INTRODUCTION
Current knowledge about the family Tritoniidae is incomplete, the result of a past where species descriptions were based primarily on the external anatomy. According to Odhner (Reference Odhner1936, Reference Odhner1963), the genera considered valid for the family Tritoniidae are: Tritonia Cuvier, 1798; Duvaucelia Risso, 1826 (later considered a synonym of Tritonia); Tritoniopsis Eliot, 1905; Tritoniella Eliot, 1907; Marionia Vayssière 1877; Marioniopsis Odhner, 1934; Paratritonia Baba, 1949; and Tochuina Odhner, Reference Odhner1963.
Members of the family Tritoniidae feed mainly on cnidarians of the subclass Octocorallia, such as gorgonians, soft corals and sea pens (McDonald & Nybakken, 1999), although researchers have recently reported tritoniids feeding on zoanthids (Bertsch et al., Reference Bertsch, Valdés and Gosliner2009). Tritoniids are often cryptic animals with rhinophores and/or gills similar to polyps of their prey. Some species even assimilate the chemical defences of their prey to use them for their own defence (Cronin et al., Reference Cronin, Hay, Fenical and Lindquist1995; Avila et al., Reference Avila, Kelman, Kashman and Benayahu1999).
Seven species of the family Tritoniidae are presently recorded for the western Atlantic: Tritonidoxa wellsi Marcus (1961); Tritonia (Candiella) bayeri Marcus (1967); Tritonia bayeri Marcus (1967); Tritoniopsis frydis Marcus (1970); Tritonia odhneri Marcus (1959); Marionia cucullata Couthouy (1852); and Marionia tedi Marcus (Reference Marcus1983). Only Tritonidoxa wellsi Marcus, Marionia cucullata Gould and Tritonia odhneri Marcus are recorded from Brazil (Marcus, Reference Marcus1983).
Of the genera validated by Odhner (Reference Odhner1936, Reference Odhner1963), only Marionia, Marioniopsis and Paratritonia bear stomach plates, making these structures an important taxonomic character for these three genera. Odhner defined the diagnosis of Marionia as having a digestive gland in two masses leaving the stomach uncovered, a jaw with 3 to 6 rows of fine denticles, and a radula possessing tricuspid central teeth and differentiated first lateral teeth.
This paper describes a new species of nudibranch belonging to the genus Marionia.
MATERIALS AND METHODS
The animals were collected by hand-picking during a spring tide at the Praia de Caponga, Cascavel (04°02′S 38°11′W), State of Ceará, and the Praia de Baixa Grande, Areia Branca (4°55′S 37°4′W), Rio Grande do Norte, 2011 (Figure 1). The samples were taken to the Laboratory of Marine Invertebrates (Universidade Federal do Ceará), where they were photographed with a Nikon 4500 digital camera coupled to a stereoscopic microscope, and measured with calipers of 0.1 mm precision. The animals were anaesthetized in a saturated solution of seawater (salinity 35) + fresh water (1:1) + magnesium chloride for 2 hours and fixed in 70% ethanol. The animal was dissected from the right side of the body. The internal organs were observed and photographed under an optical microscope, and compared with the data in the literature. The radula and jaw were extracted and treated with potassium hydroxide to remove residues of tissue. The radula was examined in a JEOL scanning electron microscope at the Laboratory of Scanning Electron Microscopy, Department of Invertebrates at the National Museum. The material was deposited in the Professor Henry Ramos Matthews Malacological Collection of the Instituto de Ciências do Mar (LABOMAR), Universidade Federal do Ceará.
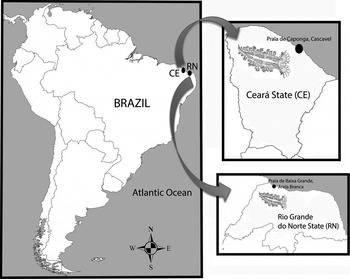
Fig. 1. Marionia limceana sp. nov., distribution map.
RESULTS
SYSTEMATICS
Infraclass OPISTHOBRANCHIA
Order NUDIBRANCHIA Cuvier, 1817
Infraorder DENDRONOTIDA Odhner, 1934
Family TRITONIIDAE Lamarck, 1804
Genus Marionia Vayssière, 1877
Marionia limceana sp. nov.
(Figures 2–8)
TYPE MATERIAL
Holotype: CMPHRM 4412. May 2011, coll. F. Vasconcelos S., 1.2 m depth, 31.0 mm long alive, dissected.
