Introduction
Major depressive disorder (MDD) is a severe psychiatric illness characterised by persistent and predominant low mood and anhedonia. Lifetime prevalence estimates of MDD vary worldwide, with 14.6% [ranging from 6.6 to 21.0%, standard error (s.e.) 0.2%] in high-income countries and 11.1% (ranging from 6.5 to 18.4%, s.e. 0.2%) in low-income countries, according to the World Health Organization World Mental Health Survey (Kessler & Bromet, Reference Kessler and Bromet2013). In terms of years lived with disability, depressive disorder was among the top three leading causes in 2017 (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018).
A number of measures are adopted in the management of MDD, including psychological, pharmacological, and physical therapies. However, 29–46% of depressed patients do not respond entirely after antidepressant administration (Fava & Davidson, Reference Fava and Davidson1996). STAR*D trial suggests that patients who do not respond adequately to initial antidepressant agents may benefit from subsequent treatment (Howland, Reference Howland2008). Such strategies for suboptimal response include using a combination with psychotherapy or electroconvulsive therapy, increasing the antidepressant dose, switching to or combining with another antidepressant with a new mechanism of action, and augmentation. Nevertheless, evidence is insufficient to differentiate which strategies were considered priorities for depressive patients with various symptoms.
Augmentation refers to the addition of a second medication to enhance the efficacy of an already prescribed antidepressant. Canadian Network for Mood and Anxiety Disorder Treatments (CANMAT) guidelines recommend atypical antipsychotics as adjunctive strategies (Kennedy et al., Reference Kennedy, Lam, McIntyre, Tourjman, Bhat and Blier2016). When combined with antidepressants, a few atypical antipsychotics (e.g. olanzapine and quetiapine) play a role in sedating and have anxiolytic effects, as well as countering some of the acute side effects of antidepressants (Malhi & Mann, Reference Malhi and Mann2018). However, atypical antipsychotics themselves also sometimes induce treatment-related adverse events (AEs). It is important to indicate that the augmented effect of antipsychotics when prescribing antidepressants remains unknown, and much of the administration is empirical (Malhi & Mann, Reference Malhi and Mann2018). Clinicians need evidence-based instructions when choosing various kinds of antipsychotics as adjunctive treatments for MDD patients with unsatisfactory antidepressant responses.
The majority of published randomised controlled trials (RCTs) have evaluated the efficacy and safety of atypical antipsychotics as antidepressant augmentation therapy compared with a placebo. However, few studies have focused on the direct comparison between different antipsychotics. As a novel statistical approach, network meta-analysis (NMA) integrates direct and indirect evidence. Here, we conducted an NMA of RCTs to evaluate the comparative efficacy and acceptability of second-generation antipsychotics in combination with antidepressants as augmentation therapy in patients with suboptimal antidepressant responses.
Materials and methods
Search strategy and selection criteria
The following databases were systematically searched: PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials, Medline, Medline in Process, PsycINFO, Cochrane Review, and Cochrane Trial for articles published prior to 25 May 2021. We utilised the search terms ‘depression’ OR ‘dysthymia’ OR ‘mood disorder’ OR ‘affective disorder’ OR ‘major depressive disorder’ OR ‘MDD’ OR ‘treatment-resistant depression’ OR ‘TRD’ combined with ‘atypical antipsychotic’ OR ‘second-generation antipsychotic’ OR other concrete names of specific antipsychotic drugs. We set the filter to restrict the article type to ‘RCT’ and ‘meta-analysis’. In addition, we conducted an advanced search in ClinicalTrials.gov considering the strategy as followed: ‘studies with results’ AND ‘interventional studies’ AND ‘depression, unipolar’ AND ‘augmentation OR adjunctive OR add-on OR combination OR co-administration’.
Only double-blind RCTs comparing antipsychotics with a placebo or another antipsychotic augmenting the action of antidepressants as oral administration for patients who were diagnosed with unipolar non-psychotic depression according to any versions of the Diagnostic and Statistical Manual of Mental Disorders (DSM) were included. Patients with other physical or mental comorbidities were excluded. For the meta-analysis retrieved, we searched the reference lists to include additional eligible studies. Two reviewers worked independently in record selection, data collection, and risk of bias evaluation.
