Introduction
Study of a host–parasite relationship can lead to a better understanding of how present parasite distributions have formed and in turn shed light on the biogeography and evolutionary history of the parasite (Hoberg and Brooks, Reference Hoberg, Brooks, Morand and Krasnov2010). Migratory vertebrates, especially birds, have been deemed to be major promoters of long-distance dispersal (LDD) events for parasites (Dietrich et al., Reference Dietrich, Gomez-Diaz and McCoy2011; Gillespie et al., Reference Gillespie, Baldwin, Waters, Fraser, Nikula and Roderick2012; Viana et al., Reference Viana, Santamaria and Figuerola2016), and birds provide many habitats for diverse ecto- and endo-parasitic metazoans (Crompton, Reference Crompton, Clayton and Moore1997). Leeches, which are blood-feeding/predatory annelid worms, include several bird–parasitic species (Sawyer, Reference Sawyer1986; Davies et al., Reference Davies, Govedich, Moser, Atkinson, Thomas and Hunter2008). The latest systematic framework has been used to classify leeches into five suborders (Tessler et al., Reference Tessler, de Carle, Voiklis, Gresham, Neumann, Cios and Siddall2018a): the proboscidate Glossiphoniiformes and Oceanobdelliformes, jawed Hirudiniformes, and jawless Americobdelliformes and Erpobdelliformes. Members of Glossiphoniiformes and Hirudiniformes are known to parasitize birds. Glossiphoniiform leeches, belonging to the genus Theromyzon, infest the nasopharynges and eyes of various waterfowls (see Davies et al., Reference Davies, Govedich, Moser, Atkinson, Thomas and Hunter2008) and are believed to use their avian hosts as transport for circumboreal distribution (Sawyer, Reference Sawyer1986). A hirudiniform Parapraobdella lineata (Sciacchitano, 1959), which inhabits southern Africa and is classified within the family Praobdellidae (Phillips et al., Reference Phillips, Oosthuizen and Siddall2011), was found infesting an orbital cavity of a migratory bird, Phoenicurus phoenicurus (Linnaeus, 1758) (Siddall et al., Reference Siddall, Rood-Goldman, Barrio and Barboutis2013); this highlighted the possibility that praobdellid leeches promote and shape their distribution via bird migrations. The hirudiniform species of Ornithobdellidae are also considered to feed on birds' blood (Benham, Reference Benham and Chilton1909).
Blood-sucking terrestrial leeches belonging to the hirudiniform family Haemadipsidae are widely distributed throughout Indo-Pacific forests (Borda and Siddall, Reference Borda and Siddall2011) and have been observed to feed on avian species (Sawyer, Reference Sawyer1986; Janovy, Reference Janovy, Clayton and Moore1997; Davies et al., Reference Davies, Govedich, Moser, Atkinson, Thomas and Hunter2008). Recent molecular phylogenetic studies revealed that haemadipsids are composed of three phylogroups (Borda and Siddall, Reference Borda and Siddall2011; Tessler et al., Reference Tessler, Barrio, Borda, Rood-Goldman, Hill and Siddall2016; Huang et al., Reference Huang, Liu, Gong, Wu, Liu, Deng, Zhang, Peng, Zhang and Liu2019): (1) the trignathous (three-jawed) genus Haemadipsa, (2) the trignathous Sinospelaeobdella and Tritetrabdella, and (3) the duognathous (two-jawed) Chtonobdella. In contrast to the trignathous haemadipsid leeches, whose distribution is restricted to Oriental and Sino-Japanese regions, the duognathous haemadipsids are widely distributed throughout the Indo-Pacific area (Borda and Siddall, Reference Borda and Siddall2011), ranging from Madagascar in the southwestern Indian Ocean (Borda, Reference Borda2006) to the Juan Fernandez Islands in the southeastern Pacific (Johansson, Reference Johansson and Skottsberg1924). Therefore, the passive LDD of Chtonobdella leeches by birds has been suggested, given the leeches' wide distribution and endemicities to Indo-Pacific oceanic islands (Lande, Reference Lande1994; Borda et al., Reference Borda, Oceguera-Figueroa and Siddall2008; Borda and Siddall, Reference Borda and Siddall2011). However, little is known about the host–parasite relationships between birds and leeches. Migratory seabirds belonging to Procellariiformes appear to be potential hosts for Chtonobdella skottsbergi (Johansson, Reference Johansson and Skottsberg1924) inhabiting the Juan Fernandez Islands (Ringuelet, Reference Ringuelet1955). To our knowledge, however, almost all birds recorded as being infested by Chtonobdella leeches have been non-migratory passerines (Passeriformes) and flightless cassowaries (Casuariiformes) (Richardson, Reference Richardson1975). Although invertebrate-derived DNA (iDNA) analyses have accelerated our understanding of the hidden host–parasite relationships among vertebrate hosts and haemadipsid leeches (Schnell et al., Reference Schnell, Sollmann, Calvignac-Spencer, Siddall, Yu, Wilting and Gilbert2015; Tessler et al., Reference Tessler, Weiskopf, Berniker, Hersch, McCarthy, Yu and Siddall2018b), the avian host species detected by previous iDNA studies (Schnell et al., Reference Schnell, Bohmann, Schultze, Richter, Murray, Sinding, Bass, Cadle, Campbell, Dolch, Edwards, Gray, Hansen, Hoa, Noer, Heise-Pavlov, Sander Pedersen, Ramamonjisoa, Siddall, Tilker, Traeholt, Wilkinson, Woodcock, Yu, Bertelsen, Bunce and Gilbert2018; Fahmy et al., Reference Fahmy, Ravelomanantsoa, Youssef, Hekkala and Siddall2019) were also sedentary or flightless.
