Introduction
The Gerreidae (Perciformes), commonly known as mojarras or silverbiddies, are a family of primarily marine fishes comprising eight genera and 53 species. They typically inhabit the coastal waters of tropical seas, occasionally entering brackish waters, and are predators of benthic invertebrates (Froese & Pauly, Reference Froese and Pauly2021). Gerreids are host to a wide variety of digenean trematodes, including representatives of at least 13 families, including the Monorchiidae Odhner, 1911. Just four monorchiids have been reported from gerreids: Alloproctorema gerres Machida, 1973, Hurleytrema shorti (Nahhas & Powell, 1965) Overstreet, 1969, Postmonorchis orthopristis Hopkins, 1941 and Pseudohurleytrema eucinostomi (Manter, 1942) Yamaguti, 1954 (see Manter, Reference Manter1942; Siddiqi & Cable, Reference Siddiqi and Cable1960; Nahhas & Cable, Reference Nahhas and Cable1964; Overstreet, Reference Overstreet1969; Machida, Reference Machida1973; Wallet & Kohn, Reference Wallet and Kohn1987; Machida, Reference Machida2011). The overall paucity of monorchiids in gerreid fishes is perhaps surprising, considering that they prey on invertebrates, which are known monorchiid intermediate hosts.
Ten gerreid species are known in Australia, and there have been no reports of monorchiid infections in these species from the region. Of these, Gerres oyena (Forsskål) and Gerres subfasciatus (Cuvier) have the widest distribution and are the most commonly encountered species on the east coast of Australia. Here, we report a new monorchiid species from these gerreids and partly elucidate its life cycle. We also report an unidentified monorchiid infection from a tellinid bivalve and provide a description and molecular sequence data for it.
Materials and methods
Host and parasite collection
Specimens of G. oyena and G. subfasciatus were collected from Moreton Bay (south-eastern Queensland) and off Heron Island (southern Great Barrier Reef), Queensland, Australia. In total, 80 specimens of G. oyena were collected from Heron Island and 41 from Moreton Bay, and 147 specimens of G. subfasciatus were collected from Moreton Bay. Fishes from Moreton Bay were collected via tunnel-netting and seine netting, and those from off Heron Island only via seine netting. The two species are morphologically similar and were differentiated by the presence of scales between the eyes and posterior to the nostrils in G. subfasciatus and the absence of these scales in G. oyena (Froese & Pauly, Reference Froese and Pauly2021). Fishes were euthanized via an overdose of anaesthetic (AQUI-S®, AQUI-S New Zealand Ltd, Lower Hutt, New Zealand). The gastrointestinal tract was removed and examined for parasites using the gut wash method described by Cribb & Bray (Reference Cribb and Bray2010). Trematodes were washed in vertebrate saline, fixed by pipetting into near-boiling saline and preserved in 80% ethanol for parallel morphological and molecular characterization. Hologenophores and paragenophores (sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) were prepared for several specimens.
Specimens of Codakia paytenorum (Iredale) and Jactellina clathrata (Deshayes) were collected from the reef flat on the southern side of Heron Island; 383 specimens of C. paytenorum and 208 specimens of J. clathrata were collected. Bivalves were collected by digging and sifting sand at low tide and identified according to Lamprell & Whitehead (Reference Lamprell and Whitehead1992). Bivalves were either shucked or cracked open with a hammer, and the viscera was carefully teased apart with forceps and examined for the presence of intramolluscan digenean stages. Trematode material was separated from bivalve host tissue and fixed by pipetting into near-boiling saline and preserved in 80% ethanol for parallel morphological and molecular characterization. Isogenophores (sensu Pleijel et al., Reference Pleijel, Jondelius, Norlinder, Nygren, Oxelman, Schander, Sundberg and Thollesson2008) were prepared for some specimens.
