Introduction
Aberrant salience is a prominent explanation for the development of psychosis symptoms. According to Kapur (Reference Kapur2003), dysregulated dopaminergic firing results in the creation, or exaggeration, of the importance of external objects or events and internal thoughts or perceptions. Dopaminergic firing is associated with motivational salience, the cognitive process of directing attention and behaviour. In the absence of a relevant stimulus context, Kapur (Reference Kapur2003) proposed dopaminergic firing leads to impairments in motivational salience and the assignment of importance to irrelevant stimuli. Kapur (Reference Kapur2003) suggested delusions serve an explanatory function for patients, explaining the importance of aberrant stimuli, whereas hallucinations manifest from aberrant visual and auditory percepts.
There is some evidence for aberrant salience in schizophrenia, however results are inconsistent. Studies show increased emotional arousal (Haralanova, Haralanov, Beraldi, Moller, & Hennig-Fast, Reference Haralanova, Haralanov, Beraldi, Moller and Hennig-Fast2012) and neural activation (Horan, Hajcak, Wynn, & Green, Reference Horan, Hajcak, Wynn and Green2013) to neutral stimuli in schizophrenia – a pattern that is observed in unaffected individuals who are exposed to negative stimuli. Compared to unaffected individuals, those with schizophrenia rate environmental sounds as more invasive, and artificial abstract sounds as more familiar (Micoulaud-Franchi et al., Reference Micoulaud-Franchi, Aramaki, Merer, Cermolacce, Ystad, Kronland-Martinet and Vion-Dury2012). Data from measures of aberrant salience, such as the Aberrant Salience Inventory (ASI; Cicero, Kerns, & McCarthy, Reference Cicero, Kerns and McCarthy2010) and Salience Attribution Test (SAT; Roiser et al., Reference Roiser, Stephan, den Ouden, Barnes, Friston and Joyce2009), show heightened aberrant salience in schizophrenia compared to controls (Cicero et al., Reference Cicero, Kerns and McCarthy2010; Katthagen et al., Reference Katthagen, Dammering, Kathmann, Kaminski, Walter, Heinz and Schlagenhauf2016; Pankow et al., Reference Pankow, Katthagen, Diner, Deserno, Boehme, Kathmann and Schlagenhauf2015). Additionally, aberrant salience is associated with abnormal beliefs (Roiser, Howes, Chaddock, Joyce, & McGuire, Reference Roiser, Howes, Chaddock, Joyce and McGuire2013), perceptual aberration, and magical ideation (Cicero et al., Reference Cicero, Kerns and McCarthy2010; Cicero, Becker, Martin, Docherty, & Kerns, Reference Cicero, Becker, Martin, Docherty and Kerns2013) in individuals with no history of psychosis, suggesting a link between aberrant salience and psychosis risk. Conversely, SAT data from other studies suggest that salience is normal in schizophrenia (Abboud et al., Reference Abboud, Roiser, Khalifeh, Ali, Harrison, Killaspy and Joyce2016; Roiser et al., Reference Roiser, Stephan, den Ouden, Barnes, Friston and Joyce2009).
Reasons for inconsistent findings vary. Aberrant salience may be more prominent during the prodromal phase than other illness phases (Roiser et al., Reference Roiser, Howes, Chaddock, Joyce and McGuire2013) or be attenuated by medication (Roiser et al., Reference Roiser, Stephan, den Ouden, Barnes, Friston and Joyce2009). However, these accounts have not been replicated (Smieskova et al., Reference Smieskova, Roiser, Chaddock, Schmidt, Harrisberger, Bendfeldt and Borgwardt2015; Walter et al., Reference Walter, Suenderhauf, Smieskova, Lenz, Harrisberger, Schmidt and Borgwardt2016). Alternatively, there is limited evidence of the validity of aberrant salience measures in schizophrenia. ASI scores correlate with psychotic-like experiences, such as magical ideation, in unaffected individuals and discriminate schizophrenia from other psychopathologies, such as bipolar disorder (Cicero et al., Reference Cicero, Kerns and McCarthy2010). The SAT shows good construct validity in unaffected individuals (Schmidt & Roiser, Reference Schmidt and Roiser2009) and concurrent validity (Katthagen et al., Reference Katthagen, Dammering, Kathmann, Kaminski, Walter, Heinz and Schlagenhauf2016). However, more robust validity studies are needed, examining whether aberrant salience measures correlate with cognitive processes associated with dopamine function, such as motivational salience and reinforcer sensitivity.