Migratory procellariiform seabirds with eyes heavily infested by segmented worms have been found at several localities in the Bonin Islands, Honshu Island and Okinawa Island of Japan. Because these parasites possessed obvious caudal suckers, five pairs of eyes forming a parabolic arc in the head region and laterally situated nephridiopores, they were definitively identified as haemadipsid species. Haemadipsa zeylanica ivosimae Oka, Reference Oka1930 was described from Kita-Ioto Island in the Volcano Islands (Oka, Reference Oka1930), located more than 150 km southwest of the Bonin Islands (Fig. 1); however, its systematic status remains uncertain (Nakano, Reference Nakano, Motokawa and Kajihara2017). The current study provides a taxonomic account of newly collected haemadipsids for both molecular phylogenetic analyses and morphological examination. The host–parasite relationships between the migratory seabirds and the haemadipsids presented in this study will help us to elucidate features of the passive LDD of these terrestrial blood-suckers.
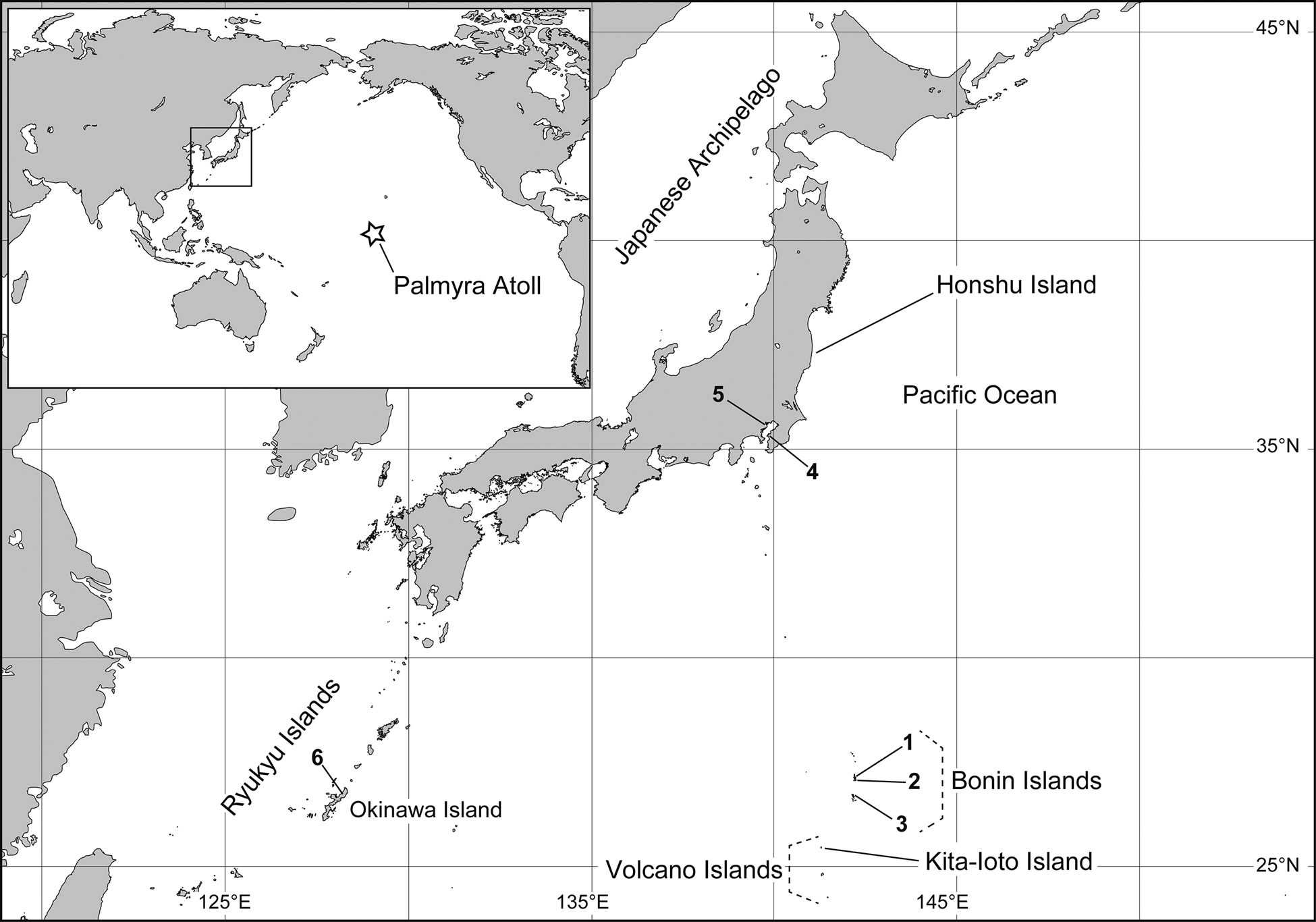
Fig. 1. Map showing sampling localities for Chtonobdella palmyrae (Richardson) infesting seabirds from Japan; the white star denotes the type locality of the species.