Morphological analysis
Specimens were washed in fresh water, stained in Mayer's haematoxylin, destained in 1.0% hydrochloric acid and neutralized in 1.0% ammonium hydroxide solution. Specimens were then dehydrated through a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Measurements were made using an Olympus SC50 digital camera mounted on an Olympus BX-53 compound microscope (Olympus, Notting Hill, Australia) with cellSens Standard imaging software (https://www.olympus-lifescience.com/en/software/cellsens), and are in micrometres (μm) and presented as the range followed by the mean in parentheses. Where length is followed by breadth, the two measurements are separated by ‘×’. Line drawings were made with a drawing tube fitted to the same compound microscope and digitized in Adobe Illustrator CC 2018 software (https://www.adobe.com/au/products/illustrator.html). Type specimens are lodged in the Queensland Museum, Brisbane. To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new taxon have been submitted to ZooBank; the Life Science Identifier (LSID) is reported in the taxonomic summary.
Molecular sequencing
Genetic sequence data were generated for two barcoding regions: the complete second internal transcribed spacer unit (ITS2) ribosomal DNA (rDNA) noncoding region and the partial cytochrome c oxidase 1 (cox1) mitochondrial DNA (mtDNA) region, and one phylogenetically informative region: the partial large ribosomal subunit (28S) rDNA region. Amplification of the two rDNA regions was performed following the protocols of Wee et al. (Reference Wee, Cutmore, Yong and Cribb2017b) using the primers 3S (5′-GGT ACC GGT GGA TCA CGT GGC TAG TG-3′; Morgan & Blair, Reference Morgan and Blair1995) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′; Cribb et al., Reference Cribb, Anderson, Adlard and Bray1998) for the ITS2 amplification, and LSU5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC A-3′; Littlewood, Reference Littlewood1994) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′; Snyder & Tkach, Reference Snyder and Tkach2001) for the 28S amplification. Amplification of the mtDNA region was performed following the protocols of Wee et al. (Reference Wee, Cribb, Bray and Cutmore2017a) using the primers Dig_cox1Fa (5′-ATG ATW TTY YTD ATG CC-3′; Wee et al., Reference Wee, Cribb, Bray and Cutmore2017a) and Dig_cox1R (5′-TCN GGR TGH CCR AAR AAY CAA AA-3′; Wee et al., Reference Wee, Cribb, Bray and Cutmore2017a). All amplifications were conducted on a Takara TP-650 PCR Thermocycler (Takara, Otsu, Shiga, Japan). Sanger sequencing was conducted using the amplification primers for the ITS2 and cox1 regions, and an internal pair of primers for the 28S regions: 300F (5′-CAA GTA CCG TGA GGG AAA GTT G-3′; Littlewood et al., Reference Littlewood, Curini-Galletti and Herniou2000) and ECD2 (5′-CCT TGG TCC GTG TTT CAA GAC GGG-3′; Littlewood et al., Reference Littlewood, Rohde and Clough1997). Contiguous sequences were assembled and edited with Geneious® version 11.0.5 (Kearse et al., Reference Kearse, Moir and Wilson2012). For ITS2 rDNA sequences, the start and end of the region were annotated via the ITS2 Database (Keller et al., Reference Keller, Schleicher, Schultz, Müller, Dandekar and Wolf2009; Ankenbrand et al., Reference Ankenbrand, Keller, Wolf, Schultz and Förster2015) using the ‘Metazoa’ model. GenBank accession numbers for novel sequence data are provided in the taxonomic summaries.
Alignments for the ITS2 and cox1 datasets were conducted in MEGA version X with MUSCLE algorithm and UPGMA clustering for iterations 1 and 2 (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018). The cox1 alignment was checked for stop codons following translation with the echinoderm/flatworm mitochondrial code, and the correct reading frame was identified. The first column was then removed so that the reading frame began on position one, simplifying position-coding in downstream analyses. The alignment was then tested for non-stationarity and substitution saturation with a χ2 test run on PAUP* (Swofford, Reference Swofford2002) and Xia's test run on DAMBE7 (Xia et al., Reference Xia, Xie, Salemi, Chen and Wang2003; Xia & Lemey, Reference Xia, Lemey, Lemey, Salemi and Vandamme2009; Xia, Reference Xia2018), respectively; no significant non-stationarity and substitution saturation was detected. Neighbour-joining analyses were also conducted in MEGA version X using the ITS2 and cox1 datasets to explore the number of base pair differences between samples and to determine species boundaries. The parameters used for both analyses were: ‘test of phylogeny = bootstrap’, ‘no. of bootstrap replications = 10,000’, ‘model/method = no. of differences’, ‘substitutions to include = d: Transitions + Transversions’ and ‘rates among sites = Uniform rates’.