Paratypes: CMPHRM 4413, May 2011, 4 specimens, coll. F. Vasconcelos S., type locality, 1.2 m depth, dissected. CMPHRM 4414, June 2011, 5 specimens, coll. F. Vasconcelos S., Praia de Baixa Grande, Rio Grande do Norte, Brazil, 1.2 m depth, partially dissected.
TYPE LOCALITY
Praia de Caponga, Cascavel, State of Ceará, north-eastern coast of Brazil (04°02′S/38°11′W).
ETYMOLOGY
The specific epithet honours the Laboratory of Marine Invertebrates of Ceará (LIMCE).
DIAGNOSIS
Tritoniidae with branched papillae in the veil, a sturdy white body of which the notum is covered with two rows of red polygons running from the rhinophores to the tail, separate digestive glands, 18 hard stomach plates, 11–14 pairs of gills, and a jaw with 3–4 rows of denticles on the inner lips.
DESCRIPTION
External anatomy: ten animals were examined and five animals were dissected. The holotype specimen was 31 mm long when alive. The animal has a robust, whitish body (Figure 2). Small tubercles can be found throughout the notum. The notum is covered by a pattern of two rows of red polygons extending from the veil to the tail. The transparent ventral region reveals the orange digestive glands and white ovotestis. A white stripe runs from the region between the rhinophores to near the tail, in the centre of the notum, branching to involve some gills. The polygons present in this stripe are less defined. Small silver patches cover the notum in both adults and juveniles. The pigment also covers the white stripe, mainly above the pericardium, next to the centre of the body. This silver pigment is likely some effect of feeding (Figure 3).
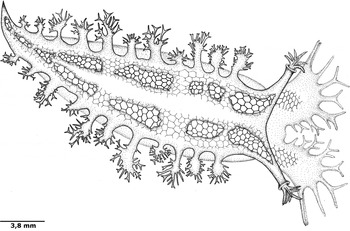
Fig. 2. Marionia limceana sp. nov., holotype CMPHRM 4412, line drawing. Mature adult.
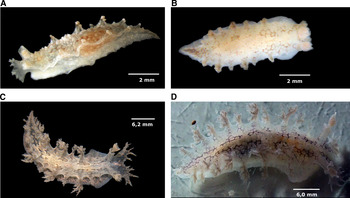
Fig. 3. Differences in pigmentation in Marionia limceana sp. nov., digital photographs: (A) juvenile specimen (CMPHRM 4413, specimen 3) with silver spots, 10 mm in length; (B) juvenile specimen (CMPHRM 4414, specimen 10) without silver spots, 10 mm in length; (C) adult specimen (CMPHRM 4412), specimen 1) with silver spots, 31 mm in length; (D) adult specimen (CMPHRM 4413, specimen 2) without silver spots, 30 mm in length.
The veil is bilobed, with eight pairs of symmetrical appendages (papillae): one pair of papillae is small and located on the inner edge of both lobes, assuming a stick form. The second, third and fourth pairs are larger than the others, and take the form of a stick with up to two main branches growing from it. The fifth and sixth pairs are formed as a staff branched at its tip. The seventh pair is similar to the first in shape and size. The eighth pair is located on the outer edge of the lobes of the veil and is in the form of a cone open laterally. The rhinophores have a typical tritoniid form, each with a bulbous clavus surrounded by seven bipinnate plume-like projections. The sheath is chalice-shaped and semitransparent, and the rhinophores are retractable. The gill plumes number up to 14 pairs, but seven of the ten specimens examined for the description had 11 pairs, according to the following standard sizes: the first pair, the closest to the rhinophores, is small with few branches. The next pair is inserted between small and large gills. This pattern changes from the 9th pair, with following pairs small and little branched. The plumes branch off into five branches—one main and two pairs—which in turn may branch into up to three branches. The anus is located below the 4th gill on the right side, while the genital opening is under the 3rd gill. The foot is straight and broad, with a rounded anterior margin. The mouth is located ventrally and anterior to the foot, between the lips.