Materials and methods
Sampling and morphological examination
Haemadipsid leeches were obtained from the eyes or other mucous membranes of procellariiform seabirds captured at six locations around Japan from August 2000 to October 2016. The captured host seabirds had been weakened by leech infestations. Most of the leech specimens were directly fixed in 70–90% ethanol. For DNA extraction, botryoidal tissue was removed from around the anterior oral sucker or posterior caudal sucker, and preserved in absolute ethanol. The reminder of the body was re-fixed in 10% formalin and preserved in 70% ethanol.
Four measurements were taken: body length (BL) from the anterior margin of the oral sucker to the posterior margin of the caudal sucker, maximum body width (BW), caudal sucker length (CL) from the anterior to the posterior margin of the caudal sucker and caudal sucker width (CW) from the right to the left margin of the caudal sucker. Examination, dissection and drawing of the specimens were conducted using a stereoscopic microscope with a drawing tube (Leica M125). The leech specimens and a seabird host examined in this study were deposited in the Zoological Collection of Kyoto University (KUZ).
The type series of H. z. ivosimae was not found in Oka's small leech collection kept at The University Museum, The University of Tokyo (Nakano and Itoh, Reference Nakano and Itoh2011). The reminder of the collection housed at the National Museum of Nature and Science, Tsukuba, Japan (NSMT) (see Nakano, Reference Nakano2010) was also searched to locate the type series, however, it is believed that the series may have been lost or destroyed in the past.
The numbering convention is based on Moore (Reference Moore, Harding and Moore1927): body somites were denoted by Roman numerals, and the annuli in each somite were given alphanumeric designations.
PCR and DNA sequencing
Genomic DNA was extracted from the botryoidal tissues preserved in absolute ethanol following the methods described by Nakano (Reference Nakano2012a). The primer sets for the polymerase chain reaction (PCR) and cycle sequencing reactions for the nuclear 18S rRNA (18S), 28S rRNA (28S) and histone H3 (H3) and mitochondrial cytochrome c oxidase subunit I (COI) used in this study were taken from previous studies (Nakano, Reference Nakano2016; Nakano et al., Reference Nakano, Jeratthitikul, Nguyen and Panha2016). PCR and DNA sequencing were performed using a modified version of a method mentioned in Nakano (Reference Nakano2012b), and detailed conditions were identical to those in previous studies (Nakano, Reference Nakano2016; Nakano et al., Reference Nakano, Jeratthitikul, Nguyen and Panha2016). In total, 27 new sequences were obtained from the haemadipsid leeches and deposited in the International Nucleotide Sequence Database Collaboration (INSDC) through the DNA Data Bank of Japan with the following INSDC accession numbers: 18S sequence LC414431, 28S sequence LC414432, H3 sequence LC414433 and 24 COI sequences LC414407–LC414430.
Molecular phylogenetic and network analyses
The phylogenetic position of the newly collected haemadipsid leeches within the genus Chtonobdella was estimated based on the 18S, 28S and COI sequences; the H3 sequence of the present specimen was not included due to the lack of those of other in-group taxa. The in-group taxa were selected from the datasets analysed in previous molecular phylogenetic studies of Haemadipsidae (Borda and Siddall, Reference Borda and Siddall2011; Tessler et al., Reference Tessler, Barrio, Borda, Rood-Goldman, Hill and Siddall2016) (Table S1 in Supplementary material). According to the previous results (Borda and Siddall, Reference Borda and Siddall2011; Tessler et al., Reference Tessler, Barrio, Borda, Rood-Goldman, Hill and Siddall2016), four Tritetrabdella species and Sinospelaeobdella cavatuses (Yang, Mo and Wang, 2009) were used as the outgroup. The 18S, 28S and COI sequences were aligned using MAFFT version 7.402 L-INS-i (Katoh and Standley, Reference Katoh and Standley2013). The lengths of the 18S, 28S and COI sequences were 1833, 2093 and 1246 bp, respectively. The concatenated sequences yielded 5172 bp of aligned positions.
Phylogenetic trees were inferred using maximum likelihood (ML) and Bayesian inference (BI). The best-fit partition scheme and substitution models were identified with the Bayesian information criterion using PartitionFinder version 2.1.1 (Lanfear et al., Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2017) with the ‘greedy’ algorithm (Lanfear et al., Reference Lanfear, Calcott, Ho and Guindon2012): for 18S, SYM + I + G; for 28S, GTR + I + G; for COI first position, TIM + I + G (GTR + I + G for BI); for COI second position, F81 + I and for COI third position, TRN + G (GTR + G for BI). The ML phylogeny was inferred using IQ-TREE version 2.0.5 (Minh et al., Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020) with non-parametric bootstrapping (BS) conducted with 1000 replicates. BI and Bayesian posterior probabilities (PPs) were estimated using MrBayes version 3.2.7a (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Two independent runs of four Markov chains were conducted for 20 million generations, and the tree was sampled every 100 generations. The parameter estimates and convergence were checked using Tracer version 1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018), and the first 50 001 trees were discarded based on the results.