Phylogenetic analyses
The newly generated partial 28S rDNA sequences were aligned with sequences of other monorchiid taxa available on GenBank (table 1) using MUSCLE version 3.7 (Edgar, Reference Edgar2004) with ClustalW sequence weighting and UPGMA clustering for iterations 1 and 2. The resultant alignment was trimmed manually, and indels larger than three bases and affecting >5% of sequences were removed; the removed bases amounted to less than 3% of the final alignment, which comprised 1275 base positions. Bayesian inference (BI) and maximum likelihood (ML) analyses were conducted using the implementations of MrBayes version 3.2.6 (Ronquist et al., Reference Ronquist, Teslenko and van der Mark2012) and RAxML version 8.2.6 (Stamatakis, Reference Stamatakis2014), respectively, in the CIPRES portal (Miller et al., Reference Miller, Pfeiler and Schwartz2010). Both analyses were run with the closest estimation of the GTR + I+Γ model of evolution, based on implementation of the Akaike Information Criterion in jModelTest version 2.1.10 (Darriba et al., Reference Darriba, Taboada, Doallo and Posada2012). The BI analysis was run over 10,000,000 generations (ngen = 10,000,000) with two runs each containing four simultaneous Markov chain Monte Carlo (MCMC) chains (nchains = 4) and every 1000th tree saved. The following parameters were used in the analysis: nst = 6, rates = invgamma, ngammacat = 4 and the priors parameters of the combined dataset were set to ratepr = variable. Samples of substitution model parameters were ‘sump burnin’ = 3000 and ‘sumt burnin’ = 3000. The ML analysis was run with 1000 bootstrap pseudoreplicates. Sequence data for the Lissorchiidae, the sister family to the Monorchiidae, were included in this dataset, and the deropristid Skrjabinopsolus nudidorsalis Sokolov, Voropaeva & Atopkin, 2020 was designated as the outgroup, based on phylogenetic findings of Sokolov et al. (Reference Sokolov, Voropaeva and Atopkin2020).