Digestive system (Figure 4): the lips are in a prone position, connecting the tube to the mouth opening. The jaw is concave, narrow, amber-coloured, with three or four rows of denticles on its inner lips (Figure 5). The pharynx includes the radular mass, where the radula (Figure 6) is inserted, connecting the oesophagus in the dorsal region of the buccal mass. The radula of the holotype measured 2 mm, even curved. The radular formula is 26 × 26–32.1.1.1.26–32 teeth. The rachidian tooth is tricuspid, with a triangular central cusp and blind lateral cusps, as usual for this genus. The first lateral tooth is different, being short, broad and blind. The remaining lateral teeth have long sharp cusps. A pair of salivary glands lies on the outer wall of the distal end of the oesophagus, opening into the pharyngeal cavity. The oesophagus extends from the buccal mass, storing many octocoral polyps in its proximal region, close to the reproductive system, until the connection to the stomach. The octocorals are easily distinguished among the viscera by their pink spikes, as discussed later. The stomach is ‘U'-shaped, with the digestive gland opening into its middle region. A girdle of stomach plates can be seen in the proximal stomach; these are 18 detachable plates (Figure 4) of similar sizes, except for a larger and thicker pair that marks the entrance of the typhlosole. The stomach plates are conical, large and semitransparent. The typhlosole runs through the intestine proximal to the anus. The intestine arises from the proximal stomach, surrounding the dorsal region, first widening and then narrowing until it connects to the anus. The digestive gland is divided into two parts; the larger posterior part fills much of the posterior region of the animal. The anterior part of the digestive gland is much smaller, is surrounded by the intestine, and connects to the posterior digestive gland through a flat channel. It also connects to the distal part of the intestine, immediately after the girdle of stomach plates. The anterior and posterior parts of the digestive gland have an orange tint, easily identifiable from outside the animal. A pore opens below the 5th gill on the left side of the animal, and connects to the digestive gland through a transparent and delicate channel. It was not possible to determine the function of this channel, and we did not find mention of it in the literature.
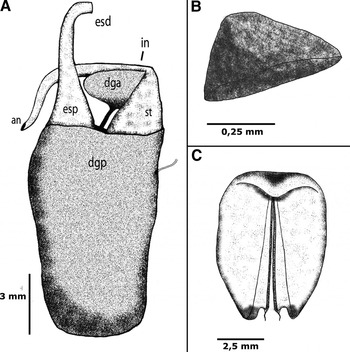
Fig. 4. Marionia limceana sp. nov. Drawing from holotype CMPHRM 4412: (A) digestive system (ventral view) (an, anus; dga, anterior digestive gland; dgp, posterior digestive gland, esd, distal oesophagus, esp, proximal oesophagus; in, intestine; st, stomach); (B) isolated stomach plate; (C) jaw, 5 mm in length.
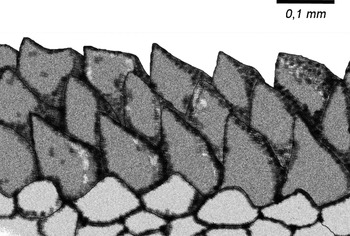
Fig. 5. Marionia limceana sp. nov. Drawing from holotype CMPHRM 4412. Denticle rows from masticatory border of the jaws.
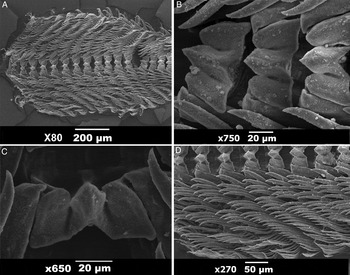
Fig. 6. Marionia limceana sp. nov. Scanning electron microscopy images of radula of holotype CMPHRM 4412: (A) overview of radula; (B) central portion of radula; (C) rachidian teeth; (D) outer laterals.
Reproductive system (Figure 7): the reproductive system is triaulic. The ovotestis covers the top of the posterior digestive gland, appearing white against the orange-coloured digestive gland. A thin transparent duct, the hermaphroditic duct, connects the ovotestis to the proximal portion of the ampulla. The ampulla is large and recurved on itself, leaving the proximal and distal portions close to each other. The distal portion connects to the female gland mass, housing the mucous and membrane gland, along with the albumen gland. A long vas deferens emerges from the female gland mass, curving and finally connecting to the penis. The penis is round and unarmed. The vagina is similar in size to the penis and is connected to the spermatheca (bursa copulatrix) through a long duct. The bursa copulatrix is large and slightly tapered. The oviduct is connected to the female gland mass. The three orifices opening to the exterior unite in a common chamber located below the third gill.
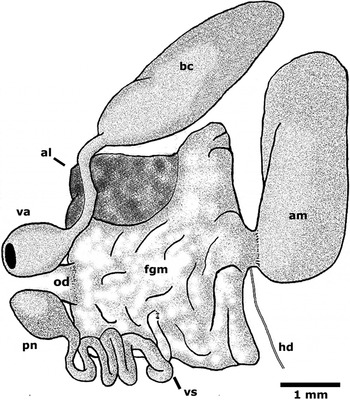
Fig. 7. Marionia limceana sp. nov. Drawing from holotype CMPHRM 4412. Reproductive system (al, albumin gland; am, ampulla; bc, bursa copulatrix; fgm, female gland mass; hd, hermaphroditic duct; od, oviduct; pn, penis; va, vaginal atrium; vd, vas deferens). Ventral view.