Relationships among the COI haplotypes (1271 bp) obtained from the present leech specimens were estimated by a statistical parsimony network using PopART version 1.7 (Leigh and Bryant, Reference Leigh and Bryant2015). According to the results of our molecular phylogenetic analyses, further relationships between the Japanese COI haplotypes and the previously published COI sequence (HQ203158) of Chtonobdella palmyrae (Richardson, Reference Richardson1975) were calculated based on 985 bp alignment positions by statistical parsimony network using PopART.
Results
Leech infestation records
In total, 25 haemadipsid leeches were collected from their attachment sites on the eyes or in the throat of procelariid or hydrobatid seabirds (Table 1) from six localities in Japan. Among the specimens, 16 leeches were collected from seabirds captures in the Bonin Islands (Fig. 1). One specimen (KUZ Z1648) was obtained from the eye of a Bonin petrel, Pterodroma hypoleuca (Salvin, 1888), captured on Chichijima Island on 16 August 2012; and the other three individuals (KUZ Z2044–Z2046) were obtained from the eyes of a Bonin petrel from Hahajima Island on 25 September 2015. A heavy infestation of leeches in the eyes of a Tristram's storm petrel, Oceanodroma tristrami Salvin, 1896, was observed on Anijima Island on 17 November 2013 (Fig. 2); in total, 12 leeches (KUZ Z1636–1647) were removed from the eyes of this petrel. When the leeches were collected from the petrel's eyes by the second and third authors, they were not sensitive to the exhaled human breath. The bird was already debilitated and died soon after the leeches were removed; therefore, this specimen was preserved in KUZ (KUZ B491).

Fig. 2. Heavy infestation of C. palmyrae (Richardson) in the eyes of a petrel, Oceanodroma tristrami Salvin (KUZ B491), from Anijima Island, Bonin Islands; arrow heads indicate respective leeches.
Table 1. Samples of Chtonobdella palmyrae (Richardson) infesting seabirds in Japan
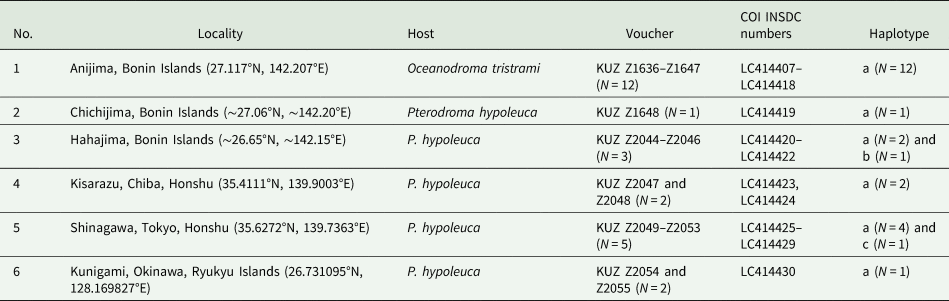
KUZ, Zoological Collection of Kyoto University.
Locality numbers (see Fig. 1) are accompanied by collection locality, geographical coordinates, individual counts, voucher, INSDC accession numbers for COI and COI haplotype.
Leeches were also collected on Honshu Island of the Japanese Archipelago. Two individuals (KUZ Z2047, Z2048) were collected from eyes of a P. hypoleuca petrel captured in Kuroto, Kisarazu, Chiba Prefecture, on 11 August 2000. Additionally, five individuals (KUZ Z2049–Z2053) were extracted from the eyes of a single P. hypoleuca captured in Shinagawa, Tokyo, on 10 August 2002. The other two individuals (KUZ Z2557 and Z2558) were found in an oral cavity or the throat of a young bird identified as P. hypoleuca caught in Kunigami, Okinawa Prefecture (Okinawa Island, Ryukyu Islands), on 23 October 2016; these two leeches were found loose from the petrel's mouth and collected.
Molecular phylogenies and COI networks
The BI tree (mean ln likelihood[L] = −20 241.493; Fig. 3A) for estimating the phylogenetic position of the present specimens had an identical topology to that of the ML tree (ln L = −20 222.492; not shown). The topology of the present phylogenies was almost concordant with those described in previous reports (Borda and Siddall, Reference Borda and Siddall2011; Tessler et al., Reference Tessler, Barrio, Borda, Rood-Goldman, Hill and Siddall2016). The haemadipsid leech (KUZ Z1636) formed a fully supported clade with C. palmyrae, which was collected from Palmyra Atoll (BS = 100%, PP = 1.0).

