Background
Body dysmorphic disorder (BDD) is a psychiatric disorder in which individuals are preoccupied with perceived defects of their appearance or are excessively concerned about a slight physical abnormality, which causes distress and/or functional impairment (APA, 2000). Perceived defects can involve any body area but most often involve the face or head (Phillips et al. Reference Phillips, McElroy, Keck, Pope and Hudson1993). Individuals experience obsessive thoughts about their appearance and tend to engage in repetitive, time-consuming behaviors such as checking their appearance in the mirror and scrutinizing details of others' appearances to compare to their own (Phillips, Reference Phillips2005). BDD is estimated to affect approximately 0.7–2.4% of the population (Faravelli et al. Reference Faravelli, Salvatori, Galassi, Aiazzi, Drei and Cabras1997; Otto et al. Reference Otto, Wilhelm, Cohen and Harlow2001; Rief et al. Reference Rief, Buhlmann, Wilhelm, Borkenhagen and Brahler2006; Koran et al. Reference Koran, Abujaoude, Large and Serpe2008; Buhlmann et al. Reference Buhlmann, Glaesmer, Mewes, Fama, Wilhelm, Brahler and Rief2010) and is associated with high lifetime rates of psychiatric hospitalization (48%) (Phillips & Diaz, Reference Phillips and Diaz1997) and suicide attempts (22–27.5%) (Veale et al. Reference Veale, Boocock, Gournay, Dryden, Shah, Willson and Walburn1996; Phillips & Diaz, Reference Phillips and Diaz1997; Phillips et al. Reference Phillips, Coles, Menard, Yen, Fay and Weisberg2005a). Very few have good insight and 36–60% are delusional (Gunstad & Phillips, Reference Gunstad and Phillips2003; Phillips et al. Reference Phillips, Menard, Fay and Weisberg2005b; Mancuso et al. Reference Mancuso, Knoesen and Castle2010). Despite the prevalence and severity of the disorder, relatively little is known about the pathophysiology underlying the various symptom domains.
One potentially important clinical symptom domain in BDD is distorted perception of appearance. Individuals with BDD perceive certain features to be defective (APA, 2000), for example their nose is crooked or their skin is pockmarked, contrary to what others perceive of them. In most cases, they seem to focus excessively on details of their appearance at the expense of global or configural aspects (Phillips, Reference Phillips2005). A possible explanation of these misperceptions might lie in visual perceptual abnormalities.
Neuropsychological and brain imaging studies suggest that abnormal information processing may contribute to perceptual abnormalities in BDD (Deckersbach et al. Reference Deckersbach, Savage, Phillips, Wilhelm, Buhlmann, Rauch, Baer and Jenike2000; Yaryura-Tobias et al. Reference Yaryura-Tobias, Neziroglu, Chang, Lee, Pinto and Donohue2002; Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007b, Reference Feusner, Moody, Townsend, McKinley, Hembacher, Moller and Bookheimer2010). Early evidence of abnormalities in own-face processing comes from a study in which individuals with BDD perceived distortions of a digital photograph of their face that were not in fact present (Yaryura-Tobias et al. Reference Yaryura-Tobias, Neziroglu, Chang, Lee, Pinto and Donohue2002). Additional evidence of abnormal visual processing of own faces comes from a functional magnetic resonance imaging (fMRI) study that demonstrated hyperactivity in frontostriatal systems for unaltered images and hypoactivity in visual cortical systems for low spatial frequency (LSF) information that conveys holistic and configural elements (Feusner et al. Reference Feusner, Moody, Townsend, McKinley, Hembacher, Moller and Bookheimer2010). Another fMRI study using others' faces demonstrated left hemisphere hyperactivity relative to healthy controls in higher-order visual processing regions, suggesting predominant detailed and analytic processing (Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007b). These studies suggest abnormalities in visual processing in BDD, at least for symptom-relevant stimuli of own and others' faces.
Aside from abnormalities of face processing, whether individuals with BDD experience general abnormalities in visual processing is not yet clear. Early evidence that this may be the case comes from a study using the Rey–Osterrieth Complex Figure Test (RCFT) to compare visuospatial performance of individuals with BDD relative to healthy controls (Deckersbach et al. Reference Deckersbach, Savage, Phillips, Wilhelm, Buhlmann, Rauch, Baer and Jenike2000). The BDD cohort demonstrated selective recall of details rather than larger organizational design features. This search strategy may implicate a deficit in executive functioning mediated by frontal–striatal circuits or other higher-order perceptual processing systems. Alternatively, deficits may have resulted from encoding an initially distorted lower-order visual perception, or from aberrant retrieval and reconstruction. However, in an earlier study Hanes et al. (Reference Hanes1998) did not find abnormal performance on the RCFT in individuals with BDD relative to healthy controls. Thus, it remains unclear whether individuals with BDD demonstrate abnormal visual processing of objects or figures and, if so, what systems may mediate this. No study to date has investigated the neural correlates of visual processing of non-face/non-body objects in individuals with BDD.
