Introduction
Toxocariasis is a worldwide zoonotic parasitic disease caused by the larval stage of intestinal nematodes of dogs and cats, namely Toxocara canis and Toxocara cati (Despommier, Reference Despommier2003). Human infection occurs by the accidental ingestion of embryonated eggs present in the soil, in vegetables or on other contaminated surfaces, as well as via consumption of the uncooked meat of paratenic hosts such as chicken, cattle, pigs or earthworms (Ito et al., Reference Ito1986; Cianferoni et al., Reference Cianferoni2006; Azizi et al., Reference Azizi2007; Choi et al., Reference Choi2012; Zibaei et al., Reference Zibaei, Sadjjadi and Maraghi2017). Dogs and cats are definitive hosts of Toxocara, whose expelled eggs become embryonated and infective in the soil after three to six weeks, depending on soil type and climatic conditions. Infective Toxocara eggs can remain viable in the soil for months to years under optimal conditions (Overgaauw, Reference Overgaauw1997; Lescano et al., Reference Lescano, Nakhle and Chieffi1998). Therefore, the soil is considered to be the source of transmission of Toxocara infection to humans, especially children at play in public parks and playgrounds contaminated with Toxocara eggs (Despommier, Reference Despommier2003).
The clinical symptoms of toxocariasis may vary from an asymptomatic infection to severe infection and there are several forms of toxocariasis, namely, visceral larva migrans (VLM), ocular larva migrans (OLM), covert toxocariasis and neurotoxocariasis (Rubinsky-Elefant et al., Reference Rubinsky-Elefant2010). VLM is caused by the inflammatory response to the larval migration through vital organs and tissues of the body. OLM can lead to partial or total loss of vision (Schantz, Reference Schantz1989; Tan, Reference Tan1997; Zibaei et al., Reference Zibaei, Sadjjadi and Jahadi-Hosseini2014). Epidemiological studies have indicated that the prevalence of human toxocariasis in various parts of Iran ranges from 1.39 to 34.48% (Sadjjadi et al., Reference Sadjjadi2000; Akhlaghi et al., Reference Akhlaghi2006; Fallah et al., Reference Fallah, Azimi and Taherkhani2007; Sharif et al., Reference Sharif2007; Nourian et al., Reference Nourian2008; Alavi et al., Reference Alavi2009; Maraghi et al., Reference Maraghi2012; Hosseini-Safa et al., Reference Hosseini-Safa2015; Shahraki et al., Reference Shahraki2017).
Epidemiological surveys have confirmed the infection of dogs and cats with Toxocara in Iran (Zibaei & Sadjjadi, Reference Zibaei and Sadjjadi2017). The prevalence of T. cati in cats and T. canis in dogs has been estimated to vary from 8–78% and 6.3–29%, respectively, in different parts of Iran (Sadjjadi et al., Reference Sadjjadi2001; Changizi et al., Reference Changizi2007; Zibaei et al., Reference Zibaei, Sadjjadi and Sarkari2007; Mikaeili et al., Reference Mikaeili2013; Emamapour et al., Reference Emamapour, Borji and Nagibi2015; Sardarian et al., Reference Sardarian2015; Hajipour et al., Reference Hajipour2016; Vafae Eslahi et al., Reference Vafae Eslahi2017). There have been some reports of contamination of the soil with Toxocara eggs in public areas in Iran. Examination of soil samples from public parks in Shiraz indicated that seven out of 112 samples (6.3%) were infected with Toxocara eggs (Motazedian et al., Reference Motazedian2006). An investigation was carried out for the presence of Toxocara spp. eggs in public parks in the city of Urmia and the results showed a contamination rate of 7.8% (Tavassoli et al., Reference Tavassoli2008). In Khoramabad, the distribution of Toxocara spp. eggs in samples collected from public parks was 63.3% (Zibaei et al., Reference Zibaei2010). In the city of Tehran 10–38.7% of public places were found to be contaminated with Toxocara spp. eggs (Khazan et al., Reference Khazan2012; Tavalla et al., Reference Tavalla2012). The contamination rate of Toxocara spp. eggs in the public parks of Tabriz city was found to be 9.3% (Garedaghi & Shabestari, Reference Garedaghi and Shabestari2012). A study of the contamination of soil in public parks in Abadan showed that 29.2% samples were infected with Toxocara spp. eggs (Maraghi et al., Reference Maraghi2014). In an evaluation of 195 soil samples from Mashhad and 145 soil samples from Khaf city, 18 (9.2%) and 16 cases (11.3%) of contamination with Toxocara spp. eggs were detected, respectively (Berenji et al., Reference Berenji2015). Studies in Kermanshah found that 13.5–18% of soil samples were infected with eggs of Toxocara spp. (Ghashghaei et al., Reference Ghashghaei2016; Maleki et al., Reference Maleki2016). Pezeshki et al. (Reference Pezeshki2017) showed that Toxocara spp. eggs were found in 14 (7%) out of 200 soil samples collected from public places in Ardabil city.