Fig. 3. Molecular phylogenetic position and haplotype networks of C. palmyrae (Richardson) infesting seabirds in Japan. (A) BI tree for 5172 bp alignment positions of nuclear 18S rRNA, 28S rRNA and mitochondrial COI markers; numbers on nodes indicate bootstrap values for ML and Bayesian PPs. Statistical parsimony networks of (B) the 985 bp COI haplotypes of Japanese (Jpn) leeches and C. palmyrae from Palmyra Atoll, and (C) the 1271 bp COI haplotypes only between the Japanese leeches; filled circles indicate missing haplotypes; each numeral in parentheses denotes the sample size of the respective haplotype.
The COI data of the 985 bp alignment positions showed there was a low-genetic diversity between the Japanese individuals (N = 24) and the C. palmyrae from the atoll (N = 1) (Fig. 3B); 3/985 (0.30%) nucleotides were polymorphic. There was no variation among the Japanese specimens based on the COI sequences with lengths of 985 bp. The COI sequences of 1271 bp alignment positions also highlighted the low-genetic diversity within the Japanese Chtonobdella leeches; 2/1271 (0.16%) nucleotides were polymorphic. Three sequence types were present in the Japanese populations, and the number of nucleotide differences between any two types ranged from one to two substitutions (Fig. 3C). The dominant sequence type (‘a’) was observed in 22 samples from six localities. The other two sequence types were observed in samples from Hahajima Island in the Bonin Islands (‘b’: KUZ Z2046) and from Tokyo (‘c’: KUZ Z2053). These two localities were also where the dominant sequence type leeches, along with the unique haplotype sample from a single host seabird, were collected. The substitution detected in the sample from Tokyo (KUZ Z2053) was nonsynonymous (leucine in the dominant haplotype to isoleucine in the minor haplotype).
Morphological characteristics
The 25 haemadipsid specimens (KUZ Z1636–Z1648, Z2044–Z2055), with BLs ranging from 5.1 to 20.5 mm, BWs ranging from 1.7 to 6.0 mm, CLs ranging from 0.8 to 3.0 mm and CWs ranging from 1.0 to 2.7 mm without obvious clitellum, possessed the following characteristics (Figs 4 and 5).

Fig. 4. Preserved specimen of C. palmyrae (Richardson) (KUZ Z1636) infesting O. tristrami Salvin (KUZ B491) from Anijima Island, Bonin Islands: (A) dorsal and (B) ventral views. Scale bar: 2 mm.

Fig. 5. Drawings of C. palmyrae (Richardson) (KUZ Z1636) infesting O. tristrami Salvin (KUZ B491) from Anijima Island, Bonin Islands. (A) Dorsal, (B) lateral and (C) ventral views of somites I–X, (D) ventral view of somites XI and XII, (E) dorsal, (F) lateral and (G) ventral views of somites XXI–XXVII and caudal sucker and (H) dorsal view of median reproductive systems, including positions of ganglia XI–XIII. at, atrium; fg, female gonopore; fr, friction ray; mg, male gonopore; np, nephridiopore; phl, prehensile lobe; ra, respiratory auricle; vd, vaginal duct; and vs, vaginal sac. Scale bars: (A–G) 0.5 mm and (H) 0.1 mm.
External morphology
Somites I completely merged with prostomium. Somites II (=peristomium) to IV uniannulate. Somite V biannulate, (a1 + a2) > a3; each of interocular plates of V (a1 + a2) subdivided. Somite VI dorsally triannulate, a1 = a2 = a3, ventrally biannulate, (a1 + a2) > a3; somite V to somite VI (a1 + a2) forming posterior margin of oral sucker. Somite VII triannulate, a1 = a2 < a3 (rarely with slight dorsal furrow, b5 = b6), or quadrannulate, a1 = a2 = b5 = b6. Somite VIII quadrannulate, a1 (occasionally with slight furrow, b1 = b2) > a2 = b5 = b6. Somites IX–XXII quinquannulate, b1 = b2 = a2 = b5 = b6. Somite XXIII generally quadrannulate, b1 = b2 = a2 < a3 (occasionally with slight furrow, b5 = b6). Somite XXIV triannulate, a1 = a2 = a3. Somite XXV–XXVII uniannulate; ambilateral margins of each somites XXV–XXVII forming lobes of one pair of respiratory auricles. Anus at posterior margin of somite XXVII. Caudal sucker ventral, elliptical; prehensile lobe developed, sharply hooked; with 74–82 friction rays. Male gonopore in somite XI b5/b6 or rarely in middle of somite XI b6. Female gonopore in somite XII a2/b5. Gonopores separated by 4, or rarely 1/2 + 3 annuli. Eyes in five pairs, in parabolic arc: first pair on somite II, second pair on III, third pair on IV, fourth pair on V (a1 + a2) and fifth pair on VI a2. Nephridiopores, when detectable, in 13–15 pairs, one each situated laterally at posterior margin of a1 of each somite in IX–XXIII. Nephridiopores of somite XXIV under respiratory auricles.
Internal morphology (based on KUZ Z1636, BL 9.3 mm)
Jaws duognathous. Genital organs undeveloped; testisacs undetectable; atrium small, globular; vaginal sac undeveloped tubular; vaginal duct short.
Colouration
In life, uniformly dark brown. Colour faded in preservative.