The objective of the current fMRI study was to test whether individuals with BDD have abnormal visual processing for stimuli unrelated to their symptoms, that is non-face/non-body objects. We used houses as the object stimuli because they contain a combination of holistic (entire house as one precept), configural (e.g. spatial relationships between windows and roof) and detailed (e.g. shingles) information from which to understand different elements of visual processing. In addition, multiple previous studies have examined object processing in healthy controls using houses as stimuli, providing a framework of expected networks that includes the parahippocampal place area, fusiform gyrus, lateral occipital cortex, cuneus and lingual gyrus (Epstein et al. Reference Epstein, Harris, Stanley and Kanwisher1999; Ewbank et al. Reference Ewbank, Schluppeck and Andrews2005; Pourtois et al. Reference Pourtois, Schwartz, Seghier, Lazeyras and Vuilleumier2005).
We used digital photographs of houses that were either unaltered or altered to contain only high spatial frequency (HSF) or only LSF information. Detailed visual analysis relies on fine visual resolution, which is conveyed by HSF information (Norman & Ehrlich, Reference Norman and Ehrlich1987; Schyns & Oliva, Reference Schyns and Oliva1999). Configural and holistic elements are primarily conveyed by LSF information (Sergent, Reference Sergent1985; Costen et al. Reference Costen, Parker and Craw1996). We therefore used HSF, LSF and normal spatial frequency (NSF) houses to functionally dissect visual processing systems. Our hypothesis was that individuals with BDD would demonstrate abnormalities for processing houses, similar in nature to that observed in the previous other-face study and therefore possibly representing a general underlying abnormal visual processing phenotype. Specifically, we hypothesized that the BDD group would demonstrate greater left-hemisphere activity in secondary and higher-order occipital and temporal object- and house-processing regions, such as the cuneus, lingual gyrus, fusiform gyrus and parahippocampal place area. Based on what was observed for others' faces, we predicted the greatest degree of abnormalities for LSF images, followed by NSF images, and no difference from controls for HSF images. Based on what was observed for others' and own faces, we predicted that greater symptom severity would be associated with lower activity in the dorsal visual stream for LSF images.
Method
Participants
The UCLA Institutional Review Board approved the protocol for the study. Fourteen participants with BDD and 14 healthy controls, ages 20–48 years, provided informed consent. (All participants had participated in the previous own-face BDD fMRI study; Feusner et al. Reference Feusner, Moody, Townsend, McKinley, Hembacher, Moller and Bookheimer2010.) BDD participants and controls were recruited from the community and were of equivalent gender, age and level of education. All were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, Reference Oldfield1971). BDD participants met DSM-IV criteria for BDD, diagnosed by J.D.F., who has clinical expertise with this population. Diagnoses were made using the BDD module (Phillips et al. Reference Phillips, Atala and Pope1995), a reliable diagnostic module modeled after the Structured Clinical Interview for DSM. In addition, we performed a clinical psychiatric evaluation and screened all participants with the Mini International Neuropsychiatric Inventory (MINI; Sheehan et al. Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller, Hergueta, Baker and Dunbar1998). All BDD participants were required to have a score of ⩾20 on the BDD version of the Yale–Brown Obsessive–Compulsive Scale (BDD-YBOCS; Phillips et al. Reference Phillips, Hollander, Rasmussen, Aronowitz, DeCaria and Goodman1997b). The BDD-YBOCS is a validated scale that is a widely used standard to evaluate symptom severity in BDD, with a range of scores from 0 to 48 (Phillips et al. Reference Phillips, Hollander, Rasmussen, Aronowitz, DeCaria and Goodman1997a). It has excellent inter-rater and test–retest reliability (intra-class correlation coefficients for total score=0.99 and 0.88 respectively), internal consistency (Cronbach's α=0.80), and convergent validity [r=0.55 with the Clinical Global Impression (CGI) scale] (Phillips et al. Reference Phillips, Hollander, Rasmussen, Aronowitz, DeCaria and Goodman1997b). We also administered the 17-item Hamilton Depression Rating Scale (HAMD-17; Hamilton, Reference Hamilton1960), with scores ranging from 0 to 54, and the Hamilton Anxiety Rating Scale (HAMA; Hamilton, Reference Hamilton1969), with scores ranging from 0 to 56. The HAMD-17 and the HAMA have similar standards for validity and reliability, and they were included to provide a broader understanding of the clinical severity of participants. We included participants with delusional beliefs about their appearance (score of 4 on item 11 of the BDD-YBOCS: ‘Lacks insight, delusional’).
Exclusion criteria for all participants included: substance abuse or dependence within the past 12 months, lifetime neurological disorder, pregnancy, or any current medical disorder that may affect cerebral metabolism. We excluded BDD participants with other Axis I disorders [current or past bipolar disorder, current or past panic disorder, current social phobia, current obsessive–compulsive disorder (OCD), current post-traumatic stress disorder, current or past psychotic disorders, current anorexia nervosa or current bulimia nervosa]. We did not exclude those with co-morbid dysthymia, major depressive disorder or generalized anxiety disorder; as depression and anxiety are so frequently co-morbid in this population, we thought it would not be a representative sample to exclude these. However, we required that BDD be the primary diagnosis as defined by the MINI: ‘Which problem troubles you the most or dominates the others or came first in the natural history?’ We excluded any participants whom the investigator judged were suicidal. Healthy controls could not have any current or past Axis I disorder, as determined by the MINI.