The identification of Toxocara species is important for planning of prevention and control programmes in human and animal communities (Deplazes et al., Reference Deplazes2011; Gawor et al., Reference Gawor2014). The differentiation of Toxocara spp. eggs based on morphological features is difficult because of their similarity to other species in terms of morphology and size (Fogt-Wyrwas et al., Reference Fogt-Wyrwas, Jarosz and Mizgajska-Wiktor2007, Borecka & Gawor, Reference Borecka and Gawor2008; Zibaei & Sadjjadi, Reference Zibaei and Sadjjadi2010). Therefore, molecular methods such as polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) and PCR-sequencing are recommended for the identification of Toxocara spp. eggs from soil samples. The study conducted by Borecka (Reference Borecka2004) for the differentiation of Toxocara spp. eggs isolated from soil by molecular methods showed that internal transcribed spacer 2 (ITS2) PCR products of T. cati and T. canis were similar in size, and the PCR-RFLP technique produced specific banding patterns, with three fragments for T. cati and two fragments for T. canis. Therefore, this method is useful for characterization to the species level of Toxocara spp. eggs isolated from soil. Khademvatan et al. (Reference Khademvatan, Abdizadeh and Tavalla2014) used microscopy and molecular methods for detection and identification of soil contamination by Toxocara eggs in Ahvaz. Toxocara eggs were found in 64 (30.4%) and 71 (33.8%) out of 210 soil samples using microscopy and PCR methods, respectively. Based on ITS2 PCR-sequencing, 28% of the samples were diagnosed as T. cati and 5.7% as T. canis; no mixed contamination was reported in their observations.
Shiraz is a city with a high prevalence of Toxocara infection in cats (26.7–52.8%) (Sadjjadi et al., Reference Sadjjadi2001; Zibaei et al., Reference Zibaei, Sadjjadi and Sarkari2007; Mikaeili et al., Reference Mikaeili2013). However, no previous study has conducted molecular identification of Toxocara spp. eggs isolated from the soil in Shiraz. The objective of this study was to evaluate soil contamination in public parks and playgrounds in Shiraz using microscopy and molecular methods simultaneously.
Materials and methods
Sample collection
This cross-sectional study was carried out during June–August 2015 in Shiraz, the capital city of Fars province, in southern Iran. A total of 150 soil samples were collected from 50 public parks and playgrounds from different areas (north, south, east, west and centre) of Shiraz. The soil samples were collected randomly from three sites in each park, from a depth of 10 cm. Samples (200 g each) were placed in labelled containers and transported to the laboratory.
Microscopy method
For detection of Toxocara spp. eggs, the soil samples were dried at room temperature for 24 hours and sifted through 80 and 100 μm mesh sieves. The samples were treated with saturated zinc sulphate solution (specific gravity 1.8) according to the method described by Borecka (Reference Borecka2004).
Molecular method
DNA extraction and nested PCR
The genomic DNA from all 150 soil samples was extracted using the Tissue Genomic DNA Extraction Mini kit with proteinase (Yekta Tajhiz Azma, Tehran, Iran), according to the manufacturer's instructions, with a minor modification. After zinc sulphate flotation, all contents on the slide were washed with TG1 buffer into a tube. The samples were subjected to three freeze–thaw cycles followed by proteinase K digestion overnight.