Discussion
Taxonomic account
The leeches in this study, which were obtained from the procellariiform seabirds P. hypoleuca and O. tristrami, unquestionably belonged to a Chtonobdella species, based on the presence of two jaws within the oral cavity. Additionally, molecular phylogenetic and network analyses identified the leeches as C. palmyrae, which was originally described based on an individual collected from Palmyra Atoll in the Northern Line Islands (Richardson, Reference Richardson1975; Fig. 1). Therefore, this study provides additional distribution records of this species well beyond its original known range; only trignathous Haemadipsa leeches have previously been recorded in the Far East (Lai et al., Reference Lai, Nakano and Chen2011; Seo et al., Reference Seo, Eun, Park, Kim, Won, Kim, Kim, Chae and Nakano2013; Morishima and Aizawa, Reference Morishima and Aizawa2019).
The present specimens revealed the intraspecific variation of the body somite annulation and male gonopore position in C. palmyrae. While the precedence descriptions of the material from Palmyra Atoll stated that almost all of them had a quadrannulate somite VII (Richardson, Reference Richardson1975, Reference Richardson1981), the Japanese specimens generally possessed the triannulate somite VII with a slight secondary furrow on its last annulus. Although most Japanese specimens had its male gonopore in the annular furrow of somite XI b5/b6 as with the individuals from Palmyra Atoll (Richardson, Reference Richardson1975, Reference Richardson1981), some of them possessed the male gonopore opening in the middle of somite XII b6. The previous study also stated that the colouration of the specimens from Palmyra Atoll was variable (Richardson, Reference Richardson1981): the holotype and paratype of C. palmyrae had slight longitudinal stripes with/without oval patches on the dorsal surface, but the small specimens were uniformly darkish brown without any stripes or markings. The colouration of the Japanese specimens is similar to that of the Richardson's (Reference Richardson1981) small leeches.
The molecular analyses support that the Japanese specimens are clearly conspecific with C. palmyrae, but nonetheless, their characteristics of the nephridiopores, respiratory auricles, and friction rays of the caudal sucker are different from those described by Richardson (Reference Richardson1981). In all Japanese specimens, nephridiopores opening between somites IV/V were not observed, contrary to the specimens from Palmyra Atoll that possessed the first nephridiopores in somites IV/V. In addition, the present specimens possessed the observable unlobate respiratory auricles (vs no developed respiratory auricles in the specimens from Palmyra Atoll), and 74–82 friction rays on the ventral surface of caudal sucker (vs 90 friction rays in the holotype of C. palmyrae). The continuation of faunal surveys on islands in the Pacific Ocean and morphological and genetic studies based on the evaluation of additional specimens are necessary to investigate the morphological discordance between the specimens from Japan and those from Palmyra Atoll.
The original description of H. z. ivosimae stated that the leeches on Kita-Ioto Island sucked the blood of birds' eyes and, moreover, rendered their hosts blind (Oka, Reference Oka1930). The colouration of H. z. ivosimae was also reported to be uniform reddish or greenish (Oka, Reference Oka1930). However, H. z. ivosimae was unquestionably described as a trignathous species clearly excluding it from the duognathous Chtonobdella (Oka, Reference Oka1930). The original description of H. z. ivosimae also mentioned that this species possesses five annuli between the gonopores, and the original drawing depicted trilobate respiratory auricles. These characteristics are also inconsistent with those of C. palmyrae including the present material, which generally possess four annuli between the gonopores as well as unlobate or undeveloped respiratory auricles (Richardson, Reference Richardson1975, Reference Richardson1981). The systematic account of H. z. ivosimae should be clarified in a future study. Because the type series of this taxon is deemed to be lost, newly collected haemadipsid specimens from Kita-Ioto Island are essential to unveil the true diversity of haemadipsid species of the Far East.
LDD of leeches via seabirds
The present results revealed that the duognathous haemadipsid C. palmyrae is an avian-specific blood-sucking parasite that infests the eyes and other mucus membranes of procellariiform migratory seabirds, and they corroborated the passive LDD of duognathous Chtonobdella leeches suggested by previous studies (Lande, Reference Lande1994; Borda et al., Reference Borda, Oceguera-Figueroa and Siddall2008; Borda and Siddall, Reference Borda and Siddall2011). The following avian host–Chtonobdella parasite relationships were recorded by Richardson (Reference Richardson1975): the leg of the passeriform Eopsaltria australis (Shaw, 1790) was parasitized by Chtonobdella limbata Grube, 1866; the head of the passeriform Gymnorhina tibicen G. R. Gray, 1840 was parasitized by Chtonobdella bilineata (Richardson, Reference Richardson1975); and the nasal chambers of the casuariiform Casuarius species were infested with an unidentified haemadipsid species and Chtonobdella novabritanniae (Richardson, Reference Richardson1975). In addition to the flightless Casuarius birds, both of the recorded passeriformes are known to be non-migratory birds (Boles, Reference Boles, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Russell et al., Reference Russell, Rowley, Christie, del Hoyo, Elliott, Sargatal, Christie and de Juana2020).