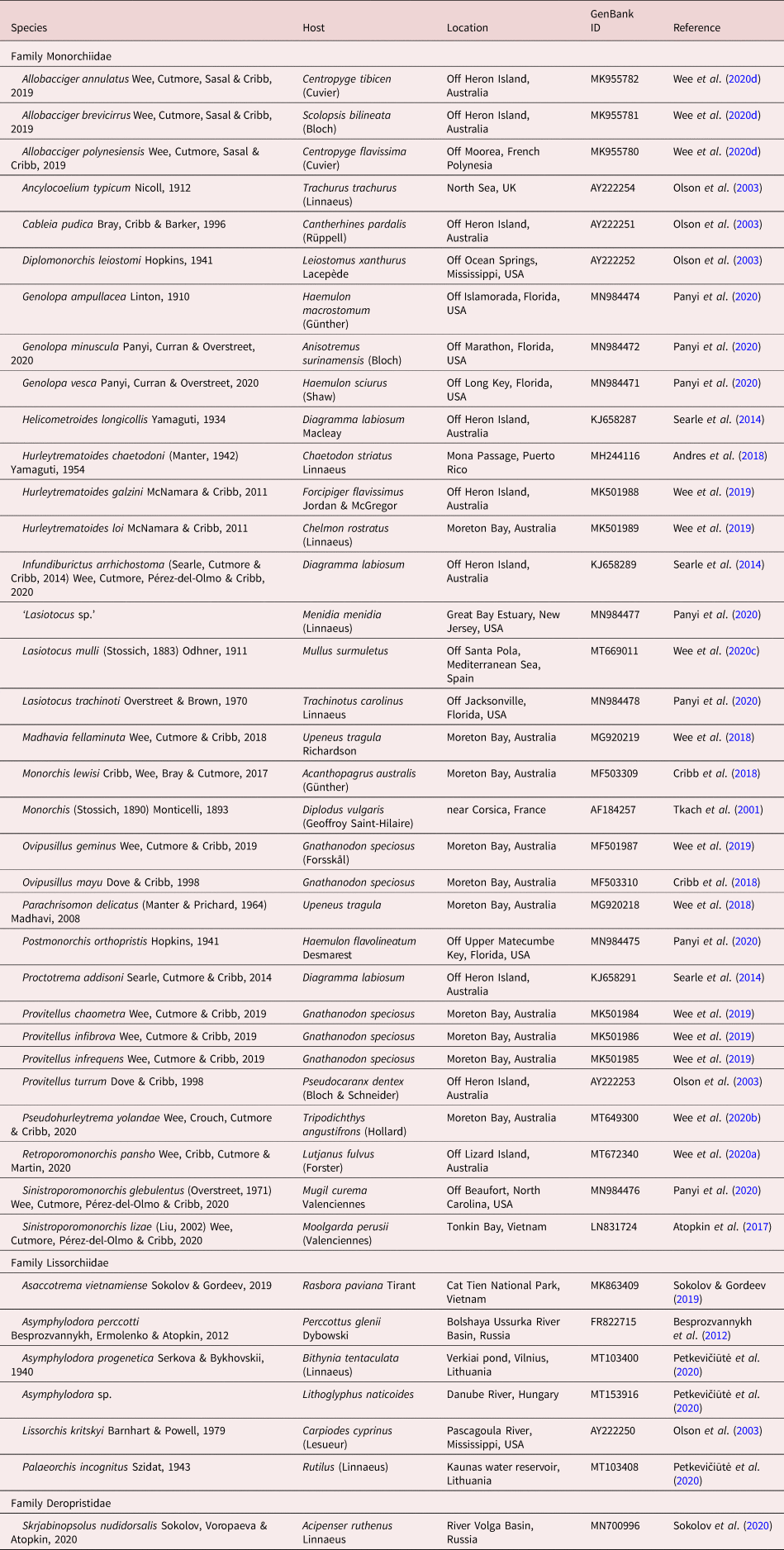
Table 1. Collection data for 28S sequences from GenBank analysed in this study.
Results
Of the examined gerreid specimens, 32 specimens of G. oyena from Heron Island, seven of G. oyena from Moreton Bay and 30 of G. subfasciatus were infected with adult trematodes consistent with the concept of the Monorchiidae, and all specimens appeared to conform morphologically to a single species. For the ITS2 rDNA region, eight identical sequences were generated (all from G. oyena, seven from Heron Island and one from Moreton Bay). For the cox1 mtDNA region, six sequences were generated (four from G. oyena from Heron Island, one from G. oyena from Moreton Bay and one from G. subfasciatus from Moreton Bay), representing three genotypes differing by 1–4 base pairs. We interpret these small differences as consistent with intraspecific variation. For the 28S rDNA region, three identical sequences were generated (all from G. oyena, two from Heron Island, one from Moreton Bay).
Of the examined specimens of the bivalve C. paytenorum from Heron Island, we found a single infection of monorchiid sporocysts and cercariae. ITS2 and cox1 sequence data generated for these intramolluscan stages were used to match them to the adult forms. The ITS2 sequence was identical to the sequences from sexual adults infecting G. oyena and G. subfasciatus, and the cox1 sequence was identical to two sequences from sexual adults infecting G. oyena from Heron Island. The 28S sequence for these intramolluscan stages differed from sequences from sexual adults at a single base position. Of the examined specimens of the bivalve J. clathrata from Heron Island, we found a single infection of monorchiid sporocysts and cercariae. ITS2, cox1 and 28S sequence data generated for this intramolluscan infection do not match any publicly available monorchiid sequence on GenBank.
Taxonomic summary
-
Family Monorchiidae Odhner, 1911
-
Subfamily Monorchiinae Odhner, 1911
Gerricola n. g.