Schizophrenia is associated with impairments in motivational salience, such as a reduced ability to unlearn previous associations (Waltz, Frank, Robinson, & Gold, Reference Waltz, Frank, Robinson and Gold2007), aberrant reward-related behaviour (Barch, Treadway, & Schoen, Reference Barch, Treadway and Schoen2014; Fervaha et al., Reference Fervaha, Graff-Guerrero, Zakzanis, Foussias, Agid and Remington2013; Gold et al., Reference Gold, Strauss, Waltz, Robinson, Brown and Frank2013; McCarthy, Treadway, Bennett, & Blanchard, Reference McCarthy, Treadway, Bennett and Blanchard2016; Reddy et al., Reference Reddy, Horan, Barch, Buchanan, Dunayevich, Gold and Green2015; Strauss et al., Reference Strauss, Frank, Waltz, Kasanova, Herbener and Gold2011), and disrupted loss sensitivity (Currie et al., Reference Currie, Buruju, Perrin, Reid, Steele and Feltovich2017; Scholten, van Honk, Aleman, & Kahn, Reference Scholten, van Honk, Aleman and Kahn2006; Trémeau et al., Reference Trémeau, Brady, Saccente, Moreno, Epstein, Citrome and Javitt2008). Reduced activation of reward-related neural regions in schizophrenia is associated with reward receipt (Gradin et al., Reference Gradin, Waiter, O'Connor, Romaniuk, Stickle, Matthews and Steele2013; Schlagenhauf et al., Reference Schlagenhauf, Sterzer, Schmack, Ballmaier, Rapp, Wrase and Heinz2009), impaired reward anticipation (Radua et al., Reference Radua, Schmidt, Borgwardt, Heinz, Schlagenhauf, McGuire and Fusar-Poli2015), and reward-seeking behaviour (Wolf et al., Reference Wolf, Satterthwaite, Kantrowitz, Katchmar, Vandekar, Elliott and Ruparel2014).
Kapur (Reference Kapur2003) proposed that motivational salience mediates aberrant salience. However, evidence of an association between motivational salience and measures of aberrant salience is limited. Ceaser and Barch (Reference Ceaser and Barch2016) found incorrect identification of a distractor stimulus as salient was associated with increased dorsal striatal activation in schizophrenia, with the degree of activation positively correlating with ASI score. Boehme et al. (Reference Boehme, Deserno, Gleich, Katthagen, Pankow, Behr and Schlagenhauf2015) found high SAT implicit aberrant salience in unaffected individuals was associated with reduced ventral striatum activation during reward prediction error.
We previously reported finding no evidence that the ASI and SAT indices are correlated among undergraduates with no history of psychosis (Neumann & Linscott, Reference Neumann and Linscott2018). At most, the ASI and SAT indices predicted aspects of willingness to expend effort under different task conditions. For example, higher ASI scores predicted greater willingness to expend effort for small, less likely rewards, and the SAT behavioural measure of aberrant salience predicted less effort for large, more likely rewards. The primary finding, that the ASI and SAT were unrelated, may reflect: (a) the effects of range restriction in aberrant salience in unaffected individuals; or (b) a typical pattern of reinforcer sensitivity and motivation in unaffected individuals compared to that seen in individuals with schizophrenia.
Therefore, our aim was to investigate relationships among measures of aberrant salience, motivation, and reinforcer sensitivity in schizophrenia and anxiety groups and in unaffected individuals. We elected to compare schizophrenia to anxiety because of evidence that anxiety results from the conflict of competing goals during reward processing (Corr & McNaughton, Reference Corr and McNaughton2012; Gray & McNaughton, Reference Gray and McNaughton2000). That is, although reward processing is associated with anxiety and psychosis, the sources of symptom development are thought to differ between these disorders. If aberrant salience is specific to schizophrenia, predicted relationships between measures should be evident in schizophrenia but not in anxious and unaffected individuals.