The ribosomal DNA region (partial sequence of ITS1 and ITS2) was subjected to analysis using nested PCR reaction. The forward primer (NC5: 5´-GTAGGTGAACCTGCGGAAGGATCATT-3´) and reverse primer (NC2: 5´-TTAGTTTCTTTTCCTCCGCT-3´) were used for amplification of the ITS region for the first step of nested-PCR (Li et al., Reference Li2007). The first PCR reactions were performed in a final volume of 25 μl. Each reaction contained 12.5 μl of PCR mix (2× Master Mix RED; Ampliqon, Denmark), which included 1.25 U Taq DNA polymerase, 200 μM of deoxynucleotide triphosphate (dNTP) and 1.5 mM MgCl2; 12.5 pmol of each primer and 5 μl of template DNA in an automated thermocycler. The temperature profile was one cycle of 95°C for 6 minutes to denature the double-stranded DNA, followed by 35 cycles of 94°C for 45 s (denaturation), 60°C for 1 minute (annealing), 72°C for 1 minute (extension) and a final extension of 72°C for 6 minutes.
The second step of nested-PCR reactions was performed using forward primer (FM1: 5´-TTGAGGGGAAATGGGTGAC-3´) and reverse primer (FM2: 5´-TGCTGGAGGCCATATCGT-3´) in a 25 μl reaction volume (Mikaeili et al., Reference Mikaeili2017). 5 μl of the product obtained in the first step of nested-PCR was used as a template for the second step. An initial denaturation step at 94°C for 12 minutes was followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extended at 72°C for 1 minute, with a final extension step at 72°C for 5 minutes. Double-distilled water (DDW) instead of template DNA was included in each set of PCR reactions as negative control. The PCR products were separated by electrophoresis on a 1.5% agarose gel and stained with GelRed nucleic acid gel stain (Biotium, Fremont, USA), 10,000× in water.
PCR-RFLP method
Nested PCR products (5 μl) were digested directly with 0.5 μl restriction enzyme SalI (ER0551, ER0641; Thermo Fisher Scientific, Waltham, USA) in a final volume of 15 μl for 3 h at 37°C. Restriction fragments of amplicons were separated on 2% agarose gel in triethanolamine (TAE) buffer. Gels were transilluminated in ultraviolet light and photographed. Digestion of the ITS products of the two Toxocara species with SalI endonuclease showed specific banding patterns, i.e. producing two fragments for T. canis but no digestion for the ITS PCR products of T. cati isolates (Mikaeili et al., Reference Mikaeili2017).
Sequencing and phylogenetic analysis
PCR-sequencing was performed to confirm the results of the PCR-RFLP method. The PCR products were purified using the EasyPure Quick Gel Extraction Kit (TransGen Biotec, Beijing, China), according to the manufacturer's instructions, and were submitted to sequencing in two directions using the primers employed in the second step of nested-PCR. Sequence results were edited using the Geneious software (www.geneious.com) and compared with GenBank reference sequences using BLAST (http://www.ncbi.nlm.nih.gov/). A phylogenetic tree was constructed with ITS sequences obtained in the present study along with reference sequences deposited in GenBank and reference sequences of Toxascaris leonina as outgroup using the maximum likelihood method in the MEGA 5.0 software (Tamura et al., Reference Tamura2011). Bootstrap analyses (using 1,000 replicates) were carried out to determine the robustness of the finding. The sequences reported here were deposited in GenBank.
Results
Eggs of Toxocara spp. were found in six (4%) of the 150 soil samples using the zinc sulphate flotation method (fig. 1). All soil samples were examined by the nested-PCR method using the ribosomal DNA region. From 150 soil samples, six (4%) amplicons of about 1000–1100 bp were successfully produced by the first step of nested-PCR, whereas 24 (16%) samples produced amplicons of about 700 bp only after the second step of nested-PCR; no amplification was observed in the negative controls (fig. 2). Table 1 shows the contamination rate of the soil of public parks and playgrounds in Shiraz according to geographical area.

