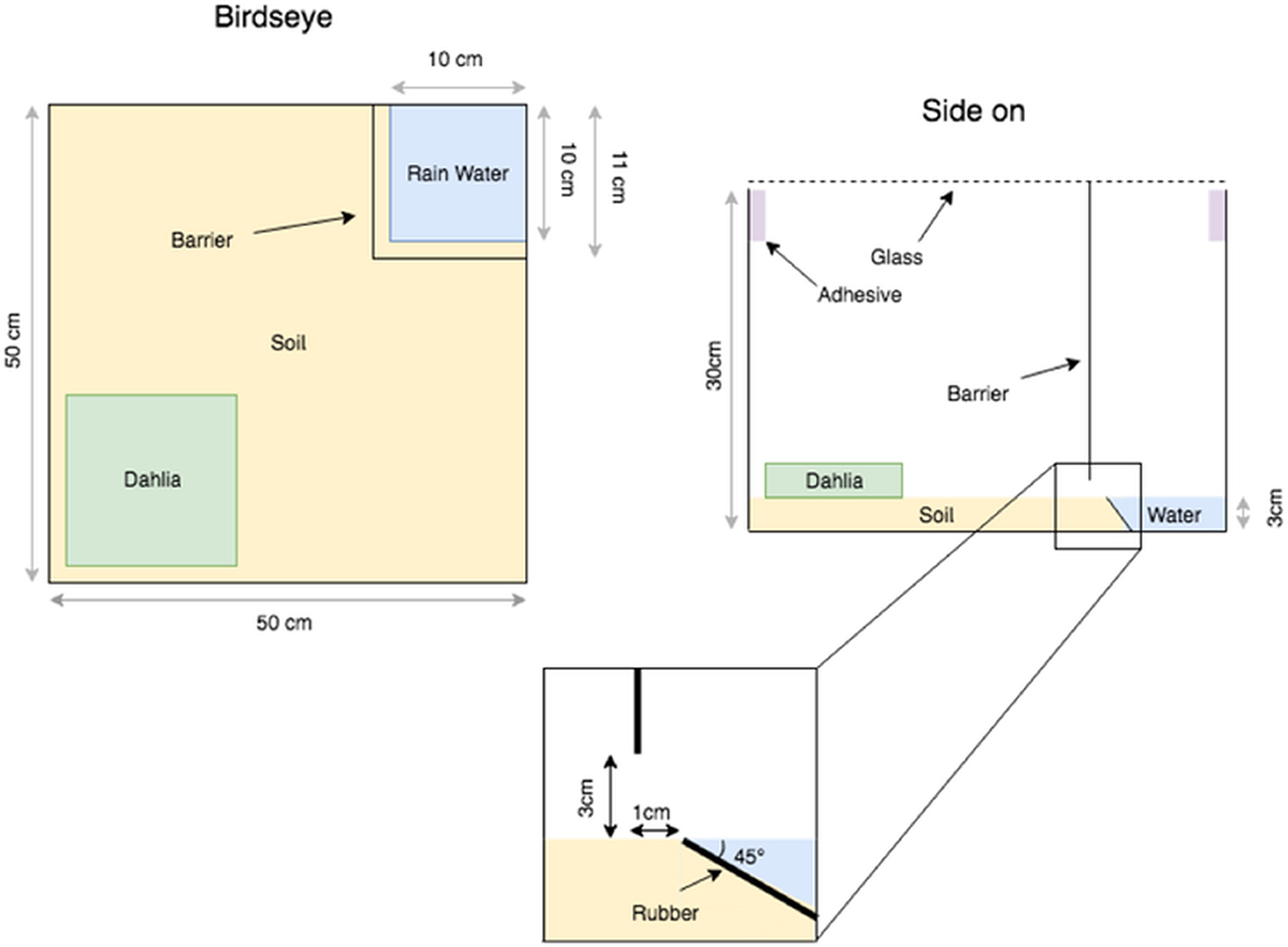
Fig. 1. Schematics of the behaviour test arena designed to present a pool of water as the only stimulus to Forficula auricularia infected with Mermis nigrescens. The foundation of the area was a repurposed 50 by 50 cm glass aquarium. The barrier (metal grating, hole <1 mm) and angled rubber ramp were designed to reduce accidental entry into the water. The barrier could be slid down to close off the pool area if needed. Two dead Dhalia spp. heads and soil from the sampling sites were added to the arenas as displayed in the figure. To prevent escape, the outer edge of the walls was lined with adhesive and the aquarium was closed off with a glass lid while observations were not being performed.