Participants with schizophrenia, anxiety, and unaffected individuals completed measures of motivational salience [Effort Expenditure for Rewards Task (EEfRT); Treadway, Buckholtz, Schwartzman, Lambert, & Zald, Reference Treadway, Buckholtz, Schwartzman, Lambert and Zald2009] and reinforcer sensitivity [Stimulus Chase Task (SCT); Hall, Chong, McNaughton, & Corr, Reference Hall, Chong, McNaughton and Corr2011] along with the ASI and SAT. We predicted that the schizophrenia group would exhibit greater aberrant salience on the ASI and SAT than would unaffected or anxiety groups (Cicero et al., Reference Cicero, Kerns and McCarthy2010; Katthagen et al., Reference Katthagen, Dammering, Kathmann, Kaminski, Walter, Heinz and Schlagenhauf2016; Pankow et al., Reference Pankow, Katthagen, Diner, Deserno, Boehme, Kathmann and Schlagenhauf2015). We expected greater variance of aberrant salience in schizophrenia, resulting in relationships between measures of aberrant salience that would not be present in the unaffected and anxiety groups. We also examined the relationship between measures of motivation and aberrant salience. We hypothesised that, in schizophrenia, aberrant salience would predict comparatively greater and lesser engagement in hard tasks when these were poorly and richly rewarded, respectively (Fervaha et al., Reference Fervaha, Graff-Guerrero, Zakzanis, Foussias, Agid and Remington2013). Finally, we predicted that relative sensitivity to gain and loss would correlate with aberrant salience indices (Currie et al., Reference Currie, Buruju, Perrin, Reid, Steele and Feltovich2017; Scholten et al., Reference Scholten, van Honk, Aleman and Kahn2006; Trémeau et al., Reference Trémeau, Brady, Saccente, Moreno, Epstein, Citrome and Javitt2008). We also explored whether relationships among measures were consistent across groups or specific to schizophrenia.
Methods
Participants and procedures
Patients (n = 34) with working diagnoses of schizophrenia, schizoaffective disorder, schizophreniform disorder, or first-episode psychosis were recruited by referral from mental health clinicians. Patients with anxiety (n = 37) and individuals with no mental disorder (n = 31) were recruited using posters in hospital staff areas and an online advertisement in a local newspaper. Inclusion criteria for the anxiety group were meeting diagnostic criteria for an anxiety disorder and reporting no prior or current psychotic symptoms. The inclusion criterion for the unaffected group was the absence of current or past experience of mental health issues. All participants were aged 18 to 65 years and indicated no history of neurological injury or disease. For the large effects (f 2 = 0.35) expected between measures of the same construct, where α = 0.05, a sample of 30 per group affords a power of ~0.84. For large effects (r = 0.5) between measures within groups, where α = 0.05, a sample of 30 affords a power of ~0.83.
In the clinical groups, the Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., Reference Sheehan, Lecrubier, Janavs, Harnett-Sheehan, Sheeha and Gray2010) was used, in conjunction with other measures and clinical evidence, to confirm the current working diagnosis and assess symptom expression. The schizophrenia group included one participant who met criteria for schizoaffective disorder and two who denied any clinically relevant mental health symptoms yet had working diagnoses of schizophrenia and histories of in- and out-patient psychiatric care and treatment for schizophrenia. Five referrals to the schizophrenia group were excluded: one who reported faking symptoms of psychosis; two who met the MINI criteria for primary mood disorder rather than a psychotic disorder; one with current (<3 months) alcohol dependency; and one who was unable to complete the assessment. All anxiety participants met the MINI diagnostic criteria for an anxiety disorder. Two were reassigned to the schizophrenia group as they met the MINI diagnostic criteria for schizophrenia and two others were excluded because of age or current alcohol dependence. One unaffected individual was excluded due to prior diagnosis of mental health issues. Table 1 shows demographic characteristics of participants included in the final analysis. Table 2 shows MINI output and current positive symptom summary for schizophrenia and anxiety participants.
Table 1. Demographics for unaffected, anxiety and schizophrenia groups

Table 2. Clinical characteristics of anxiety and schizophrenia groups

a Classification based on the MINI.
b These patients had working diagnoses of schizophrenia and schizoaffective disorder, respectively.