Fig. 1. Egg of Toxocara spp. isolated from one of the soil samples taken from public parks and playgrounds in Shiraz, Iran.

Fig. 2. Agarose gel electrophoresis of (a) the first step and (b) the second step of nested-PCR products. M: 100 bp DNA marker; N: negative control; lane1: positive control; lanes 2–13: soil samples.
Table 1. Contamination rate of soil samples from public parks and playgrounds in Shiraz, Iran with Toxocara spp. eggs, based on microscopy and molecular methods.

The nested-PCR products of Toxocara spp. were similar in size, therefore the PCR-RFLP differentiated T. canis and T. cati from each other and showed specific banding patterns. Digestion with SalI endonuclease produced two fragments of 320 and 394 bp for T. canis and an undigested band of 736 bp for T. cati (fig. 3). Based on the PCR-RFLP method, a total of 23 out of 24 isolates were confirmed as T. cati and one out of 24 as T. canis.

Fig. 3. PCR-RFLP pattern of Toxocara after digestion with SalI restriction enzyme. M: 100 bp DNA marker; N: negative control; lane 1: positive control (T. cati); lanes 2, 3, 4: positive control (T. canis); lanes 5, 6, 7, 8: soil samples.
Sequence analysis was performed for 12 randomly selected nested-PCR products to confirm the PCR-RFLP results. The edited sequences were compared with other available sequences in GenBank using the BLAST system, and 11 isolates were identified as T. cati and only one as T. canis. The consensus sequences determined in this study were deposited in GenBank with the accession numbers MF592391–MF592402.