Behavioural experiment
At 8:50 am on 7 February 2018, the pools were filled with rainwater and at 9:00 am the barriers were lifted and pools were accessible to the earwigs; the arenas were thereafter observed continuously until 5:00 pm. The time at which each earwig entered the pool was recorded. Entering was defined as having the entire body in the water. If they remained in the water for over 1 min or a worm began to egress, they were removed and individually placed in a labelled 1.7-mL Eppendorf tube. At the end of the observation period, remaining earwigs were put into falcon tubes. All earwigs in Eppendorf and falcon tubes were then euthanized in a −70 °C freezer overnight. The following day they were freeze-dried, then sexed (based on the shape of their cerci), measured against a ruler for total body length, and then dissected under a microscope. When found, mermithids were photographed with a microscope-mounted camera and measured with image J (version 1.52i). This entire process from sampling to dissection was repeated again the following week (12–14 February 2018). In total, 800 earwigs were put through the behavioural trials. All earwigs were dissected, but host sex and size were only recorded for those with worms.
Statistical analyses
Infected hosts were split into three categories relative to worm length (short <10 mm, medium ⩾10 ⩽100 mm, and long >100 mm) based on past observations of egressed mermithid lengths (Christie, Reference Christie1937; Poulin and Latham, Reference Poulin and Latham2002). Data on worm length were log transformed prior to analysis to normalize their distribution. Analyses included only infected individuals. Firstly, whether or not an earwig entered water was treated as a categorical response variable, therefore a logistic regression was used to test whether this response was influenced by host size, host sex and worm length. Secondly, using only those individual hosts that ended up in the water, the time it took a host to enter the water from the start of the experiment was used as a response variable in a generalized linear model (multiple regression) testing the effects of host size, host sex and worm length. Given the close proximity in both space and time at which the eight batches of earwigs were collected, and the lack of any difference among them during preliminary data exploration, batch ID was not included in the analyses. All analyses were conducted in JMP version 11.0 (SAS Institute Inc., Cary, NC, USA).
Results
Water entry rate
Of the 800 earwigs, 62 were infected (7.8% prevalence). Seven of the infected individuals had multiple worms ranging from two to more than five per host. Fifteen of the 26 earwigs (58%) harbouring a long worm entered the pool of water and remained there for a minute before being extracted (only one had a double worm infection). This is a substantially higher frequency than the other categories (uninfected, short worm infection and medium worm infection) which averaged around 7% entering the water (Fig. 2). The water entry rate of uninfected earwigs was the lowest at 3% relative to the other categories.

Fig. 2. Percentage of Forficula auricularia (n = 800) which entered the water and stayed there (⩾1 min) relative to their infection status with Mermis nigrescens. Uninfected denotes uninfected earwigs that entered the water during the trials. Short, medium and long worm infections found in earwigs were defined by the lengths <10 mm, ⩾10 ⩽100 mm, and >100 mm, respectively. Fractions above the bars indicate the numbers of earwigs that entered the water, against the total number of individuals in that category (i.e. 23/738 = 23 uninfected earwigs entered the water out of 738 individuals).
Logistic regression confirmed worm length had a very strong influence on the chance an earwig would enter the water, while the sex of the host played a weaker role (Table 1), with males having a greater probability of entering the water. Long worms ranged from 102 to 257 mm in length, with an average of 151.2 ± 37.9 mm (mean ± s.d.), with medium worms measuring 53.4 ± 25.9 mm and short worms measuring 6.3 ± 1.7 mm.
Table 1. Results of the logistic regression analysis testing sex, body length and Mermis nigrescens length impacting the chance of Forficula auricularia entering the water

Timing of water entry
Uninfected and infected earwigs appeared to enter the water at random times (Fig. 3). However, multiple regression analysis of host sex, host body length and worm length as predictors of time to water entry revealed worm length to be a weak predictor (Table 2).

Fig. 3. The time at which Forficula auricularia entered the water in each trial (100 earwigs per trial), colour coded to their infection status by Mermis nigrescens (see legend). Only earwigs which entered the water were included in this graph. Infected host categories: short worm (n = 1) <10 mm, medium worm (n = 3) ⩾10 ⩽100 mm, long worm (n = 15) >100 mm, uninfected (n = 23). Data points were jittered to make all of them visible.
Table 2. Results of the multiple regression analysis conducted on what factors (sex, body length, Mermis nigrescens length) had the most influence on how fast Forficula auricularia entered the water

Only hosts that entered the water were included in this analysis.
Discussion
Earwigs infected by long worms were far more likely to enter the water than any other earwigs (Fig. 2) and worm length was a very strong predictor of entry into the water (Table 1). This substantiates, to a degree, the predictions regarding how hydrophilia will manifest in mermithid-infected hosts. We now have correlational evidence that suggests the terrestrial mermithid, Mermis nigrescens, can induce positive hydrotaxis, despite observations indicating it egresses into water-saturated soil, and not open water (Christie, Reference Christie1937; Baylis, Reference Baylis1944, Reference Baylis1947). This could indicate a non-specific response to water when in a hydrophilic state and/or a flexible, opportunistic strategy leading to mating aggregations in or immediately around water puddles. These results parallel the observations of Maeyama et al. (Reference Maeyama, Terayama and Matsumoto1994) in the terrestrial mermithid Mermis sp. infecting Colobopsis sp. ants, where infected ants drowned themselves in water. Furthermore, recent proteomic evidence suggests that mature Mermis nigrescens may modulate axon connections and synapses in Forficula auricularia, coupled with induction of hyperactivity, to make earwigs more likely to end up in water (Herbison et al., Reference Herbison, Kleffman, Algie, Evans and Poulin2019).