Diagnosis
Body moderately elongate, pyriform. Tegument thin, spined. Eye-spot pigment present in forebody. Oral sucker terminal, almost round, with opening subterminal. Ventral sucker spherical, in middle third of body. Prepharynx short. Pharynx smaller than oral sucker, subspherical. Oesophagus simple, moderately long. Intestine bifurcates immediately anterior to ventral sucker. Intestinal caeca blind, long, extend into post-testicular region, near to posterior extremity of body. Testis single, entire, in posterior half of hindbody. Cirrus sac subcylindrical, in middle third of body, extends from anterior margin of testis to level of anterior portion of ventral sucker. Seminal vesicle unipartite, cylindrical. Pars prostatica short to moderately long, simple. Cirrus prominent, spined. Genital atrium aspinous. Common genital pore median, immediately anterior to ventral sucker. Ovary entire or weakly lobed, dextro-submedial, antero-dextral to and contiguous with testis. Uterine seminal receptacle present. Vitellarium composed of two, well-separated lateral clusters of regular, dense follicles in mid-hindbody. Uterus extensive in hindbody, extends beyond gonads posteriorly, without discernible metraterm, enters terminal organ at junction of spined and unspined sections. Terminal organ bipartite, smaller than cirrus sac; posterior section saccular, unspined; anterior section spined, without gap in spination. Eggs small, unfilamented. Excretory vesicle small, saccular, restricted to post-gonadal region. Excretory pore terminal. In intestine of gerreid fishes.
Type and only species. Gerricola queenslandensis n. g., n. sp.
ZooBank registration. The LSID for Gerricola n. g. is: urn:lsid:zoobank.org:act:F60871B3-9007-4F02-963B-1EC6A48DDD51.
Etymology. The name is derived from the definitive host genus, Gerres, and the Latin word ‘cola’, for inhabiting. The genus is to be treated as masculine.
Gerricola queenslandensis n. g., n. sp.
Adults
Type host. Gerres oyena (Forsskål), the blacktip silverbiddy (Perciformes: Gerreidae).
Other host. Gerres subfasciatus Cuvier, the common silverbiddy (Perciformes: Gerreidae).
Type locality. Off Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E).
Other locality. Eastern Moreton Bay, Queensland, Australia (27°24′S, 153°20′E).
Site of infection. Intestine.
Prevalence. Gerres oyena, 32/80 (40.0%) (Heron Island), 7/41 (17.1%) (Moreton Bay); G. subfasciatus, 30/147 (20.4%) (Moreton Bay).
Deposition of specimens. Holotype (QM G239139) and 27 paratypes, comprising two hologenophores (QM G239165–6) and 25 paragenophores (QM G239140–63).
Molecular sequence data. ITS2 rDNA, eight identical replicates, all from G. oyena (seven from Heron Island, one from Moreton Bay), one submitted to GenBank (GB MZ271998); cox1 mtDNA, six sequences (four from G. oyena from Heron Island, and one each from G. oyena and G. subfasciatus from Moreton Bay), comprising three genotypes, one replicate of each host locality combination from each genotype submitted to GenBank (GB MZ295277, MZ295279–81); 28S rDNA, three identical replicates (all from G. oyena, two from Heron Island, one from Moreton Bay), one submitted to GenBank (GB MZ271999).
ZooBank registration: The Life Science Identifier (LSID) for Gerricola queenslandensis n. g., n. sp. is urn:lsid:zoobank.org:act:73AD8BD8-97D5-4EF5-8FF8-ACBA09A32F77.
Etymology. The specific name queenslandensis refers to the state of Australia where the species is found.
Description
Based on 28 gravid, unflattened specimens: 15 from G. oyena from Heron Island, six from G. oyena from Moreton Bay, seven from G. subfasciatus from Moreton Bay (fig. 1a–c).