Clinical group participants attended two ~90-min sessions. In the first, participants completed a demographics questionnaire and the ASI, SAT, EEfRT and SCT. The performance task order was counterbalanced across participants. During the second session, conducted within a week of the first, participants completed the screening and symptom measures. Unaffected participants attended one 2-hr session during which they completed all measures except the MINI.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the University of Otago Human Ethics Committee (H16/026). All participants provided written informed consent. Participants were offered a payment of $25 per appointment.
Measures
Screening and symptoms
The Alcohol and Drug Abuse and Dependence Screen (ADDS; Muthén, Reference Muthén1995) was used to screen for problematic substance use. The ADDS contains items that address the quantity and impact of consumption of drugs and alcohol. Outcome scores range from 0 (no use) to 240 (severe dependency).
The Depression Anxiety Stress Scales (DASS; Lovibond and Lovibond, Reference Lovibond and Lovibond1995) were used to quantify mood and anxiety symptoms. The DASS contains 42 self-report items that respondents rate from 0 = did not apply at all to 3 = applied most of the time, with 14 items relating to each of depression, anxiety, and stress.
Nine items comprising the Anxiousness facet of the Personality Inventory for DSM-5 (PID-5A; American Psychiatric Association, 2013b) were used to assess anxious temperament. Respondents rate items from 0 = very false or often false to 3 = very true or often true.
The DSM-5 Self-Rated Level 1 Cross-Cutting Symptom Measure (L1SM; American Psychiatric Association, 2013a) was used to screen for current mental health issues. The L1SM is a 23-item screen for key features of depression, anger, mania, anxiety, somatic symptoms, suicidal ideation, psychosis, sleep problems, memory impairment, repetitive thoughts and behaviours, dissociation, personality functioning, and substance use experienced in the past 2 weeks. Respondents rate items from 0 = none/not at all to 4 = severe/nearly every day.
The MINI (Sheehan et al., Reference Sheehan, Lecrubier, Janavs, Harnett-Sheehan, Sheeha and Gray2010) was used to evaluate current and past diagnoses. The MINI is a semi-structured brief interview covering DSM-IV and ICD-10 Axis I psychiatric disorders.
Aberrant salience
The ASI (Cicero et al., Reference Cicero, Kerns and McCarthy2010) was used as a self-report measure of aberrant salience. The ASI consists of 29 dichotomous-response items about lifetime experiences and beliefs characterised by aberrant salience. The ASI score is the number of endorsed items. ASI scores have high test-retest reliability over 2 weeks (0.96; Lelli et al., Reference Lelli, Godini, Lo Sauro, Pietrini, Spadafora, Talamba and Ballerini2015), high internal consistency (α = 0.89; Cicero et al., Reference Cicero, Kerns and McCarthy2010; Lelli et al., Reference Lelli, Godini, Lo Sauro, Pietrini, Spadafora, Talamba and Ballerini2015), and predict psychotic experiences (Cicero et al., Reference Cicero, Kerns and McCarthy2010).
The SAT (Roiser et al., Reference Roiser, Stephan, den Ouden, Barnes, Friston and Joyce2009) was used as a performance measure of adaptive and aberrant salience. The SAT is a computerised task that uses implicit and explicit associative learning of stimulus-reinforcement contingencies to measure adaptive and aberrant salience. The task comprises two blocks of 64 trials. In each trial, a red or blue line drawing of an animal or household object appears above and below a fixation cross. When the fixation cross is replaced by a black box, respondents are to press a button as fast as possible. Following each trial, feedback indicates whether the trial was reinforced and, if so, the amount of reward.
Participants are informed that response latency determines reward magnitude (reward; between 10c and $1) whereas stimulus type determines reward probability (probability). For the latter, the stimulus-reward contingencies vary randomly across participants. That is, for a participant, only one of the two dimensions (colour = red or blue; semantic category = household object or animal) is task relevant. On the task-relevant dimension, one level has a high reinforcement probability (87.5%) and the other a low reinforcement probability (12.5%); on the task-irrelevant dimension, both levels have a 50% reinforcement probability.