The consensus phylogenetic tree indicated that 11 isolates of T. cati obtained in the current study based on ITS sequences divided into two haplotypes, and intraspecies variation was 0–0.2%. In this study, the ITS sequence of T. canis isolated from soil samples had a 100% homology with T. canis isolated from fox in China (accession no. JF837169) and dog in Iran (accession no. KF577855) (fig. 4). Interspecies sequence differences among T. cati and T. canis isolated from soil samples were significantly higher, being 15–15.3%.
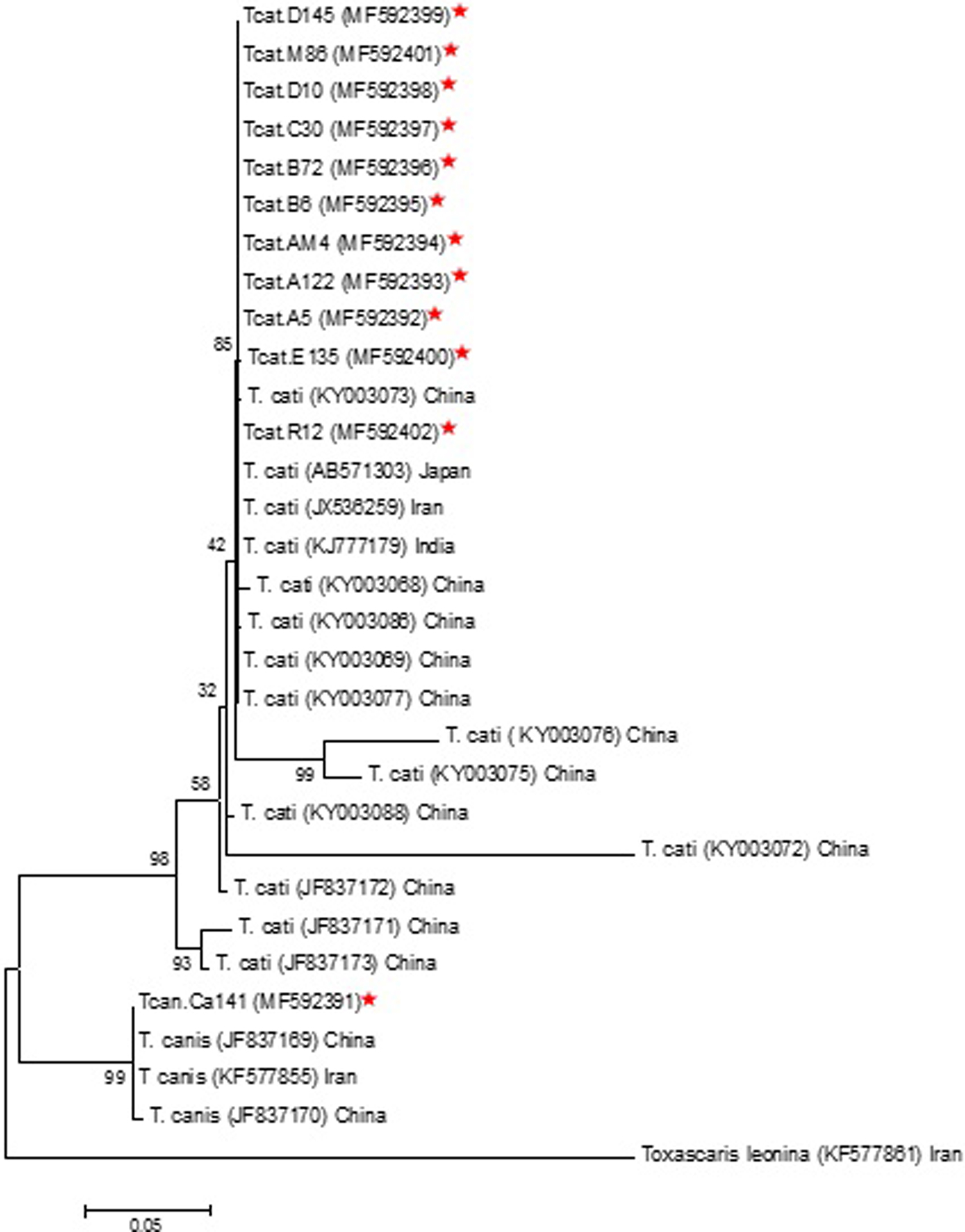
Fig. 4. Phylogenetic relationship of ITS sequences of Toxocara cati and Toxocara canis isolates obtained in this study and reference sequences retrieved from GenBank. Toxascaris leonina (AN: KF577861) was used as the outgroup.
Discussion
The infection of dogs and cats with T. canis and T. cati in various parts of Iran has been reviewed by Zibaei & Sadjjadi (Reference Zibaei and Sadjjadi2017). A study in Shiraz found that a total of 57 out of 108 stray cats (52.8%) were infected with T. cati (Sadjjadi et al., Reference Sadjjadi2001). Zibaei et al. (Reference Zibaei, Sadjjadi and Sarkari2007) reported the prevalence of T. cati and other intestinal helminths in stray cats in Shiraz and their results revealed that T. cati was one of the most frequently detected intestinal helminths (42.6%). Another study showed that the infection rate of T. cati among 30 stray cats in Shiraz was 26.7% (Mikaeili et al., Reference Mikaeili2013). In a study of intestinal helminths in stray dogs in Mashhad, north-east Iran, the prevalence of infection with T. canis was 29% (Emamapour et al., Reference Emamapour, Borji and Nagibi2015). A similar study investigated the prevalence of zoonotic intestinal parasites in household and stray dogs in rural areas of Hamadan, in western Iran; the results indicated that T. canis was the most frequently detected parasite, with a prevalence of 6.3% (Sardarian et al., Reference Sardarian2015).