Nervous system (Figure 8): the main ganglia of the central nervous system lie on the dorsal surface of the distal oesophagus. They consist of paired cerebral and pleural ganglia (cerebropleurals) joined together by a connective; a pair of pedal ganglia on either side of the cerebropleurals, connected to them through a short connective and connected to each other by the circum-oesophageal nerve ring. A pair of buccal ganglia was found on the ventral oesophagus, joined by a short connective, and connected with the pair of pedal ganglia by long connectives. Giant neurons, typical of the family, are present on all ganglia, and are most visible in the posterior portions. A statocyst is located under the short connective between each pair of cerebropleural and pedal ganglia.
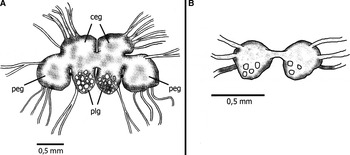
Fig. 8. Marionia limceana sp. nov. Drawing from holotype CMPHRM 4412: (A) central nervous system (ceg, cerebral ganglia; peg, pedal ganglia; plg, pleural ganglia); (B) buccal ganglia.
The heart lies within the pericardium. It is a flat, transparent and membranous structure on the dorsal part of the viscera, just above the stomach.
MEASUREMENTS (MM)
Holotype: CMPHRM 4412 = 31 mm; paratypes: CMPHRM 4413 = 30 mm (specimen 2), 10 mm (specimen 3), 7 mm (specimen 4), 7 mm (specimen 5); CMPHRM 4414 = 3 mm (specimen 6), 5 mm (specimen 7), 5 mm (specimen 8), 4 mm (specimen 9), 10 mm (specimen 10).
GEOGRAPHICAL AND BATHYMETRIC DISTRIBUTION
Marionia limceana is found on the north-eastern Brazilian coast (on the Praia de Caponga, Ceará) and the Praia de Baixa Grande, Rio Grande do Norte. So far the species is known only from the intertidal zone.
DISCUSSION
Tables comparing characters of the internal anatomy and morphology of Marioniopsis, Marionia and Paratritonia were presented in four papers (Jensen, Reference Jensen and Morton1994; Avila et al., Reference Avila, Kelman, Kashman and Benayahu1999; Smith & Gosliner, Reference Smith and Gosliner2005, Reference Smith and Gosliner2007). Avila et al. (Reference Avila, Kelman, Kashman and Benayahu1999) focused on the genus Marioniopsis and a new species; Jensen (Reference Jensen and Morton1994) went further and added the data for Marionia, along with a new species. Smith & Gosliner (Reference Smith and Gosliner2005, Reference Smith and Gosliner2007) added newly described species and Paratritonia lutea. We have combined the available data in an updated table of the species of Marionia, Marioniopsis, Paratritonia and our proposed species (Table 1).
Table 1. Comparison of species of the genera Marionia, Marioniopsis and Paratritonia (u, under; b, between). Data compiled from Jensen (Reference Jensen and Morton1994); Avila et al. (Reference Avila, Kelman, Kashman and Benayahu1999); and Smith & Gosliner (Reference Smith and Gosliner2005, Reference Smith and Gosliner2007).
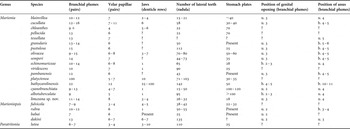
Recent authors (Willan, Reference Willan1988; Avila et al., Reference Avila, Kelman, Kashman and Benayahu1999; Wägele & Willan, Reference Wägele and Willan2000; Smith & Gosliner, Reference Smith and Gosliner2003, Reference Smith and Gosliner2005) discussed the difficulty of studying the family Tritoniidae using only the characters proposed by Odhner (Reference Odhner1963), particularly regarding the division of the digestive gland. Bertsch et al. (Reference Bertsch, Valdés and Gosliner2009) drew attention to this problem by calculating a parsimonious phylogenetic tree for the family Tritoniidae, concluding that although the family presently appears to be monophyletic, the current genera are not, which revealed serious problems in the distinctions between the genera. Here, we assigned M. limceana to the genus Marionia due to its digestive gland divided into two lobes, arborescent papillae in the veil, and hard stomach plates (Marcus, Reference Marcus1983).