BDD participants were free from psychoactive medications for at least 8 weeks prior to the study and were not receiving cognitive-behavioral therapy. Healthy controls were excluded if they were taking any psychiatric medication or were in any psychotherapy treatment. We only included participants with normal or corrected vision, as verified by the Snellen eye chart. Any participants with ferromagnetic metal implantations or devices or weight greater than 280 lb were excluded because of safety and capacity requirements of the MRI scanner.
Stimuli and task
We obtained digitized, frontal view photographs of houses from the Internet and used Adobe Photoshop CS3 software to convert to grayscale. We created HSF and LSF images, as described previously (Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007b). This included using ImageJ software (http://rsbweb.nih.gov/ij/) to Fourier transform digital photographs, alter these images in their frequency domains, and then reverse Fourier transform them to create high-pass and low-pass filtered images (Iidaka et al. Reference Iidaka, Yamashita, Kashikura and Yonekura2004). A ‘high-pass filter’ creates images that contain only the HSF information. A ‘low-pass filter’ creates images that contain only LSF information. We normalized luminosity across stimuli (Fig. 1).

Fig. 1. Examples of the three different categories of house stimuli.
Three different categories of houses comprised the tasks: (A) NSF, (B) HSF and (C) LSF. The control condition consisted of gray rectangular and square shapes, approximately the same size as the houses. We used MacStim 3.0 (White Ant Occasional Publishing, Australia) to program the stimuli presentation and to record accuracy and reaction time. The matching task is a forced-choice, two-alternative task. Each set of stimuli consisted of three houses or shapes: a target house or shape on top and two selection houses or shapes on the bottom. We instructed participants to: ‘Choose the house or shape on the bottom that is the same house or shape as the one on the top, by pressing the right or left button. Make your selection as rapidly and as accurately as possible.’ Each set of houses or shapes appeared on the screen for 4 s [sufficient time to allow for visual inspection and for the task to be easy, yet short enough to minimize habituation of the blood oxygen level-dependent (BOLD) response], with a 1-s interstimulus interval.
Each block consisted of four sets of unique houses of the same spatial frequency that were (A) NSF, (B) HSF or (C) LSF, or four sets of the control task. Each set of four blocks (A–B–C–Control) was repeated four times in each run. The total time for each run was 6 min 8 s. There were two runs, the second presented in a different order. Between participants, differently ordered runs were counterbalanced using a Latin Square design, to avoid possible order effects. Participants wore fMRI-compatible goggles to view the stimuli. If participants wore eyeglasses, appropriate corrective lenses for the goggles were used. After each run, participants rated their anxiety level during the tasks on a Likert scale of 0–10.
fMRI
We used a 3-T Allegra (Siemens) MRI scanner to evaluate BOLD contrast, using a T2*-weighted echo planar imaging (EPI) gradient–echo pulse sequence [repetition time (TR)=2.5 s, echo time (TE)=35 ms, flip angle=90°, matrix=64×64, field of view=24×24 cm, in-plane voxel size 3.1 mm×3.1 mm, slice thickness 3 mm, 1 mm intervening spaces, and 28 total slices]. We obtained matched-bandwidth T2 and Magnetization Prepared Rapid Acquisition Gradient Echo (MP-RAGE) T1-weighted images to provide detailed anatomy during the structural image acquisition.
Image processing included motion correction, skull stripping, spatial smoothing of the 5-mm full-width at half-maximum Gaussian kernel, mean-based intensity normalization of all volumes by the same factor, and high-pass temporal filtering. We co-registered functional images of each participant to corresponding matched-bandwidth structural images in native space, then performed a second-stage registration to their higher-resolution MP-RAGE scans, and finally registered these to structural standard images, defined by the Montreal Neurological Institute (MNI) averaged 152 standard brain.
Data analysis
Behavioral data
We performed a two-way repeated-measures ANOVA to compare response times (RTs) and accuracy rates between groups, using proc glm in SAS (SAS Institute Inc., USA). Group (BDD and healthy controls) was the between-subject factor and stimulus type (NSF, HSF, LSF, and control stimuli) was the within-subject factor.
Anxiety levels
A two-sample t test was used to compare mean subjective anxiety scores between groups.
Functional neuroimaging data
We used FMRI Expert Analysis Tool (FEAT) version 5.4, part of the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl).
For within-group analyses, we performed a random-effects analysis with subject as the random factor to identify typical patterns of brain activity in BDD participants and healthy controls. We modeled the hemodynamic response function by performing a simple convolution of the blocked experimental paradigms of each condition versus control task with the canonical hemodynamic response function and its temporal derivative (Aguirre et al. Reference Aguirre, Zarahn and D'Esposito1998). To determine the patterns of activation in the BDD group and in the control group, we analyzed the normalized data with multiple regression by using three regressors to model hemodynamic changes associated with the HSF, LSF and NSF tasks, each contrasted to the control task.
Model fitting generated whole-brain images in native space of parameter estimates and corresponding variance, representing average signal change during each particular contrast. We used FMRIB's Improved Linear Model (FILM) for time-series statistical analysis, using local autocorrelation correction (Woolrich et al. Reference Woolrich, Brady and Smith2001). We thresholded Z statistic images using clusters determined by Z>2.0 and a (corrected) cluster significance threshold of p=0.05 (Worsley et al. Reference Worsley, Evans, Marrett and Neelin1992).