A precedence iDNA study using Haemadipsa leeches collected from Southeast Asia and Chtonobdella individuals from Australia and Madagascar could detect several avian species from the leeches' bloodmeals (Schnell et al., Reference Schnell, Bohmann, Schultze, Richter, Murray, Sinding, Bass, Cadle, Campbell, Dolch, Edwards, Gray, Hansen, Hoa, Noer, Heise-Pavlov, Sander Pedersen, Ramamonjisoa, Siddall, Tilker, Traeholt, Wilkinson, Woodcock, Yu, Bertelsen, Bunce and Gilbert2018). From the Australian Chtonobdella leeches, the flightless emu Dromaius novaehollandiae (Latham, 1790) (Casuariiformes) (Folch et al., Reference Folch, Christie, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020) and sedentary lyrebirds of Menura (Lill and Boesman, Reference Lill, Boesman, del Hoyo, Elliott, Sargatal, Christie and de Juana2020a, Reference Lill, Boesman, del Hoyo, Elliott, Sargatal, Christie and de Juana2020b) were identified (Schnell et al., Reference Schnell, Bohmann, Schultze, Richter, Murray, Sinding, Bass, Cadle, Campbell, Dolch, Edwards, Gray, Hansen, Hoa, Noer, Heise-Pavlov, Sander Pedersen, Ramamonjisoa, Siddall, Tilker, Traeholt, Wilkinson, Woodcock, Yu, Bertelsen, Bunce and Gilbert2018). Two avian genera Dryolimnas (Rallidae) and Mystacornis (Vangidae) were identified from the Malagasy Chtonobdella samples (Schnell et al., Reference Schnell, Bohmann, Schultze, Richter, Murray, Sinding, Bass, Cadle, Campbell, Dolch, Edwards, Gray, Hansen, Hoa, Noer, Heise-Pavlov, Sander Pedersen, Ramamonjisoa, Siddall, Tilker, Traeholt, Wilkinson, Woodcock, Yu, Bertelsen, Bunce and Gilbert2018); but birds belonging to the genera are deemed to be sedentary (Collar et al., Reference Collar, Robson, Schulenberg, Schulenberg and Keeney2020; Taylor, Reference Taylor, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). Schnell et al. (Reference Schnell, Bohmann, Schultze, Richter, Murray, Sinding, Bass, Cadle, Campbell, Dolch, Edwards, Gray, Hansen, Hoa, Noer, Heise-Pavlov, Sander Pedersen, Ramamonjisoa, Siddall, Tilker, Traeholt, Wilkinson, Woodcock, Yu, Bertelsen, Bunce and Gilbert2018) detected the other three genera (Bycanistes, Chalcophaps and Gallirallus) from bloodmeals of the Malagasy Chtonobdella leeches; but species of these genera are flightless or basically sedentary, and moreover not indigenous to Madagascar (Lepage, Reference Lepage2020a). Another iDNA analysis utilizing Malagasy Chtonobdella species identified the avian brachypteraciid species Atelornis crossleyi Sharpe, 1875 and Atelornis pittoides Lafresnaye, 1834 and the muscicapid Copsychus albospecularis (Eydoux and Gervais, 1836) (Fahmy et al., Reference Fahmy, Ravelomanantsoa, Youssef, Hekkala and Siddall2019); however, all of birds species were also determined to be sedentary (Collar and Kirwan, Reference Collar, Kirwan, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Langrand, Reference Langrand, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). Although Fahmy et al. (Reference Fahmy, Ravelomanantsoa, Youssef, Hekkala and Siddall2019) detected five additional avian species, they were either domesticated species, or species not indigenous to Madagascar (Lepage, Reference Lepage2020a). Therefore, the present findings finally provide insights into how Chtonobdella leeches have achieved a widespread distribution throughout the Indo-Pacific region, including on oceanic islands.
The present results suggest the possibility that at least one population of C. palmyrae is maintained somewhere in the Bonin Islands and Volcano Islands (hereinafter Bonin-Volcano Islands), particularly given the fact that (1) breeding sites of P. hypoleuca and those of O. tristrami overlap in the Bonin and Volcano Islands (Momiyama, Reference Momiyama1930; Chiba et al., Reference Chiba, Kawakami, Suzuki and Horikoshi2007, Reference Chiba, Sasaki and Horikoshi2012) and (2) the present samples had unique COI sequences compared with the sequence of C. palmyrae collected in Palmyra Atoll (Borda and Siddall, Reference Borda and Siddall2011). Although both P. hypoleuca and O. tristrami are known to breed in a western part of the Hawaii Islands (Seto and O'Daniel, Reference Seto, O'Daniel, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Slotterback, Reference Slotterback, del Hoyo, Elliott, Sargatal, Christie and de Juana2020), haemadipsids have not been recorded in the Hawaiian Islands (Eldredge and Miller, Reference Eldredge and Miller1997).