Data extraction
We extracted the characteristics of the included studies. Relevant information consisted of article characteristics (such as first author, year of publication, register number, and recruitment), participant characteristics (such as diagnostic criteria, history of inadequate antidepressants response, mean age, sex, duration of current episode, and severity of depression), and intervention characteristics (data related screen phase, antidepressant lead-in phase, and randomised phase).
Our primary outcome was the response rate for efficacy and the all-cause dropout rate for acceptability. Response was defined as the number of patients with more than a 50% decrease in total scores on the standardised depression scale. We employed the Montgomery–Asberg Depression Rating Scale (MADRS). If MADRS was unavailable, we chose the Hamilton Rating Scale for Depression (HAM-D) or alternative scales. If the response rate was absent, we imputed missing data according to a verified imputation algorithm (Furukawa, Barbui, Cipriani, Brambilla, & Watanabe, Reference Furukawa, Barbui, Cipriani, Brambilla and Watanabe2006; Furukawa, Cipriani, Barbui, Brambilla, & Watanabe, Reference Furukawa, Cipriani, Barbui, Brambilla and Watanabe2005). All-cause dropout rate was defined as the percentage of participants quitting the trial for whatever reason, and it encompassed efficacy and tolerability (Cipriani et al., Reference Cipriani, Furukawa, Salanti, Geddes, Higgins, Churchill and Barbui2009). Our secondary outcomes were remission rates (defined as the total number of patients whose standardised depression scale score was less than a particular cut-off value) and discontinuation due to AEs (defined as the portion of patients leaving the trial early from AEs, which only reflected tolerability).
We recorded the outcome data at approximately 8 weeks from the augmentation phase. If information was not available at 8 weeks, the time point closest to 8 weeks was considered. Only results from the intention-to-treat analysis were extracted. We preferred outcomes using mixed method repeat measures for imputing dropout patients rather than the last observation carried forward (Furukawa et al., Reference Furukawa, Salanti, Atkinson, Leucht, Ruhe, Turner and Cipriani2016b).
Data analysis
Stata/SE (version 15.1) in a frequentist framework was used for the data analysis. Since our outcomes were all dichotomous variables, we calculated odds ratios (ORs) with corresponding 95% credible intervals (CrIs). The random-effect model was used when performing the NMA. We presented the results of each comparison based on direct and indirect evidence in a two-dimensional graph and tabular form. To rank the probability of efficacy and acceptability for treatments, we used the surface under the cumulative ranking curve (SUCRA). We evaluated the heterogeneity of each comparison by quantifying I 2 statistics (Higgins & Thompson, Reference Higgins and Thompson2002), and the visualised form was presented by a predictive interval (PrI) plot, where differences between CrIs and PrIs indicated the size of heterogeneity. According to the Cochrane Handbook, a value of 0–40% was insignificant, 30–60% indicated moderate heterogeneity, 50–90% suggested essential heterogeneity, and 75–100% represented appreciable heterogeneity (Deeks, Higgins, & Altman, Reference Deeks, Higgins and Altman2021). Inconsistency, representing the heterogeneity of direct and indirect evidence, was evaluated using global and local network methods. We evaluated local inconsistency by node-splitting and loop-specific methods and global inconsistency using a design-by-treatment test (Higgins et al., Reference Higgins, Jackson, Barrett, Lu, Ades and White2012). We assessed the included RCTs using version 2 of the Cochrane risk-of-bias tool for randomised trials (RoB 2) (Higgins, Savović, Page, Elbers, & Sterne, Reference Higgins, Savović, Page, Elbers and Sterne2021). There are five domains in RoB 2 assessing bias coming from the randomisation procedure, proposed intervention deviations, absent outcome data, outcome measurement, and selection of the reported result. We set a cut-off value of 80% rather than the 95% recommended in the Cochrane Handbook when evaluating whether the outcome data of all participants were completed (Furukawa et al., Reference Furukawa, Salanti, Atkinson, Leucht, Ruhe, Turner and Cipriani2016b). We conducted a sensitivity analysis of the conclusions for two primary outcomes by excluding (1) studies with small sample sizes (number of randomised patients of <30) and (2) studies with a high risk of bias. We performed network meta-regression in R (version 4.1.0) to adjust the effect of study years and baseline depression severity. We used comparison-adjusted funnel plots to investigate published bias.