For between-group analyses, we compared BDD participants and controls directly using a voxel-wise, mixed-effects analysis in FSL. After performing the within-group analyses, we used FMRIB's Local Analysis of Mixed Effects (FLAME) stages 1 and 2 (Beckmann et al. Reference Beckmann, Jenkinson and Smith2003; Woolrich et al. Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith2004). We thresholded Z statistic images using clusters determined by Z>2.0, and a (corrected) cluster significance threshold of p=0.05 (Worsley et al. Reference Worsley, Evans, Marrett and Neelin1992). A two-sample t test identified group mean differences in activity at each voxel.
To represent graphically BOLD signal changes in regions identified from the voxel-wise analysis as differentially active between groups, we performed post-hoc percentage signal change analyses. For this we created a set of spherical regions of interest (ROIs) (6 mm radii) at local maxima for significant clusters from the between-group analyses. Parameter estimate data were then extracted from each ROI for each participant using FSL command line tools (Poldrack, Reference Poldrack2007).
To investigate the relationship between symptom severity in BDD participants and regional brain activation, we entered results from the within-group analysis into a higher-level analysis with de-meaned BDD-YBOCS scores as a covariate of interest. This produced a voxel-wise map of regions whose activity correlated with BDD symptom severity. We also extracted post-hoc percentage signal changes from local maxima (as above) to create scatterplots depicting the relationship between symptom severity and brain activity across BDD participants.
Results
Demographics and psychometrics
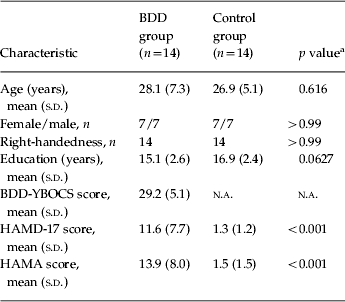
Table 1 summarizes the demographic and psychometric data. One BDD participant had co-morbid major depressive disorder, one had generalized anxiety disorder, four had major depressive disorder and generalized anxiety disorder, and one had both dysthymic disorder and generalized anxiety disorder. All participants had preoccupations with perceived facial defects.
Table 1. Demographics and psychometric scores
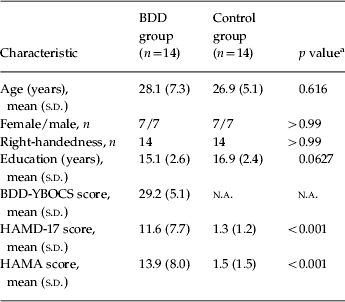
BDD, Body dysmorphic disorder; BDD-YBOCS, BDD version of the Yale–Brown Obsessive–Compulsive Scale; HAMD-17, 17-item Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Rating Scale; s.d., standard deviation; n.a., not applicable.
a t test for all comparisons except gender and handedness (χ2 test).
Behavioral data
Matching task
The BDD group had slower mean RTs across all tasks including the control task (1.28±0.22 v. 1.03±0.35 s for NSF, 1.26±0.22 v. 0.99±0.37 s for LSF, 1.65±0.33 v. 1.39±0.47 s for HSF, and 1.22±0.23 v. 0.96±0.30 s for control stimuli, for BDD and healthy control groups respectively). There were significant main effects of group (F 1,26=5.14, p=0.0320) (slower RT for the BDD group) and stimulus (F 3,78=79.41, p<0.0001) (slower RT for HSF images) but no group by stimulus interaction effect (F 3,78=0.02, p=0.996).
Mean accuracy rates were similar between groups across all stimuli (99.33±1.81% v. 99.09±1.50% for NSF, 99.33±1.33% v. 98.42±2.07% for LSF, 98.73±1.55% v. 98.88±1.56% for HSF, and 96.91±2.88% v. 96.36±3.93% for control stimuli, for BDD and healthy control groups respectively). There was a significant main effect of stimulus (F 3,78=8.02, p⩽0.0001) (lower accuracy for control stimuli) but no significant main effect of group (F 1,26=0.77, p=0.389) or group by stimulus interaction (F 3,78=0.29, p=0.829). In the BDD group, correlations were non-significant between RT (collapsed across all stimuli because of absence of a group by stimulus interaction effect) and BDD-YBOCS scores (r=0.34, p=0.24), HAMD scores (r=0.29, p=0.31), HAMA scores (r=0.16, p=0.60), and task anxiety (r=−0.14, p=0.62).
Anxiety levels
Mean anxiety during the experiment was slightly higher for the BDD than the healthy control group but not significantly different (3.32±2.45 v. 2.04±1.88, t=1.55, df=26, p=0.13). We explored using task anxiety as a covariate in the two-way repeated measures ANOVA. When controlling for anxiety, there were still significant main effects of group (F 1,25=5.88, p=0.0229) (slower RT for the BDD group) and stimulus (F 3,75=29.86, p<0.0001) (slower RT for HSF images) but no group by stimulus interaction effect (F 3,75=0.05, p=0.977) for RT. For accuracy rate, when controlling for anxiety there was a significant main effect of stimulus (F 3,75=4.83, p⩽0.0119) (lower accuracy for control stimuli) but no significant main effects of group (F 1,25=0.59, p=0.451) or group by stimulus interaction (F 3,75=0.62, p=0.543).
fMRI
Within-group analyses
For all stimulus types, the BDD and healthy control groups activated extensive bilateral visual cortex, in addition to the fusiform cortex and parahippocampal gyri. There was also activation for both groups for NSF and HSF (but not LSF) images evident in subcortical (thalamic) and prefrontal (paracingulate, supplementary motor area, and inferior frontal gyrus) regions. For HSF images, activation in bilateral frontal poles was evident for the BDD but not the healthy control group.