Fig. 1. Sexual and intramolluscan stages of Gerricola queenslandensis n. g., n. sp., and intramolluscan stages of Monorchiidae sp. from Heron Island, Queensland, Australia. (a) Gerricola queenslandensis n. g., n. sp., adult worm, ventral view; (b) G. queenslandensis n. g., n. sp., adult worm, eggs and uterus excluded, ventral view; (c) G. queenslandensis n. g., n. sp., terminal genitalia and part of uterus, ventral view; (d) G. queenslandensis n. g., n. sp., sporocyst containing cercariae; (e) Monorchiidae sp. ex Jactellina clathrata, sporocyst; (f) Monorchiidae sp. ex J. clathrata, cercaria. Scale bars: (a, b) 200 μm; (c–f) 100 μm.
Body small, pyriform, slightly tapering anteriorly and moderately rounded posteriorly, 442–789 (526) × 120–238 (163), 2.60–3.68 (3.15) times longer than wide, widest at middle of hindbody. Forebody 136–219 (171) long, occupying 29.6–39.9 (34.6)% of body length; hindbody 228–495 (299) long, occupying 51.6–66.4 (56.6)% of body length. Tegument thin, uniformly covered with small, fine, regular spines. Eye-spot pigment granules present, restricted to forebody.
Oral sucker terminal, almost round, with opening distinctly subterminal, 32–67 (48) × 40–70 (50), 0.90–1.28 (1.03) times wider than long. Ventral sucker roughly spherical, 40–65 (50) × 42–70 (52), 0.88–1.19 (1.04) times wider than long; ventral sucker 0.97–1.51 (1.08) times longer and 0.96–1.21 (1.05) times wider than oral sucker. Prepharynx short. Pharynx muscular, subspherical, 25–46 (32) × 29–49 (34), 0.85–1.06 (0.96) times longer than wide; pharynx length 61.9–78.7 (68.8)% of oral sucker length; pharynx width 57.1–82.2 (69.9)% of oral sucker width. Oesophagus moderately long, gently to strongly sinuous, 49–137 (84) long, occupies 10.7–20.9 (16.9)% of body length. Intestinal bifurcation immediately anterior to ventral sucker; pre-bifurcal zone occupies 26.7–41.3 (32.8)% of body length. Intestinal caeca blind, long, occupy 53.8–69.7 (61.1)% of body length, terminate in post-testicular zone, approximately at level of excretory vesicle, 7.6–16.8 (11.0)% of body length from posterior end of body.
Testis single, large, entire, subspherical to transversely or longitudinally ellipsoidal, mainly in posterior half of hindbody, median, can overlap either caecum, separated by 12.9–25.4 (19.6)% of body length from ventral sucker, 61–141 (96) × 71–150 (93); pre-testicular zone 52.1–71.2 (64.2)% of body length; post-testicular zone 9.7–29.5 (17.8)% of body length. Cirrus sac mainly in middle third of body, subcylindrical, mostly median, typically mostly intercaecal, slightly overlapping both caeca, with posterior end typically slightly overlapping ovary and partially overlapping testis in some specimens, with posterior end typically slightly sinistro-submedian and anterior end slightly dextro-submedian, 108–198 (131) × 24–53 (35), occupies 21.2–32.1 (26.5)% of body length. Seminal vesicle ellipsoidal, unipartite, 33–77 (51) × 18–49 (31), occupies 27.4–52.1 (38.4)% of cirrus sac length. Pars prostatica short or moderately long, simple, with few prostatic cells observed, 17–51 (27) long. Cirrus relatively narrow, subcylindrical, armed with small acicular spines, tapers slightly anteriorly, 30–69 (44) × 11–27 (17), occupies 20.7–42.6 (33.5)% of cirrus sac length. Genital atrium prominent, aspinous, ellipsoidal, simple. Common genital pore median, immediately anterior to ventral sucker.