Both C. palmyrae leeches obtained from P. hypoleuca at Chiba (locality number 4 in Fig. 1) and Tokyo (locality 5) in Honshu and an individual from P. hypoleuca at Okinawa Island (locality 6) share a COI sequence type (‘a’ in Fig. 3C) that was dominant among the obtained sequences. Their host P. hypoleuca is a migrant seabird that breeds in the Bonin-Volcano Islands and has been known to visit Honshu and Okinawa irregularly (The Ornithological Society of Japan, 2012). Moreover, Chtonobdella leeches are not considered to be endemic to either Honshu or Okinawa islands (Itoh, Reference Itoh, Nishida, Shikatani and Shokita2003; Aizawa and Morishima, Reference Aizawa and Morishima2018). Because the Bonin-Volcano Islands are respectively located approximately 1000 and 1500 km from Chiba and Tokyo and from Okinawa Island, C. palmyrae may be able to disperse around 1000 km overseas by infesting the eyes and mucus membranes of P. hypoleuca.
The COI sequences revealed there was low-genetic diversity between the Japanese individual leeches and C. palmyrae from Palmyra Atoll; nonetheless, they did not share the same sequence type, suggesting that the two populations may be genetically distinct. Neither P. hypoleuca nor O. tristrami have ever been recorded in Palmyra Atoll (Lepage, Reference Lepage2020b); therefore, it is unknown how the trans-oceanic C. palmyrae populations were established in the Bonin-Volcano Islands and Palmyra Atoll. Its trans-oceanic distribution, in which the Bonin-Volcano Islands population is approximately 6300 km from the Palmyra Atoll population, can be explained by either the direct or stepping-stone dispersal (see Saura et al., Reference Saura, Bodin and Fortin2014). If the leeches dispersed directly between these two habitats along with the migration of their avian hosts, C. palmyrae may also parasitize other procellariiform seabirds, e.g. Bulweria bulwerii (Jardine and Selby, 1828), which are known to occur in both the Bonin-Volcano Islands and Palmyra Atoll (Chiba et al., Reference Chiba, Kawakami, Suzuki and Horikoshi2007; Lepage, Reference Lepage2020b). It is also feasible that there are additional undiscovered habitats of C. palmyrae, where migratory birds occurring in Bonin-Volcano Islands or Palmyra Atoll are known to visit, that may act as stepping stones for the trans-oceanic distribution of this leech species. The western Hawaii Islands are potential candidates for undiscovered habitats of C. palmyrae, as they contain breeding grounds for both P. hypoleuca and O. tristrami.
Our knowledge of how duognathous leeches colonize remote areas is hampered by the lack of a detailed understanding of the natural history for C. palmyrae. All specimens obtained from P. hypoleuca and O. tristrami were deemed to be immature individuals because they lacked detectable clitella, and the dissected specimen, which had a body length of ca. 1 cm, lacked developed genital organs. Meanwhile, the free-living individuals, whose body lengths exceeded 1.5 cm, collected in Palmyra Atoll were deemed to be almost mature individuals, as they possessed developed female organs (Richardson, Reference Richardson1981). The bat-specific haemadipsid Sinospelaeobdella species and, in particular, avian-specific glossiphoniid Theromyzon leeches are known to detach from their hosts to copulate and deposit a cocoon (Wilkialis and Davies, Reference Wilkialis and Davies1980; Huang et al., Reference Huang, Liu, Gong, Wu, Liu, Deng, Zhang, Peng, Zhang and Liu2019). Therefore, individuals of C. palmyrae may detach from their avian hosts to undergo reproductive behaviours after they have fed on sufficient bird blood.
Haemadipsids are known to be vectors of several pathogens, including Bartonella (Kang et al., Reference Kang, Won, Kim, Kim, Park, Park, Seo and Chae2016) and Trypanosoma (see Sawyer, Reference Sawyer1986). Although no pathogens have ever been recorded in C. palmyrae, trypanosome blood-parasites have been detected in other Chtonobdella species (Richardson and Hunt, Reference Richardson and Hunt1968; Ewers, Reference Ewers1974; Richardson, Reference Richardson1974). Our knowledge of the pathogens of procellariform seabirds is severely limited; nonetheless, coccidia have been known to infest procellariform species (Yabsley, Reference Yabsley, Atkinson, Thomas and Hunter2008) and to be infectious in leeches (O'Donoghue, Reference O'Donoghue2017). Therefore, the host–parasite relationships between procellariform seabirds and Chtonobdella leeches described in this study highlight the possibility that haemadipsids may act as vectors of procellariform pathogens.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182020001729
Acknowledgements
The authors are grateful to Dr Kazuo Horikoshi (Institute of Boninology; IB), Masashi Miyagi (IB), Tsubasa Kojima, Yasuko Iwami, Miyako Tsurumi, Noboru Nakamura and Naomi Tokita for their assistance in collecting the haemadipsid specimens examined in this study. We also thank Dr Angus Bell (Brunel University London), Dr Michael Tessler (American Museum of Natural History) and two anonymous reviewers for their valuable comments and suggestions on this manuscript, and Dr Suzanne Leech (Edanz Group) for editing a draft of this manuscript. The first author extends his gratitude to Dr Hironori Komatsu (NSMT) for allowing him to survey the leech collection.
Financial support
This study was supported by Japan Society for the Promotion of Science KAKENHI (T.N., grant numbers JP13J00450, JP26840127, JP15J00720, JP17K20064 and JP18K14780).
Conflict of interest
None.
Ethical standards
Not applicable.