Between-group analyses
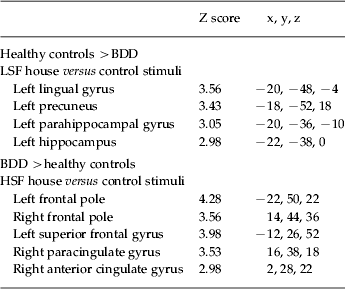
There were no significant differences between groups for NSF stimuli. For the LSF images, the BDD group relative to the healthy control group showed lower activity in the left parahippocampal cortex, the left hippocampus, left lingual gyrus, left posterior cingulate and bilateral precuneus (Fig. 2 a, Table 2). This was accounted for primarily by lower activation in the BDD group relative to healthy controls in these regions (see percentage signal changes, Fig. 2 b).

Fig. 2. Significant differences in regional brain activity between body dysmorphic disorder (BDD) and healthy control groups. (a) Lower activation for BDD relative to control participants (blue) for low spatial frequency (LSF) stimuli in the left lingual gyrus and right precuneus (top image), and left parahippocampal gyrus and left hippocampal gyrus (middle image). There was greater activation for BDD than control participants (orange) for high spatial frequency (HSF) stimuli in the left frontal pole (middle image), left superior frontal gyrus (not shown), and right anterior cingulate gyrus and right paracingulate gyrus (bottom image). (See Table 2 for list of all unique local maxima.) (b) Percentage signal changes for several regions found to be significant between groups in the voxel-wise analysis for LSF (top) and HSF (bottom) stimuli.
Table 2. Local maxima for significant between-group differences
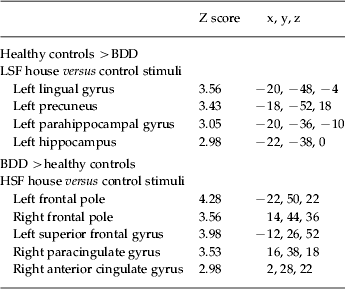
BDD, Body dysmorphic disorder; LSF, low spatial frequency; HSF, high spatial frequency.
Only local maxima from unique regions are represented. There were no significant differences for normal spatial frequency (NSF) images.
For HSF images, BDD participants relative to controls showed greater activity in the bilateral frontal pole, left superior frontal gyrus, right anterior cingulate gyrus and right paracingulate gyrus (Fig. 2 a, Table 2). This was accounted for by activation in the BDD group relative to the control task and deactivation in healthy controls relative to the control task in these regions (see percentage signal changes, Fig. 2 b). To verify whether this pattern represented deactivation in the healthy controls and activation for the BDD group for the HSF task versus ‘true’ baseline (as opposed to HSF versus control task), we performed a post-hoc analysis contrasting the HSF task and also the control task to the rest periods of the scan (during which participants viewed a small crosshair in the middle of the screen). Within-group results revealed deactivation in the healthy controls for both HSF and control tasks in the bilateral dorsal and ventral medial prefrontal cortex, precuneus/posterior cingulate and angular gyrus. The BDD group demonstrated similar deactivations in these regions for both HSF versus baseline and control task versus baseline, except for the dorsal medial prefrontal cortex (see Fig. 5 available as supplementary online material). There were no significant differences between groups for HSF versus baseline or for the control task versus baseline.
RT covariate analysis
As there were behavioral differences between groups in RTs on all tasks, we performed a between-groups analysis using RT on the tasks as a covariate of non-interest. For the LSF analysis, there were no significant differences between groups at a threshold of Z>2.0, but at Z>1.9 there was relative hypoactivation in the BDD group in the left lingual gyrus, the left parahippocampal gyrus, the left hippocampus and the left precuneus (similar to the regional activation without RT as a covariate). For the HSF analysis, the regional activation was essentially unchanged in the prefrontal regions compared to the results without RT as a covariate. For the NSF analysis, there remained no significant differences between groups.
Correlation with BDD-YBOCS
For NSF images, there were inverse associations between BDD-YBOCS scores and activity in the bilateral middle frontal gyrus, right lateral dorsal occipital cortex, bilateral orbitofrontal cortex, left anterior cingulate gyrus, left precentral gyrus and left inferior frontal gyrus (Fig. 3 a; see also Fig. 4 and Table 3). For LSF images, there were no significant associations between BDD-YBOCS scores and brain activity. For HSF images, there were inverse associations between BDD-YBOCS scores and activity in the left lateral occipital cortex, bilateral occipital pole, right dorsal cuneus, left inferior frontal gyrus and left frontal pole (Fig. 3 b; see also Fig. 4 and Table 3). There were no significant positive associations between BDD-YBOCS scores and brain activity for any image type.