Ovary in middle of hindbody, anterodextral to and partially overlapping testis, partially overlaps right caecum, smooth, roughly triangular, 9.3–16.9 (12.7)% of body length from ventral sucker, 42–100 (55) × 38–81 (54); pre-ovarian zone occupies 46.7–62.8 (57.0)% of body length; post-ovarian zone occupies 25.6–40.8 (33.2)% of body length. Mehlis’ gland large, dorsal to and easily mistaken as part of ovary, not observed in some specimens. Uterine seminal receptacle present. Vitellarium composed of two lateral masses of densely clustered follicles, at level of ovary and testis, may extend slightly anteriorly to ovary, never extends posteriorly beyond testis, ventral to and partially overlapping both caeca, mass length 42–101 (62), occupying 8.3–14.5 (12.0)% of body length. Uterus thin-walled, extensive, restricted to hindbody, ventral to ovary, testis, caeca and part of cirrus sac, coils mostly indiscernible, with ascending coil entering terminal organ at junction of spined and unspined sections. Terminal organ sinistro-ventral to and about a third the length of cirrus sac, bipartite, comprising unspined, saccular posterior chamber, and spined, tubular anterior section, 55–107 (72) × 26–46 (36). Saccular posterior chamber roughly spherical, contains fibrous mass, 30–65 (41) × 23–46 (32). Tubular anterior section with small acicular spines, approximately same size as those in cirrus, roughly subcylindrical, 16–44 (27) × 9–20 (14). Eggs small, lightly tanned, operculate, unfilamented, 13–20 (17) × 6–11 (8).
Excretory vesicle small, saccular, reaches to level of posterior ends of caeca. Excretory pore terminal.
Sporocysts and cercariae
Host. Codakia paytenorum (Iredale), Payten's Lucina Clam (Lucinidae).
Locality. Off Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E).
Site of infection. Gonad.
Prevalence. One of 383 (0.26%).
Deposition of specimens. Isogenophores (QM G239167–71).
Molecular sequence data. One replicate each of ITS2 rDNA (GB MZ271997), 28S rDNA (GB MZ272000) and cox1 mtDNA (GB MZ295278).
Description
Sporocysts. Based on ten specimens (fig. 1d). Elongate, 313–469 (381) × 62–88 (75), mostly contain germinal balls, some with one or two recognizable cercariae in various developmental stages.
Cercariae. Based on five relatively well-developed specimens (fig. 1d). Oculate distome cercaria. Body elongate, 216–340 (260) × 57–70 (61), 3.26–5.96 (4.30) times longer than wide. Tegumental spines not observed. Forebody 72–89 (82) long, occupies 25.6–35.2 (31.8)% of body length. Oral sucker terminal, roughly round, 26–39 (31) × 28–33 (31), 0.81–1.27 (1.01) times wider than long. Ventral sucker roughly spherical, in posterior third of body, 27–33 (30) × 26–33 (29), 0.79–1.22 (0.99) times wider than long; 0.85–1.04 (0.97) times length and 0.79–1.18 (0.96) times width of oral sucker. Two eye-spots at pharyngeal or oesophageal zone, recognizable from early in development. Penetration glands not observed. Prepharynx not observed. Pharynx small, 17–22 (20) × 13–18 (16). Oesophagus not observed. Caeca still in early stages of development, reach to near posterior extremity of body. Genital primordium at level of ventral sucker. Excretory bladder ovoid, large, 37–43 (41) × 31–36 (34), occupies 12.6–18.9 (16.1)% of body length. Flame cell pattern not determined. Tail long, unspecialized, 110–181 (136) × 14–22 (17), 0.42–0.61 (0.53) times length of body.
Remarks
The infection here was not patent, given all cercariae were under-developed. The cercaria has most typical monorchiid characters – distome, a pharynx, eye-spots, long tail and lacking a stylet, but we were unable to detect any tegumental spines. Notably, excysted cercariae were poorly developed or damaged beyond any practical use, and the intrasporocyst cercariae depicted in fig. 1d were the best specimens available to us.
Cercariae and sporocysts ex Jactellina clathrata (Deshayes)
Host. Jactellina clathrata (Deshayes) (Tellinidae).
Locality. Off Heron Island, southern Great Barrier Reef, Queensland, Australia (23°27′S, 151°55′E).
Site of infection. Gonad.
Prevalence. One of 208 (0.48%).
Deposition of specimens. Isogenophores (QM G239172–78).