Several studies have been carried out on contamination of soil with Toxocara spp. eggs in Iran. Motazedian et al. (Reference Motazedian2006) reported the prevalence of helminth eggs in soil samples from public places and children's playgrounds in Shiraz. In their study, T. cati eggs were found in seven out of 112 soil samples (6.3%), using saturated zinc sulphate flotation. However, it is not clear from the methodology how T. cati eggs were differentiated from other Toxocara spp. eggs, which is difficult using light microscopy. On the basis of their observations, it can be argued that the conclusions reported by Motazedian et al. (Reference Motazedian2006) are not entirely supported by their results (Zibaei & Sadjjadi, Reference Zibaei and Sadjjadi2010). In our survey, using the saturated zinc sulphate flotation method, 4% of the soil samples from public parks and playgrounds were contaminated with Toxocara spp. eggs, which was almost the same as the findings of Motazedian et al. (Reference Motazedian2006) for Shiraz. In another study, a total of 580 soil samples were collected from different areas of Sari, northern Iran. The soil contamination with Toxocara spp. eggs was reported to be 3.73%, using the saturated sucrose flotation method (Hezarjaribi et al., Reference Hezarjaribi2016). Saraei et al. (Reference Saraei2012) reported a low rate of soil contamination with Toxocara eggs (3.15%) in public parks of Qazvin, Iran. The rate of contamination of soil with Toxocara eggs in our study was lower than in some previous studies conducted in different areas of Iran. A study undertaken in Khorram Abad found that the distribution of Toxocara spp. eggs in samples collected from public parks was 63.3%, using the saturated sucrose flotation method (Zibaei et al., Reference Zibaei2010). In a study conducted in Tehran city during 2008–2009 on 150 soil samples collected from various sites, Toxocara eggs were observed in 38.7% of the samples using sodium nitrate flotation (Tavalla et al., Reference Tavalla2012). A study carried out in Khuzestan found that 29.2% of the soil samples were infected with Toxocara spp. eggs using the modified flotation method using saturated sucrose (Maraghi et al., Reference Maraghi2014).
Studies carried out in various countries have indicated that the contamination rate varies markedly in different regions of the world. A total of 5.71% of soil samples in Thailand (Wiwanitkit & Waenlor, Reference Wiwanitkit and Waenlor2004), 11.9% of soil samples in the Czech Republic (Dubná et al., Reference Dubná2007), 0.8% of soil samples in Costa Rica (Paquet-Durand et al., Reference Paquet-Durand2007), 9% of soil samples in Japan (Zibaei & Uga, Reference Zibaei and Uga2008), 16.4% of soil samples in Spain (Dado et al., Reference Dado2012), 4.75% of soil samples in India (Thomas & Jeyathilakan, Reference Thomas and Jeyathilakan2014), 77% of soil samples in the Philippines (Paller & de Chavez, Reference Paller and de Chavez2014) and 22.2% of soil samples in Iraq (Taher, Reference Taher2017) have been reported to be positive for Toxocara spp. eggs.
The differences in contamination rate depend on a variety of factors, including soil type, climate, soil collection method, flotation method, number of infected stray cats and dogs in the region, culture, and people's interest in keeping cats and dogs as pets.
Soil contamination with T. canis and T. cati eggs is considered to be the main source of human toxocariasis (Nijsse et al., Reference Nijsse2015). In most studies, soil contamination with Toxocara spp. eggs has been investigated using microscopy and the results have been reported only for the genus Toxocara (Zibaei et al., Reference Zibaei2010; Maraghi et al., Reference Maraghi2014; Hezarjaribi et al., Reference Hezarjaribi2016). Toxocara spp. eggs are similar in size and shape. Although it is possible to differentiate them by electron microscopy, their identification by light microscopy is not possible (Holland & Smith, Reference Holland and Smith2006; Zibaei & Sadjjadi, Reference Zibaei and Sadjjadi2010). Molecular methods such as PCR-RFLP, PCR-sequencing and PCR using species-specific primers are the most suitable techniques for the differentiation of Toxocara spp. eggs isolated from the soil (Borecka, Reference Borecka2004; Fahrion et al., Reference Fahrion2011). The differentiation of Toxocara spp. eggs is essential for serological studies and the planning of prevention and control programmes in human and animal communities. For the first time, Borecka (Reference Borecka2004) used a PCR-linked RFLP method for the differentiation of Toxocara spp. eggs isolated from the soil. PCR-RFLP was useful for differentiating eggs of Toxocara spp. isolated from the soil to species level. The PCR products for ITS2 of T. cati and T. canis were similar in size. Digestion of the purified ITS2 products of the two Toxocara species with RsaI endonuclease produced specific banding patterns. In our study, molecular identification of Toxocara spp. eggs isolated from the soil was performed using PCR-RFLP and PCR-sequencing methods. The binding patterns of the nested-PCR for all samples were similar in size and this method alone could not differentiate Toxocara spp. eggs. PCR-RFLP with SalI endonuclease produced two fragments of 320 and 394 bp for T. canis and an undigested band of 736 bp for T. cati. Otero et al. (Reference Otero2017) used molecular analysis and sequencing of the 18S rRNA gene to discriminate between Toxocara species in soil samples collected from public parks and playground sandpits in Portugal, and their results showed that 53% of soil samples were positive for Toxocara spp. and only T. cati eggs were found in soil samples Other authors who detected Toxocara spp. eggs in the environment used molecular methods with species-specific primers (Khademvatan et al., Reference Khademvatan, Abdizadeh and Tavalla2014; Ozlati et al., Reference Ozlati2016; Gao et al., Reference Gao2017; Studzińska et al., Reference Studzińska2017).