Fig. 3. Percentage signal change as a function of symptom severity. The percentage signal change (y axis) in regions found to be significantly associated with symptom scores on the body dysmorphic disorder version of the Yale–Brown Obsessive–Compulsive Scale (BDD-YBOCS) (x axis) for (a) normal spatial frequency (NSF) and (b) high spatial frequency (HSF) images. No regions were significantly associated with low spatial frequency (LSF) images. (Note that in b the left lateral occipital cortex, right cuneus and left occipital pole are not depicted because the local maxima were on cortical surface regions such that spherical region of interest (ROI) extraction for percentage signal change would not be valid.)

Fig. 4. Regions significantly associated with body dysmorphic disorder (BDD) symptom severity in the BDD group. Symptom severity measured by the body dysmorphic disorder version of the Yale–Brown Obsessive–Compulsive Scale (BDD-YBOCS). There were inverse relationships between symptom severity and activity for high spatial frequency (HSF) images (yellow) and normal spatial frequency (NSF) images (purple). Areas of overlap between the two image types appear in red. There were no regions positively associated with symptom severity for any image type, and no regions positively or inversely associated with symptom severity for low spatial frequency (LSF) images.
Table 3. Local maxima for regions inversely associated with BDD symptom severity

BDD, Body dysmorphic disorder; NSF, normal spatial frequency; HSF, high spatial frequency.
Local maxima associated with regions inversely associated with BDD symptom severity in the BDD group, as measured by the body dysmorphic disorder version of the Yale–Brown Obsessive–Compulsive Scale (BDD-YBOCS). Only local maxima from unique regions are represented. There were no regions positively associated for any image type and no regions inversely associated with BDD-YBOCS scores for low spatial frequency (LSF) images.
Discussion
This study demonstrates that unmedicated individuals with BDD have abnormal brain activation patterns when viewing non-face/non-body objects. The primary finding is less activation than healthy controls in secondary visual processing systems for configural and holistic elements. Moreover, greater BDD symptom severity is associated with lower activity in the dorsal visual stream and in ventrolateral prefrontal regions. Because images of houses are not symptom-related stimuli, the current study therefore suggests the possibility of general, aberrant visual processing in BDD.
These results confirmed our hypothesis of abnormal activity for LSF information in occipital and temporal visual processing systems in individuals with BDD. Contrary to our predictions, however, a pattern of predominant left hemisphere hyperactivity was not evident; instead, there was abnormal hypoactivity in the left lingual gyrus and parahippocampal cortex. The parahippocampal cortex (in addition to a distributed network in the bilateral ventral temporal and occipital cortex; Ishai et al. Reference Ishai, Ungerleider, Martin, Schouten and Haxby1999, Reference Ishai, Ungerleider, Martin and Haxby2000) has been identified as an important region for recognition and encoding of places, scenes and houses, and has been termed the ‘parahippocampal place area’ (Epstein et al. Reference Epstein, Harris, Stanley and Kanwisher1999; Ewbank et al. Reference Ewbank, Schluppeck and Andrews2005; Pourtois et al. Reference Pourtois, Schwartz, Spiridon, Martuzzi and Vuilleumier2009). The BDD group also demonstrated relative hypoactivation for LSF information in systems involved in directed and sustained attention including the posterior cingulate and precuneus (Cabeza & Nyberg, Reference Cabeza and Nyberg2000; Cavanna & Trimble, Reference Cavanna and Trimble2006). Together, these findings provide evidence that individuals with BDD may underactivate systems involved in secondary visual processing and attention, specifically for configural and holistic visual elements.
The nature of these findings fits with results from a previous neuropsychological study of complex figure processing using the RCFT, in which individuals with BDD seemed to underutilize configural and holistic elements while selectively recalling and reproducing visual details (Deckersbach et al. Reference Deckersbach, Savage, Phillips, Wilhelm, Buhlmann, Rauch, Baer and Jenike2000). It also fits with clinical observation that they often focus on details of their appearance, for example small skin blemishes or small asymmetries of their nose, at the expense of global or configural aspects.
For HSF information, there were differences in patterns of brain activation between groups. However, the post-hoc between-groups analysis of the HSF task relative to a low-level baseline (as opposed to the matching task of circles and ovals) revealed that there were no significant differences. The within-groups post-hoc analysis demonstrates that the overall pattern of deactivation in both groups represents task-related deactivation in the default mode network (DMN); this includes the medial prefrontal cortex, precuneus/posterior cingulate and inferior parietal lobule. Deactivation of the DMN is commonly observed in a wide variety of cognitive tasks, and activation is evident during rest (see Buckner et al. Reference Buckner, Andrews-Hanna and Schacter2008 for a review). The within-groups analysis in this study suggests that there may have been lower deactivation in dorsal medial prefrontal regions in the BDD group. Thus, it is possible that the BDD group may be less able to deactivate specifically the dorsal medial prefrontal portions of the DMN when engaging in the task. As a subsystem of the DMN involving the dorsal medial prefrontal cortex is thought to be involved in self-referential thinking (Castelli et al. Reference Castelli, Happe, Frith and Frith2000; Gusnard et al. Reference Gusnard, Akbudak, Shulman and Raichle2001), one speculation about these findings is that the BDD group may have difficulty in switching out of self-referential thinking during the task, perhaps related to intrusive thoughts about themselves or other spontaneous stimulus-independent thoughts (McGuire et al. Reference McGuire, Paulesu, Frackowiak and Frith1996; Andrews-Hanna et al. Reference Andrews-Hanna, Reidler, Huang and Buckner2010).