Dependent measures include implicit and explicit indices of adaptive and aberrant salience. Implicit adaptive salience is the mean difference in response latency (ms) between high and low probability trials of the task-relevant dimension; implicit aberrant salience is the absolute between-level difference in response latency on the task-irrelevant dimension. Explicit measures are obtained by asking respondents at the end of each 64-trial block to indicate the probability that each stimulus type was rewarded, with responses given on a visual analogue scale (mm).
Reward processing
The EEfRT (Treadway et al., Reference Treadway, Buckholtz, Schwartzman, Lambert and Zald2009) was used to assess decision making. The EEfRT is a computerised, multi-trial, fixed-duration task in which willingness to exert effort (determined by task choice) is assumed to reflect motivation. At the start of each trial, respondents are told the rewards for easy (set at $1) and hard (between $1.24 and $4.30) task choices and the probability of winning (12, 50 or 88%). In the easy task, respondents use the dominant index finger to complete 30 buttons presses whereas in the hard task, the non-dominant little finger to complete 100 buttons presses. After choosing and completing the selected task, trial feedback indicates whether a reward was available and how much was won. The dependent variable is task choice. As the easy task took less time to complete than the hard task, the number of trials completed during the 20-min task varied between participants.
The SCT (Hall et al., Reference Hall, Chong, McNaughton and Corr2011) was used to assess relative sensitivities to gain and loss and relative tendencies for approach and avoidance. Respondents begin the task with a nominal balance of $180 (equivalent to NZ$1.80) and are told they will be paid the cash equivalent of their end-of-task balance. The SCT has two phases, each comprising 50 blocks of 5 trials. In each trial the respondent sees a blue box on a computer screen. Gain and loss values (e.g. ‘+$6, –$2’) are displayed alongside the box. The respondent's task is to choose whether to chance a gain or loss (outcome probability = 50%) or to refrain and retain the current balance. If they take the gamble, the trial ends with feedback showing the outcome and the revised balance.
At the start of each block, respondents are told gain and loss values for the block; these do not vary within blocks. Across blocks, gain and loss values follow the sequence, (+8, −8), (+8, −6), (+8, −4), (+8, −2), (+8, −0), (+6, −8), … , (+0, −2), (+0, −0), (+0, −0), (+0, −2), (+0, −4), … , (+8, −6), (+8, −8). At the start of each phase, respondents are told how to enter or refrain from the gamble: In Phase 1, respondents click on the blue box to chance the outcome; in Phase 2, respondents chance the outcome by not clicking. If there is no response, trials end at 3000 ms. Thus, Phases 1 and 2 capture approach and avoidance behaviour, respectively. Decision speed is recorded.
The SCT yields two dependent measures based on the response time: the ratios of gain to loss sensitivities and of approach to avoidance tendencies. Sensitivity and tendency parameters are modelled with the matching law (Hall et al., Reference Hall, Chong, McNaughton and Corr2011) using Microsoft® Excel Solver. If the model fit is poor (i.e. r 2 < 0.7), participant's SCT data are excluded.
Analysis
Planned comparisons and ANOVA with Games-Howell post hoc analysis were used to test group differences in measures. Pearson's correlation coefficient (2-tailed unless otherwise stated), with the Holm adjusted significance level, was used to assess relationships among measures within each group. Holm correction for multiple comparisons was carried out on each within-group measure pair. For planned comparisons, ANOVA, and correlations, bias-corrected and accelerated bootstrapped confidence intervals (95%, 10 000 samples) were computed using SPSS version 25 (IBM Corp, 2017). SCT ratios were log-transformed for analysis. Holm's correction was applied to families of hypotheses using R psycho package (Makowski, Reference Makowski2018).
EEfRT trials were categorised by probability (12, 50 and 88%) and reward (low <$2, medium $2 to $2.99, high ⩾$3), yielding 9 trial types, and the proportion of hard task choices (effort) was obtained for each trial type. Mixed-effects modelling was used to calculate the effects of probability, reward, and reward × probability on task choice. Models included random slopes and intercepts. Mixed-effects analyses were conducted using R (R Development Core Team, 2016) with the Hmisc (Harrell, Reference Harrell2016), lme4 (Bates, Maechler, Bolker, & Walker, Reference Bates, Maechler, Bolker and Walker2015), and psych (Revelle, Reference Revelle2016) packages.