In the current study, the molecular method indicated that 16% of soil samples were contaminated with Toxocara spp., which was higher than the percentage indicated by the microscopy method (4%), while Ozlati et al. (Reference Ozlati2016) found Toxocara spp. eggs in 57 (31.6%) and 14 (7.7%) soil samples using the microscopy and PCR methods, respectively, which is inconsistent with our findings. Khademvatan et al. (Reference Khademvatan, Abdizadeh and Tavalla2014) used the microscopy and molecular methods for detection and identification of soil contamination by Toxocara eggs in Ahvaz, southwestern Iran. Nucleotide sequencing was performed to confirm the results of the PCR method. Toxocara eggs were found in 30.4% and 33.8% of soil samples using the microscopy and PCR methods, respectively, and there was no statistically significant difference between the two methods (PV = 0.279).
Based on PCR-RFLP and PCR-sequencing methods, we found a total of 23 out of 24 isolates were confirmed as T. cati and one out of 24 as T. canis without any mixed contamination. Similar to our study, Khademvatan et al. (Reference Khademvatan, Abdizadeh and Tavalla2014) indicated that T. cati was more prevalent than T. canis (28% and 5.7%, respectively), and no mixed contamination was observed. In contrast to our study, Ozlati et al. (Reference Ozlati2016) identified T. canis in 15.5% of soil samples, T. cati in 27.2% of soil samples and mixed infections in 12.2% of soil samples.
In conclusion, this study revealed that a higher contamination rate was observed using the molecular method (16%) in comparison to the microscopy method (4%). PCR-RFLP and PCR-sequencing methods are the most suitable technique for the differentiation of Toxocara spp. eggs.
As soil contamination with Toxocara vitulorum eggs is possible, and the morphological identification of Toxocara spp. eggs is difficult, molecular methods have been used for the characterization of species (Gasser et al., Reference Gasser2006; Fahrion et al., Reference Fahrion2011; Pawar et al., Reference Pawar2012). In the present study, the rate of soil contamination with T. cati eggs was much higher than that with T. canis eggs, which is in accordance with reports of the infection rate of T. cati among stray cats in Shiraz (Sadjjadi et al., Reference Sadjjadi2001; Mehrabani et al., Reference Mehrabani, Sadjjadi and Oryan2002; Zibaei et al., Reference Zibaei, Sadjjadi and Sarkari2007; Mikaeili et al., Reference Mikaeili2013). Soil contamination with Toxocara spp. eggs is considered to be the main source of toxocariasis in humans, and children are the main group exposed to contamination because of their high contact with soil when playing in public parks and playgrounds; therefore, prevention and control programmes in human and animal communities are needed. Our examination of 150 soil samples collected from various sites in 50 parks in Shiraz gives an indication of the level of soil contamination with Toxocara eggs in the town. However, the higher rate of infection of soil samples with T. cati eggs may play the most important role in human infection with toxocariasis in this urban environment.
Financial support
This study was supported by the office of the Vice Chancellor for Research at SUMS, grant number: 93-7422.
Conflict of interest
None.