Greater BDD symptom severity was associated with lower activity in the dorsal occipital cortex for the HSF and NSF images, although not for the LSF images, as hypothesized. Dorsal occipital cortex and parieto-occipital regions, part of the dorsal visual stream (Ungerleider & Mishkin, Reference Ungerleider, Mishkin, Ingle, Goodale and Mansfield1982), are involved in motion perception and extracting shapes and three-dimensional cues from objects (Sereno et al. Reference Sereno, Trinath, Augath and Logothetis2002; Peuskens et al. Reference Peuskens, Claeys, Todd, Norman, Van Hecke and Orban2004; Durand et al. Reference Durand, Nelissen, Joly, Wardak, Todd, Norman, Janssen, Vanduffel and Orban2007). Such information extracted from objects is then integrated with information related to object identification and recognition extracted through the ventral visual stream (see Farivar, Reference Farivar2009 for review). Therefore, greater BDD symptom severity may be associated with abnormalities in extracting configural visual information within unaltered and HSF images related to shape.
Greater BDD symptom severity was also associated with lower activity in ventrolateral prefrontal regions. Previous studies have found that the ventrolateral prefrontal cortex (and to a lesser extent the dorsolateral prefrontal cortex) is important for selection, comparison and judgment, as evidenced by involvement in simultaneous matching tasks (Corbetta et al. Reference Corbetta, Miezin, Dobmeyer, Shulman and Petersen1991; Haxby et al. Reference Haxby, Horwitz, Ungerleider, Maisog, Pietrini and Grady1994; Kosslyn et al. Reference Kosslyn, Alpert, Thompson, Chabris, Rauch and Anderson1994; Grady et al. Reference Grady, Horwitz, Pietrini, Mentis, Ungerleider, Rapoport and Haxby1996; McIntosh et al. Reference McIntosh, Grady, Haxby, Ungerleider and Horwitz1996; Rushworth et al. Reference Rushworth, Nixon, Eacott and Passingham1997) and in tasks involving working memory and short- and long-term memory (Haxby et al. Reference Haxby, Ungerleider, Horwitz, Rapoport and Grady1995; Petrides, Reference Petrides1996; Rushworth et al. Reference Rushworth, Nixon, Eacott and Passingham1997). These are regions that are involved in face, form, color and spatial matching (Corbetta et al. Reference Corbetta, Miezin, Dobmeyer, Shulman and Petersen1991; Haxby et al. Reference Haxby, Horwitz, Ungerleider, Maisog, Pietrini and Grady1994; Kosslyn et al. Reference Kosslyn, Alpert, Thompson, Chabris, Rauch and Anderson1994; Grady et al. Reference Grady, Horwitz, Pietrini, Mentis, Ungerleider, Rapoport and Haxby1996) and have connections to visual processing regions in the temporal and occipital lobes (Webster et al. Reference Webster, Bachevalier and Ungerleider1994; Catani et al. Reference Catani, Howard, Pajevic and Jones2002). A possible interpretation of the results, then, is that greater severity of BDD symptoms may be associated with impaired integration of visual and prefrontal systems.
As this is the first functional neuroimaging study to investigate object processing in BDD, other similar studies are not available for comparison. However, fMRI studies of other-face processing (Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007b) and own-face processing (Feusner et al. Reference Feusner, Moody, Townsend, McKinley, Hembacher, Moller and Bookheimer2010) have demonstrated both similar and different patterns. For example, in the own-face processing study, the BDD group had similar relative visual cortical hypoactivity in left primary and secondary visual processing regions for LSF images as in this study, although in the posterior and inferior regions of the occipital cortex (Feusner et al. Reference Feusner, Moody, Townsend, McKinley, Hembacher, Moller and Bookheimer2010). In contrast to the findings in this study, there were no significant differences from controls for HSF stimuli. In that study subjective aversiveness of faces was inversely associated with activity in the bilateral dorsal lateral occipital cortex and precuneous for LSF images. In addition, greater BDD symptom severity was inversely associated with activity in the left dorsal occipital cortex and the right lateral occipital cortex for the LSF images, whereas in this study there were similar inverse correlations although with HSF and NSF images.
In the other-face processing study, the BDD group, compared to healthy controls, had greater left hemisphere activity in temporal, parietal and prefrontal regions compared to healthy controls; group differences were most prominent for LSF stimuli but were also evident for NSF and to a lesser extent HSF stimuli (Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007b). By contrast, in the current study there was lower left hemisphere activity for LSF images in temporal regions. In the other-face study there was relative hypoactivity in the left and right occipital cortex for NSF stimuli, as in the current study, although not in the dorsal visual stream. Greater BDD symptom severity was found to be associated with lower activity in left occipital and parieto-occipital (dorsal) regions for LSF other-face images (Feusner et al. Reference Feusner, Townsend, Bystritsky and Bookheimer2007a), although not for HSF and NSF images, as in the current study.