This suggested induction of hyperactivity in the proteomic study is echoed in the behavioural data presented here, as earwigs infected by long, near-adult worms were not only more likely to enter the pool of water, but they tended to do this sooner after the start of trials, than uninfected earwigs or those harbouring small worms. Relative to body length and sex, worm length was the only weak predictor of how fast the infected earwigs entered the water (Table 2).
The timing of water entry suggests a possible temporal clustering for earwigs infected by long worms (see red points in Fig. 3): individuals from the same trial appeared to enter the water at roughly the same time, a pattern not seen for uninfected earwigs or those harbouring shorter worms. However, it is too early to speculate on any synchronising mechanism, as a much larger dataset would be necessary to test the validity of this apparent clustering.
It is interesting to note that 42% of earwigs harbouring long worms did not enter the water at all. Size at maturity, and thus at emergence from the host, is highly variable in M. nigrescens, ranging from a little under 60 mm to well over 100 mm (Presswell et al., Reference Presswell, Evans, Poulin and Jorge2015). This could explain why some long worms induced no behavioural change in their host leading them to enter the water: they may simply have been immature, despite their large size. In contrast, the fact that many long worms all induced their host to enter the water within hours of a pool of water being accessible suggests that whatever their size, they were developmentally ready to emerge from the host, but just waiting for the right moment to emerge. Their host's hydrophilia may have been induced some time previously, but only became apparent once they were placed in an arena containing a strong water stimulus in the form of open water. In a parallel proteomic study on this mermithid-earwig system, only half of the earwigs infected by long worms displayed significant proteomic changes relative to control earwigs or those harbouring small worms (Herbison et al., Reference Herbison, Kleffman, Algie, Evans and Poulin2019), which echoes the behavioural data presented here.
However, in the behavioural tests, we did not offer the infected earwigs a choice between water-saturated soil (a possibly more common environmental feature for earwigs) or a pool of water, only the choice between the pool and soil of normal humidity. This simplified dichotomous choice limits our ability to decipher the infected earwig's preference for water vs a saturated substrate. The mermithids may prefer saturated soil, and entering the pool of water may have been a non-natural alternative. For earwigs, being in the water does not automatically lead to drowning, as earwigs can survive several hours when submerged under water (Crumb et al., Reference Crumb, Eide and Bonn1941). However, using a pool of water created a more clear-cut stimulus, ideal for our goal of testing for hydrophilia. Also, a mechanism that selects for a specific form of water is less parsimonious than a mechanism which creates a non-specific attraction to water (higher concentrations of water should naturally result in greater attraction). Using water vapour to keep the arenas humid did not appear to weaken the strength of the water pool stimulus, as water entry matched the predicted bias towards individuals with long worms.
It must be noted that the surface tension of the water seemed to trap some earwigs in the pool: once they entered the water, it was very difficult to escape it. This may explain why a few uninfected earwigs ended up in the pool. In Maeyama et al.'s (Reference Maeyama, Terayama and Matsumoto1994) observations, infected Colobopsis ants continually re-entered the water if removed. Future experiments should include repeatability of water entry as an additional criterion. Alternatively, a shallower water pool, allowing the earwigs to walk out, could reduce the chances of misidentifying an individual entering water due to manipulation by parasites.
A complement to this behavioural test would be to assess the infected host's response to changing humidity gradients. Humidity is measured as atmospheric moisture and is essentially a marker for static forms of water. Water naturally releases into the air, creating an immediate high level of humidity that decreases with increasing distance from the source (Webb et al., Reference Webb, Pearman and Leuning1980; Gat, Reference Gat2000). Providing behavioural evidence that infected hosts are attracted towards higher humidity would suggest a mechanism by which hosts are drawn to a pool of water. As the hydrophilia induced is thought to be non-specific to open water, humidity gradients should be the most parsimonious explanation for how the hosts are attracted to large concentrations of water, or as in the case of earwigs, how they have their preference for humid habitats boosted by the parasite.
Conclusion
This study has provided the first experimental evidence that hydrophilia, or some other behavioural mechanisms leading to entry in the water, is induced in a terrestrial host-mermithid system. The behavioural change is significantly more likely to be induced by large, mature worms, relative to small worms. On multiple levels, the behavioural data presented here aligns with proteomic data from the same host–parasite system. As the phenomenon of behavioural manipulation gathers steam in both behavioural and mechanistic fields of science, the mermithid-earwig and other recently established host-manipulative parasite systems will be fundamental to advancing neuroparasitology.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.