Result
Search selection and network of evidence
The screening flowchart is presented in Fig. 1. In summary, 6842 citations were retrieved, and 83 full-text articles were selected for further review. Finally, 30 RCTs searched in the database and three RCTs searched in ClinicalTrials.gov were included.

Fig. 1. Systematic review flow diagram. RCT, randomised controlled trial; NMA, network meta-analysis.
A network plot of comparisons between eligible interventions is shown in Fig. 2. All antipsychotics except for ziprasidone had more than one placebo-controlled comparator. Only two closed loops existed in the network (aripiprazole v. olanzapine v. placebo and brexpiprazole v. quetiapine v. placebo). Four outcomes shared similar network plots; therefore, we only use the result of the response rate in this manuscript; the results of other outcomes are presented in online Supplemental Material S1. Tests of overall heterogeneity variances were relatively low and not significant for outcomes (response rate: I 2 = 0%, p = 0.991; remission rate: I 2 = 0%, p = 0.952; all-cause dropout rate: I 2 = 0%, p = 0.854; dropout due to AE: I 2 = 30.1%, p = 0.063). Overall inconsistency tests indicated identical results (response rate: χ2(3) = 0.90, p = 0.826; remission rate: χ2(3) = 3.98, p = 0.264; all-caused dropout rate: χ2(3) = 0.52, p = 0.915; dropout due to AE: χ2(3) = 1.55, p = 0.671). The detailed results are presented in online Supplementary materials S2 and S3.

Fig. 2. NMA of eligible comparisons for response rate. The node represents treatment, and the size of the node is proportional to the sample size of treatment. The line represents the comparison between two treatments, and the width of the line is proportional to the sum of the s.e. of the two treatments.
Characteristics of studies and risks of bias
Online Supplementary material S5 summarises the basic characteristics of the included studies. In total, 4543 and 6059 patients were randomised to the placebo and antipsychotics groups, respectively. All of the participants had a mean age of 44.55 years [standard deviation (s.d.) 11.55], and the proportion of females was 65%. The length of trials was 7.24 weeks (s.d. 3.42), ranging from 4 to 24 weeks. Four (12.12%) trials recruited participants from Asia, 16 (48.48%) from North America, two (6.06%) from Europe, nine (27.27%) were cross-continental, and two (6.06%) did not mention the location. All MDD diagnoses were based on the DSM criteria. Seven trials involved identical classes of drugs at different doses. The response rate was imputed in only two trials. Thirty (90.91%) trials were multicentre, and 27 (81.82%) clearly defined the criteria for the history of inadequate antidepressant response. Twenty-one (63.64%) consisted of a screening phase, in which prohibited psychotropics were discontinued, and 23 (69.70%) contained an antidepressant run-in phase in order to establish an inadequate response.
In all studies, the effect of intervention assignment was estimated on an intent-to-treat basis. One trial (3%) exhibited a high risk of bias in the randomisation process, while six (18.2%) trials might have a high risk of bias owing to missing outcome data. Overall, there were six (18.2%) RCTs with a high risk of bias, 12 (36.3%) indicating some concerns, and 15 (45.5%) with low risk. Detailed results of the risks of bias are reported in online Supplementary material S4.