Results
One outlier in the schizophrenia group fell above 1.5 × the interquartile range and singularly skewed results, creating a correlation between ASI and SAT aberrant salience. Data from this outlier were excluded. SCT data from n = 41 participants did not fit the matching law. There was no difference between SCT fit and non-fit group means for other indices (all p > 0.10) and non-fitting behaviour was not associated with the group, χ2(2) = 5.63, p = 0.06. SCT data were analysed for: schizophrenia, n = 12; anxiety, n = 23 and unaffected, n = 17. The mean number of trials completed during the EEfRT was: schizophrenia, m = 65; anxiety, m = 68 and unaffected, m = 67.
Group differences
Compared to anxiety, schizophrenia was associated with higher ASI and SAT implicit aberrant salience scores and lower explicit adaptive salience scores (Table 3, Fig. 1 and online Supplementary Fig. S1). The schizophrenia group also had higher ASI scores and lower explicit adaptive salience scores than the unaffected group. The anxiety group had higher on ASI scores than the unaffected group.
Table 3. Means and their bootstrapped (BCa) 95% confidence intervals (CI) for the ASI, SAT, SCT and EEfRT

ASI, Aberrant Salience Inventory; SAT, Salience Attribution Test; SCT, Stimulus Chase Task; EEfRT, Effort Expenditure for Reward Task; AS, aberrant salience; AdS, adaptive salience; A:A, approach-avoid; G:L, gain-loss; L, low; M, medium; H, high; Pr, probability; $, reward.
Both SCT ratios differed across groups (Fig. 1b). Ratios over 1 indicate greater sensitivity to gain than loss and greater tendency to approach than avoid, respectively. Compared to the unaffected group, schizophrenia was associated with a higher approach-avoidance ratio.

Fig. 1. Mean group scores showing significant differences in planned comparisons for (a) ASI, (b) Stimulus Chase Task gain-loss and approach-avoidance ratios, (c) SAT Attribution Test implicit and explicit aberrant salience and (d) Salience Attribution Test implicit and explicit adaptive salience; and ANOVA and post hoc differences for significant group differences in (e) reward × probability combinations of the Effort Expenditure for Reward Task. Error bars show standard error of the mean.
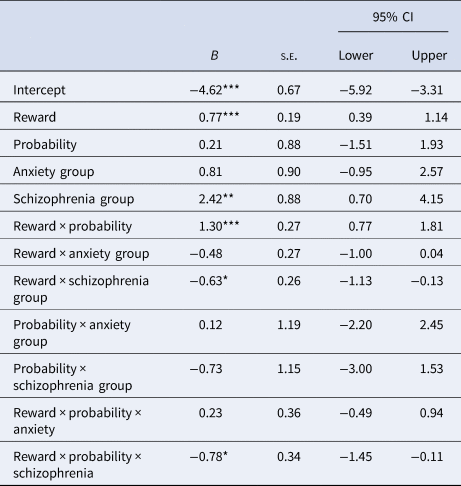
During the EEfRT task, the schizophrenia group chose the hard task more often with lower less likely rewards; and less often for higher, more certain rewards and for medium and high combinations of probability and reward (Fig. 1). There was also an effect of group, with the schizophrenia group selecting the hard task less often than the unaffected and anxiety groups. The effects of reward and reward × probability were also significantly lower in the schizophrenia group compared to the unaffected and anxiety groups (Table 4). There were no main effects of probability.
Table 4. Effect of reward, probability and reward × probability on task choicea

a Unaffected individuals and hard task choice are baseline.
*p < 0.05, **p < 0.01, ***p < 0.001.