A priori, M. limceana differs from other species listed in Table 1 by the number of stomach plates present in the proximal region of the stomach: 18 plates, the fewest reported in the genus. The species with the next fewest plates is Marioniopsis fulvicola Avila, Kelman, Kashman & Benayahu (Reference Avila, Kelman, Kashman and Benayahu1999), with 22 plates, but this species differs from M. limceana in all other characters, such as the jaws and radula. Marionia limceana also has a radula with 26–32 lateral teeth in its widest part, being one of the eight species that contains fewer than 35 lateral teeth. However, it differs from M. blainvillea Risso (1818), M. cucullata Couthouy, M. semperi Jensen (Reference Jensen and Morton1994), M. platyctenea Willan (Reference Willan1988), M. chloanthes Bergh (1902), M. pellucida Eliot (1904), M. cyanobranchiata Odhner (1828) and M. babai Odhner (Reference Odhner1936) mainly in the number of stomach plates. It also differs in all important respects from M. cucullata Couthouy, the only representative of Marionia recorded for the South Atlantic.
Marionia echinomuriceae Jensen (Reference Jensen and Morton1994) has extensive external morphological similarities to M. limceana, especially in the pattern of the notum (although M. echinomuriceae has dark-brown polygons whereas M. limceana has red polygons), bi-lobed veil, appendages, number of gills, and shape of the rhinophores. However the study of internal anatomy revealed significant differences, including the radular formula (26 × 26–32.1.1.1.26–32 in M. limceana against 43 × 65.1.1.1.65 in M. echinomuriceae), the number of stomach plates in the proximal stomach (18 in M. limceana against 28 in M. echinomuriceae), and the number of rows of denticles on the inner edge of the jaw (3–4 in M. limceana against 1 in M. echinomuriceae). Based on the distribution of M. echinomuriceae, reported from Hong Kong in the Pacific Ocean, and M. limceana from north-east Brazil in the Atlantic Ocean, we can assume that the similar external appearance is actually an evolutionary convergence.
Another interesting detail is the presence of silver pigment spots in some specimens of M. limceana. These small spots covered the entire skin of some animals, except for the foot, being present even in the rhinophores and the papillae. Several authors have reported how some members of Tritoniidae tend to appropriate chemical substances from their prey to protect themselves (Gosliner & Ghiselin, Reference Gosliner and Ghiselin1987; Gosliner et al., Reference Gosliner, Behrens and Williams1996; Willan, 1998; Avila et al., Reference Avila, Kelman, Kashman and Benayahu1999). As Cronin et al. (Reference Cronin, Hay, Fenical and Lindquist1995) showed with Tritonia hamnerorum Gosliner & Ghiselin (Reference Gosliner and Ghiselin1987), the use of chemical compounds present in gorgonians can protect nudibranchs from predators, primarily reef fishes. A similar strategy is likely for M. limceana, a relatively large nudibranch compared to other nudibranchs found off north-east Brazil, and thus more easily distinguishable in situ. Marionia limceana was found feeding only on Stragulum bicolor Ofwegen & Haddad, a recently described species and genus of Octocorallia for Brazil, and probably invasive (Van Ofwegen & Haddad, Reference Van Ofwegen and Haddad2011). Polyps were found, often intact, inside the oesophagus and stomach, among grains of sand and mica. Marionia limceana is the first animal reported to feed on this octocorallian.
Marionia limceana also demonstrated avoidance behaviour, defined as ‘escape swims', where the animal moves through the water using strong body contractions in an attempt to escape a potential predator. The movement was reported in the first 30 minutes after the capture of the animal, when it was removed from the substrate and placed in the water column. After this period, the animal stopped responding with ‘escape swims'. Wyeth & Willows (Reference Wyeth and Willows2006) studied the field behaviour of Tritonia diomedea Bergh (1894), and reported that this escape mechanism probably has a high energy cost and is not often used. The individuals of M. limceana were found only during the daylight.
It is also interesting to note the pattern of maturation of the reproductive system of M. limceana: smaller, younger animals had the spermatheca and ampulla developed, close in size to the entire female gland mass. However, glands responsible for spawning such as the mucus gland and albumen were immature or virtually non-existent. This characteristic indicates a protandric sexual development, where the male organs develop before the female organs. This suggests that only the larger specimens were able to spawn (although the smaller individuals probably participate in the mating), a hypothesis supported by Anthes & Michiels (Reference Anthes and Michiels2007) in their study of the maturation of Cephalaspidea, where individuals that laid eggs were larger than the average size of the species.
ACKNOWLEDGEMENTS
We thank Victor Azevedo, Ana Karla and Yan Torres for their assistance in the sampling, Sulla Sulani for her help with the scanning electron microscope, and Jessika Alves for making the line drawing.