Efficacy, acceptability, and tolerability
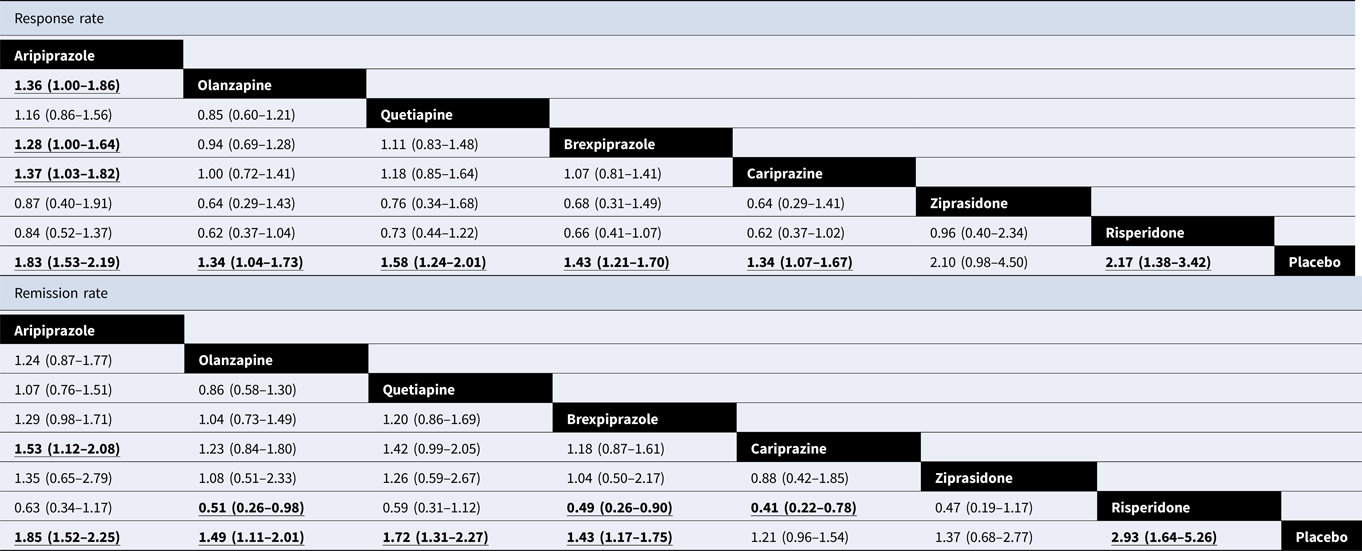
Table 1 shows the results of efficacy in the NMA. For response rate, all active antipsychotics except for ziprasidone (OR 2.10, 95% CrI 0.98–4.50) were more efficacious than the placebo, with OR ranging from 1.34 for olanzapine and cariprazine (95% CrI 1.04–1.73 and 1.07–1.67, respectively) to 2.17 for risperidone (95% CrI 1.38–3.42). In comparison among active antidepressants, aripiprazole was better than olanzapine (OR 1.36, 95% CrI 1.00–1.86), brexpiprazole (OR 1.28, 95% CrI 1.00–1.64), and cariprazine (OR 1.37, 95% CrI 1.03–1.82). In terms of the remission rate, besides ziprasidone (OR 1.37, 95% CrI 0.68–2.77), cariprazine showed no significant result (OR 1.21, 95% CrI 0.96–1.54). Olanzapine (OR 0.51, 95% CrI 0.26–0.98), brexpiprazole (OR 0.49, 95% CrI 0.26–0.90), and cariprazine (OR 0.41, 95% CrI 0.22–0.78) were less efficacious than risperidone. Table 2 shows the results of acceptability and tolerability in the NMA. In terms of acceptability, quetiapine (OR 0.68, 95% CrI 0.50–0.91), brexpiprazole (OR 0.69, 95% CrI 0.55–0.86), and cariprazine (OR 0.61, 95% CrI 0.46–0.82) were worse than the placebo. In terms of tolerability, except for olanzapine and risperidone, all antipsychotics caused AEs more frequently than the placebo, with ORs ranging from 0.04 (95% CrI 0.00–0.87) for ziprasidone to 0.43 (95% CrI 0.22–0.82) for aripiprazole. No significant differences were found in the comparison of active antipsychotics. Figure 3 integrates the two-dimensional graphs of the efficacy and acceptability.

Fig. 3. Two-dimensional graphs about efficacy and acceptability. Data are reported as ORs compared with the placebo. Individual drugs are represented in nodes with different numbers. Bold lines indicate 95% CrI of corresponding outcomes, and cross without dash line indicates significance in each dimension. Nodes located in the righter and upper show better efficacy and acceptability, respectively. OR, odds ratios. 1 = aripiprazole; 2 = olanzapine; 3 = quetiapine; 4 = brexpiprazole; 5 = cariprazine; 6 = ziprasidone; 7 = risperidone; 8 = placebo.