The number of males and females differed across groups, χ2(2) = 21.29, p < 0.001. Additionally, the mean age of the psychosis group (M = 43.5, s.e. = 2.06) was higher than the unaffected (M = 32.10, s.e. = 1.83), t(58) = −4.14, p < 0.001, r = 0.47, and anxiety (M = 34.82, s.e. = 1.83) groups, t(59.37) = −3.16, p = 0.003, r = 0.37. Multiple regression with bootstrapped confidence intervals (95%, 10 000 samples) were computed to investigate the effect of age and sex covariates on group differences. Controlling for age and sex did not make any substantive difference except that there was no longer any evidence of a schizophrenia group difference on low-probability low-reward EEfRT scores (ß = 0.07, 95% CI −.02, .16)
Within-group relationships between measures
There was no evidence that the ASI correlated with any other measure in the schizophrenia group. SAT implicit and explicit adaptive salience positively correlated, however correlations between SAT, EEfRT, and SCT indices did not survive the Holm correction (online Supplementary Table S1). Similarly, none of the correlations between measures in the anxiety group survived the Holm correction (online Supplementary Table S2). In the unaffected group, ASI and explicit aberrant salience predicted relatively lower gain:loss sensitivity (online Supplementary Table S3). No other correlations survived Holm correction.
Discussion
There was partial support for the idea that, compared to anxiety, schizophrenia is associated with greater expressed aberrant salience. Schizophrenia was associated with higher ASI and higher SAT implicit aberrant salience scores. However, contrary to our hypotheses, the ASI and SAT aberrant salience indices were negatively correlated, albeit not significantly. We found no evidence for a relationship between aberrant salience indices and reinforcer sensitivity in schizophrenia and correlations between aberrant salience and motivational salience did not survive corrections for multiple testing. Support for the construct validity of aberrant salience indices against reward processing measures was also limited in unaffected and anxiety groups.
The results have several implications for the interpretation of ASI and SAT data. First, they cast doubt on the validity of the ASI and, to a lesser extent, the SAT. The intermediate rating of the anxiety group suggests the ASI measures a trait that is not unique to schizophrenia. Furthermore, the failure to find a relationship between the ASI and EEfRT indicates that the construct measured with the ASI is not related to motivational salience. There was no evidence that implicit aberrant salience predicted effortful decision-making in the schizophrenia group. These findings rest in stark contrast to what would be expected given even modest construct validity and specificity. The lack of relationship could be due to construct validity issues with the EEfRT. However, this is unlikely given the current data from the EEfRT are consistent with evidence suggesting maladaptive behaviour in schizophrenia and contrast with the adaptive behaviour found in unaffected individuals (Barch et al., Reference Barch, Treadway and Schoen2014; Fervaha et al., Reference Fervaha, Graff-Guerrero, Zakzanis, Foussias, Agid and Remington2013; McCarthy et al., Reference McCarthy, Treadway, Bennett and Blanchard2016; Strauss, Waltz, & Gold, Reference Strauss, Waltz and Gold2014).
This notwithstanding, the schizophrenia group did exhibit a pattern of inefficient, maladaptive behaviour not seen in the anxiety or unaffected groups. Schizophrenia was associated with lower adaptive but higher aberrant reinforcement learning. During the EEfRT, the schizophrenia group exhibited less adaptive behaviour, pursuing the hard task when it was less likely to yield higher rewards but not when higher more likely rewards were available.
Several mechanisms may underlie this pattern of behaviour in schizophrenia. Impaired cost and effort computations in schizophrenia have previously been linked to impairments in working memory, value representations, and cost calculations (Strauss et al., Reference Strauss, Waltz and Gold2014). The current findings indicate reduced cognitive effort during cost and effort computations in schizophrenia. Specifically, stimuli that required greater cognitive effort (e.g. determining reward value from a scale and calculating the reward by probability interaction) had less effect on task choice in the schizophrenia compared to other groups. These findings are in line with evidence suggesting reduced activation in the striatum, a region associated with acquired salience (Esslinger et al., Reference Esslinger, Braun, Schirmbeck, Santos, Meyer-Lindenberg, Zink and Kirsch2013), contributes to impaired reward processing (Gradin et al., Reference Gradin, Waiter, O'Connor, Romaniuk, Stickle, Matthews and Steele2013; Radua et al., Reference Radua, Schmidt, Borgwardt, Heinz, Schlagenhauf, McGuire and Fusar-Poli2015; Roiser, Stephan, den Ouden, Friston, & Joyce, Reference Roiser, Stephan, den Ouden, Friston and Joyce2010; Schlagenhauf et al., Reference Schlagenhauf, Sterzer, Schmack, Ballmaier, Rapp, Wrase and Heinz2009) and effortful behaviour (Wolf et al., Reference Wolf, Satterthwaite, Kantrowitz, Katchmar, Vandekar, Elliott and Ruparel2014) in schizophrenia.