In total, these own- and other-face studies and the current houses study share evidence of abnormal visual cortical hypoactivity, in addition to lower dorsal visual stream activity associated with greater symptom severity. However, the observation that specific aspects of abnormal visual processing in BDD are not identical across house, own-face and other-face stimuli may be due to the differential relevance in the context of their symptoms. For example, own-face stimuli are directly relevant to core appearance concerns regarding the face, and therefore may evoke emotional arousal and/or urges to engage in compulsive behaviors. Other-face stimuli may also be relevant to their symptoms, albeit indirectly, as individuals with BDD typically engage in scrutiny of others' faces to compare to their own. These symptom-relevant stimuli may engage prefrontal and/or frontostriatal systems and may lead to secondary top-down modulation of visual processing systems. House stimuli, which are not related to appearance preoccupations and are less likely to elicit emotional arousal or urges to engage behavioral sequences, may therefore be less likely to result in top-down modulation (however, this remains to be tested directly). Visual cortical abnormalities in the current study of house processing suggest the possibility of general, lower-level visual processing abnormalities in BDD, although it is still possible that top-down attentional modulation may affect these secondary visual systems.
Similar experiments using non-face/non-body objects have not yet been conducted in other disorders that share overlapping phenotypes with BDD, such as OCD, other anxiety disorders and eating disorders. However, neuropsychological studies of visuospatial functioning have found similar detail-focused, piecemeal processing strategies on the RCFT in BDD (Deckersbach et al. Reference Deckersbach, Savage, Phillips, Wilhelm, Buhlmann, Rauch, Baer and Jenike2000), OCD (Savage et al. Reference Savage, Deckersbach, Wilhelm, Rauch, Baer, Reid and Jenike2000; Mataix-Cols et al. Reference Mataix-Cols, Alonso, Hernandez, Deckersbach, Savage, Manuel Menchon and Vallejo2003) and anorexia nervosa (Sherman et al. Reference Sherman, Savage, Eddy, Blais, Deckersbach, Jackson, Franko, Rauch and Herzog2006). These implicate abnormalities in executive functioning and/or visual memory encoding (Deckersbach et al. Reference Deckersbach, Savage, Phillips, Wilhelm, Buhlmann, Rauch, Baer and Jenike2000). In addition, several neuroimaging studies in OCD have found possible abnormalities in visual processing systems, including below-normal glucose metabolic activity in parieto-occipital regions (Nordahl et al. Reference Nordahl, Benkelfat, Semple, Gross, King and Cohen1989; Kwon et al. Reference Kwon, Kim, Lee, Lee, Lee, Kim, Lyoo, Cho and Lee2003) and abnormal hyperactivity when viewing biological motion in the ventral visual stream (left fusiform and left inferior temporal gyrus) (Jung et al. Reference Jung, Gu, Kang, Park, Yoo, Choi, Lee and Kwon2009). Future studies in these related disorders using similar stimuli as in the current study may be useful to explore possible shared phenotypes or endophenotypes of abnormal visual processing.
Behaviorally, the BDD group was slower on all tasks including the control task. It is unlikely that the results obtained for the BOLD signal were affected by offset of timing behaviorally because it was a blocked design and we used temporal derivatives. Moreover, using RT as a covariate of non-interest, the findings in prefrontal regions for HSF images were essentially unchanged. The findings in secondary visual processing systems for LSF images were also essentially unchanged, although the power was reduced. This suggests that the between-group differences are not just a function of slower behavioral responses for configural/holistic information.
This study has several limitations. The cross-sectional design precludes causal understandings of whether these abnormalities represent an endophenotype that predisposes individuals to develop BDD, or are secondary results of having BDD symptoms. Another limitation is that the temporal resolution of fMRI does not allow the determination of whether observed abnormalities are the result of top-down modulation of temporal and occipital systems. The sample size may have limited our power to detect small differences between groups; however, this may not have been the case as a different study of 16 individuals with autism spectrum and 16 healthy controls using similar house stimuli found statistically significant differences of 0.02% signal change (Bird et al. Reference Bird, Catmur, Silani, Frith and Frith2006). Because of the exploratory nature of this study as the first to investigate non-face/non-body object processing in BDD, and the fact that our hypotheses involved multiple regions that may be involved in distributed networks, we did not use a priori ROIs to make use of small-volume corrections. It is possible that group differences in brain activity may have been influenced by different behavioral eye-tracking patterns; the use of an eye-tracking camera in future studies would help to clarify this.
In conclusion, this study suggests that unmedicated individuals with BDD may have general abnormalities in higher- and lower-order visual processing, beyond that for their own appearance or for faces in general. These findings are consistent with a model of imbalances in global versus local processing, which may exist for symptom-related and non-related visual stimuli. Future studies using techniques with higher temporal resolution such as electroencephalography (EEG) may help to elucidate whether visual cortical abnormalities are the result of top-down modulation. Future studies will also be useful to characterize the inherited versus acquired nature of these findings, for example by studying unaffected first-degree relatives or twins or by studying individuals very early in their development of the disorder.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).
Acknowledgments
Funding for this study was provided by the National Institute for Mental Health (5K23MH079212 to J. D. Feusner). We thank S. Bookheimer and C. Sheen for their comments on the manuscript.
Declaration of Interest
None.