Table 1. Results of NMA for efficacy

Data are ORs in the column-defining treatment compared with the row-defining treatment. For response and remission, ORs lower than 1 favour row-defining treatment and vice versa. Significant results are in bold and underlined. OR, odds ratios.
Table 2. Results of NMA for acceptability and tolerability

Data are ORs (95% CrI) in the column-defining treatment compared with the row-defining treatment. For dropout rates, ORs lower than 1 favour column-defining treatment and vice versa. Significant results are in bold and underlined. OR, odds ratios; AE, adverse event.
Rank probability
Cumulative probability plots and SUCRAs are presented in online Supplementary material S6. In terms of efficacy, risperidone and aripiprazole were the best among all seven antipsychotics in terms of response or remission rates. Ziprasidone ranked in the same order as aripiprazole in response rate (mean rank = 2.6), while it ranked in the lower half for remission rate (mean rank = 5.2). In terms of acceptability and tolerability, olanzapine, aripiprazole, and risperidone ranked as the first three, in that order.
Sensitivity analysis, network meta-regression, and published bias
The results of the sensitivity analysis did not alter significantly. Only cariprazine exhibited different outcomes compared with placebo both in response (OR 1.14, 95% CrI 0.83–1.58) and acceptability (OR 1.45, 95% CrI 0.96–2.17) when excluding high-risk articles. In the network meta-regression, all regression coefficient beta showed no statistical significance. Detailed results of efficacy and acceptability and funnel plots are shown in online Supplementary materials S7–S9.
Discussion
This NMA compares efficacy (as ORs of response and remission rates) and acceptability (as ORs of dropout rates due to any cause and AEs) in the pharmacological therapy of antidepressants with adjunctive atypical antipsychotics. The data analysis was based on 33 trials, which included 10 602 patients with MDD randomly assigned to seven second-generation antipsychotics or a placebo.
Our findings indicated that all antipsychotics, with the exception of ziprasidone in the NMA, were more effective than a placebo. Among these medications, risperidone and aripiprazole had comparatively high response rates and low dropout rates. Olanzapine, a commonly used antipsychotic, had relatively low efficacious indices and dropout rates, even considering the causes of AEs. Quetiapine, brexpiprazole, and cariprazine had a moderate response rate compared to the placebo, while they were only three drugs worse than the placebo, with significant differences in all-cause dropout rates. However, in terms of remission rates, cariprazine was not better than the placebo, while quetiapine was significantly better. The response rate of ziprasidone showed a higher point estimate response rate but no significant difference compared to the placebo.
This research extends previous NMAs, regardless of the number of articles or the variety of drug types. According to an NMA involving aripiprazole, olanzapine, quetiapine, and risperidone, all antipsychotics administrated in standard doses for augmentation therapy of treatment resistant depression are beneficial in alleviating depressive symptoms, which is consistent with our results (Zhou et al., Reference Zhou, Keitner, Qin, Ravindran, Bauer, Del Giovane and Xie2015). It considered the quality of life of patients and found that risperidone and aripiprazole showed improvements, which mirrored our result that risperidone and aripiprazole were highly recommended (Zhou et al., Reference Zhou, Keitner, Qin, Ravindran, Bauer, Del Giovane and Xie2015). Our work expanded the number of patients (6436 v. 4422) to strengthen the conclusions and included other types of antipsychotics (7 v. 4) to integrate into the network. A newly published meta-analysis included seven identical drugs in data analysis compared with the placebo and concluded that all antipsychotics were shown to be more efficacious than the placebo (overall OR 1.59, 95% CrI 1.44–1.75) (Vázquez, Bahji, Undurraga, Tondo, & Baldessarini, Reference Vázquez, Bahji, Undurraga, Tondo and Baldessarini2021). Nevertheless, a subgroup analysis was not implemented by dividing the different antipsychotics, and heterogeneity was significant. According to the report, overlapping CrIs restrict their usefulness in providing recommendations for which medicine should be administered first (Vázquez et al., Reference Vázquez, Bahji, Undurraga, Tondo and Baldessarini2021). Our work solves this problem to some extent. Previous traditional meta-analyses illustrated results parallel to ours (Nelson & Papakostas, Reference Nelson and Papakostas2009; Spielmans et al., Reference Spielmans, Berman, Linardatos, Rosenlicht, Perry and Tsai2013; Wen et al., Reference Wen, Wang, Liu, Huang, Liu and Hu2014). Our findings were somewhat in accord with the recommendations of the CANMAT guidelines, which recommend aripiprazole, quetiapine, and risperidone as first-line adjunctive therapy for non-response or partial response to antidepressants with level 1 evidence (Kennedy et al., Reference Kennedy, Lam, McIntyre, Tourjman, Bhat and Blier2016).