However, alternative accounts for these findings should also be considered. First, aberrant salience may be more evident in the prodromal phase but dampened in subsequent illness phases due to medication or symptom development (Abboud et al., Reference Abboud, Roiser, Khalifeh, Ali, Harrison, Killaspy and Joyce2016). If that were the case, the relationship between aberrant salience and motivational salience may also reduce. Secondly, it may be that the schizophrenia participants here exhibited intact reinforcement learning and motivational salience. However, in line with previous findings (Fervaha et al., Reference Fervaha, Graff-Guerrero, Zakzanis, Foussias, Agid and Remington2013), the schizophrenia group exhibited aberrant effortful behaviour.
The findings should be considered in light of several limitations. First, given we examined aberrant salience indices obtained using different measurement methods, our expectation of obtaining large effects may have been unreasonable or a larger sample should have been used. However, we are confident our expectations were reasonable and our key interpretation is safe for several reasons. The SAT implicit and explicit measures, which involve performance and global judgement methodologies (respectively), did show some evidence of significant within-group relationships; even though we used common methods for the SAT explicit and ASI measures, the magnitude of observed relationships was no greater than those for the SAT implicit measures; and the SAT implicit aberrant salience measure was negatively related to ASI ratings, albeit not significantly.
We did not record information on current medication for schizophrenia or anxiety group participants. A large proportion of the current schizophrenia group, however, reported current psychotic symptoms. Antipsychotic medication is not universally effective (e.g. Gotfredsen et al., Reference Gotfredsen, Wils, Hjorthoj, Austin, Albert and Nordentoft2017) and, among those for whom it is effective, is often not completely effective. However, future research should examine the effect of antipsychotics. All but one participant in the schizophrenia group were receiving treatment within a health care system at the time of participation whereas the anxiety group were not recruited via health services and many had not sought formal treatment. Furthermore, whereas unaffected participants underwent screening for current mental health experiences and asked about past mental health in the demographic questionnaire, there may have been undiagnosed or unreported mental health issues.
The implications of data loss due to chaotic behaviour, resulting in exclusion from SCT analysis, are also worth considering. Higher than expected exclusion rates may be explained if these had been observed in individuals with schizophrenia only. In that case, non-fitting behaviour could have been attributed to impairments in reward processing and motivational salience leading to chaotic behaviour (e.g. Currie et al., Reference Currie, Buruju, Perrin, Reid, Steele and Feltovich2017; Gold et al., Reference Gold, Strauss, Waltz, Robinson, Brown and Frank2013; Reddy et al., Reference Reddy, Horan, Barch, Buchanan, Dunayevich, Gold and Green2015; Strauss et al., Reference Strauss, Frank, Waltz, Kasanova, Herbener and Gold2011). However, all groups had high SCT exclusion rates. Given this, we are now exploring whether and how to use non-fitting behaviour as an SCT task outcome. Finally, the groups were not well matched on age and sex. Although there was little evidence that the pattern of effects seen in the schizophrenia group was attributable to sex or age, matching would allow more accurate analysis of the effect.
The current findings suggest a variance in construct definition among measures of aberrant salience and challenge the construct validity of the measures. Therefore, caution should be applied when interpreting findings from currently available measures of aberrant salience. These implications, which rest in part on the failure to reject null hypotheses, should be addressed in future research. In addition to attending to limitations of power and effects of medication, it is critical that in future research investigators continue to develop and test new and diverse approaches to operationalising and measuring aberrant salience.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720000264
Acknowledgements
We are grateful to the volunteers who contributed to this research and the assistance of mental health professionals in referring participants. We also thank Prof Neil McNaughton (SCT), Prof Jon Roiser (SAT) and Dr Michael Treadway (EEfRT) for their advice and assistance.
Financial support
This research was funded by grants and awards to RJL from the New Zealand Schizophrenia Research Group and Department of Psychology, University of Otago.
Conflict of interest
None.