Our study had several strengths. As far as we know, this NMA is the most extensive of the augmentation treatments for depression administered with atypical antipsychotics. We performed a novel evidence-based medical analysis approach to rank the order of each medication based on the integration of direct and indirect comparisons. A few methods were used, such as the evaluations of heterogeneity, inconsistency, risk of bias through various domains, and up-to-date tools and implementation of sensitivity analysis, to make our results more reliable. The conclusion based on a vast majority of subjects was substantially consistent with previous reviews, and we further verified the accuracy of contemporary clinical guidelines.
Our study had several limitations. First, we did not consider the dose of drugs in the analysis since key relative information (e.g. the virtual mean dose delivered) was unavailable in the original articles. In some trials, a relatively large difference between the virtual and predefined doses in some comparator groups was observed. In addition, it was formidable to define a precise cut-off value for low and high doses of each drug in practical trials. Second, we did not focus on the analysis of head-to-head comparisons because few original articles with direct comparisons were published (2/33 articles with 317/10 602 participants). Bias is likely to occur in placebo-controlled studies. For example, in placebo-controlled trials, some patients who believe they were assigned to the placebo group left the trial early because of no response, which caused a relatively higher all-cause dropout rate than in head-to-head trials (Cipriani et al., Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson, Ogawa and Geddes2018). Conversely, depressive symptoms tend to improve over time, which causes a high number of responders in the placebo-controlled group (Furukawa et al., Reference Furukawa, Cipriani, Atkinson, Leucht, Ogawa, Takeshima and Salanti2016a). Other explanations may be associated with therapeutic settings, rater bias, etc. (Rutherford & Roose, Reference Rutherford and Roose2013).
Overall, combining adjunctive antipsychotics with antidepressants induces a high response rate but low acceptability and safety in MDD patients with inadequate responses to antidepressants. Among the commonly used drugs, risperidone and aripiprazole are more efficacious and acceptable than other atypical antipsychotics as the augmentation therapy of antidepressants for the management of MDD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722001246
Acknowledgements
We thank Peijing Yan for the methodology instruction.
Author contributions
Yushun Yan was involved in the design of the study, extraction of data, evaluation of risk of bias, statistical analysis, and drafting and revision of the manuscript. Xiao Yang was involved in the extraction of data, evaluation of risk of bias, and statistical analysis. Min Wang and Boran Chen were involved in systematic search and records selection. Li Yin was involved in revising the manuscript. Xiaohong Ma contributed to the design of the study, statistical analysis, and revision of the manuscript. All authors approved the final version of the manuscript.
Financial support
This study was supported by the Key Research and Development Program of the Science and Technology Department of Sichuan Province (Nos. 22ZDYF1531, 22ZDYF1696), the Program of Chengdu Science and Technology (No. 2021-YF05-00272-SN), National Natural Science Foundation of China (No. 82001432), China Postdoctoral Science Foundation (Nos. 2020TQ0213, 2020M683319), the Open Project Program of the National Laboratory of Pattern Recognition (No. 202000034), and West China Hospital Postdoctoral Science Foundation (No. 2020HXBH104).
Conflict of interest
The authors declare that they have no competing interests.