Molecular sequence data. One replicate each of ITS2 rDNA (GB MZ271996), 28S rDNA (GB MZ272001) and cox1 mtDNA (GB MZ295282).
Description
Sporocysts. Based on ten specimens (fig. 1e). Elongate, 907–1165 (1006) × 116–168 (149), contain 13–26 recognizable cercariae in various developmental stages and germinal balls.
Cercariae. Based on eight relatively well-developed specimens (fig. 1f). Oculate distome cercaria. Body elongate, 134–183 (165) × 57–80 (72), 1.89–2.93 (2.28) times longer than wide. Tegumental spines not observed. Forebody 75–112 (95) long, occupies 50.0–69.4 (57.5)% of body length. Oral sucker terminal, roughly round, 37–41 (39) × 33–41 (38), 0.89–1.05 (0.99) times wider than long. Ventral sucker roughly spherical, 32–41 (38) × 34–42 (38), 0.90–1.06 (1.00) times wider than long; 0.78–1.05 (0.98) times length and 0.87–1.07 (0.99) times width of oral sucker. Two eye-spots at pharyngeal or oesophageal zone, recognizable from early development. Penetration glands not observed. Prepharynx not observed. Pharynx small, 16–29 (20) × 14–22 (20). Oesophagus short. Caeca mostly still in early stages of development, reach posterior third of body. Genital primordium at level of ventral sucker. Excretory bladder ovoid, large, lined with conspicuous cells, reaches to dorsal to ventral sucker, 49–66 (58) × 31–41 (37), occupies 29.3–38.7 (35.2)% of body length. Flame cell pattern not determined. Tail long, unspecialized, 192–264 (230) × 17–22 (19), 1.05–1.57 (1.41) times length of body.
Remarks
The single infection may not have been patent, given that most cercariae were under-developed. This cercaria has most typical monorchiid characters – distome, a pharynx, eye-spots, long tail and lacking a stylet – but we were unable to detect any tegumental spines. Sequence data did not match any known monorchiid species with sequence information publicly available on GenBank.
Molecular phylogenetic analyses
BI (fig. 2) and ML analyses of the 28S rDNA dataset produced similar phylograms in which G. queenslandensis n. g., n. sp. resolved deep within a large clade predominantly comprising monorchiine taxa. Within this large clade, however, the species formed a well-supported clade with the hurleytrematine species Pseudohurleytrema yolandae Wee, Crouch, Cutmore & Cribb, 2020, but with noticeably long branch lengths separating the two. This clade is sister to a clade comprising species of Allobacciger Hafeezullah & Siddiqi, 1970, Genolopa Linton, 1910, Monorchis Monticelli, 1893 and Sinistroporomonorchis Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020, and Infundiburictus arrhichostoma (Searle, Cutmore & Cribb, 2014) Wee, Cutmore, Pérez-del-Olmo & Cribb, 2020, P. orthopristis Hopkins, 1941, Retroporomonorchis pansho Wee, Cribb, Cutmore & Martin, 2020 and ‘Lasiotocus sp.’ The monorchiid from J. clathrata formed a poorly supported clade with Diplomonorchis leiostomi and two species of Lasiotocus, resolving sister to these recognized species. There were two differences with regards to the overall topology of the Monorchiidae between the two analyses, with the first involving the position of R. pansho. In the BI analysis, R. pansho resolves sister to a clade comprising Monorchis monorchis (Stossich, 1890) Looss, 1902, ‘Lasiotocus sp.’ and species of Sinistroporomonorchis, whereas in the ML analysis, R. pansho resolves sister to a large clade comprising the same species as well as I. arrhichostoma, Monorchis lewisi Cribb, Wee, Bray & Cutmore, 2018 and species of Allobacciger; nodal support for its position in both analyses are poor. The second involves the positions of Helicometroides longicollis Yamaguti, 1934 and Cableia pudica Bray, Cribb & Barker, 1996, which were swapped in the two analyses, with the former the most basal monorchiid taxon in the BI analysis, and the latter the most basal in the ML analysis; the basal support of the derived taxon in both analyses were poor.
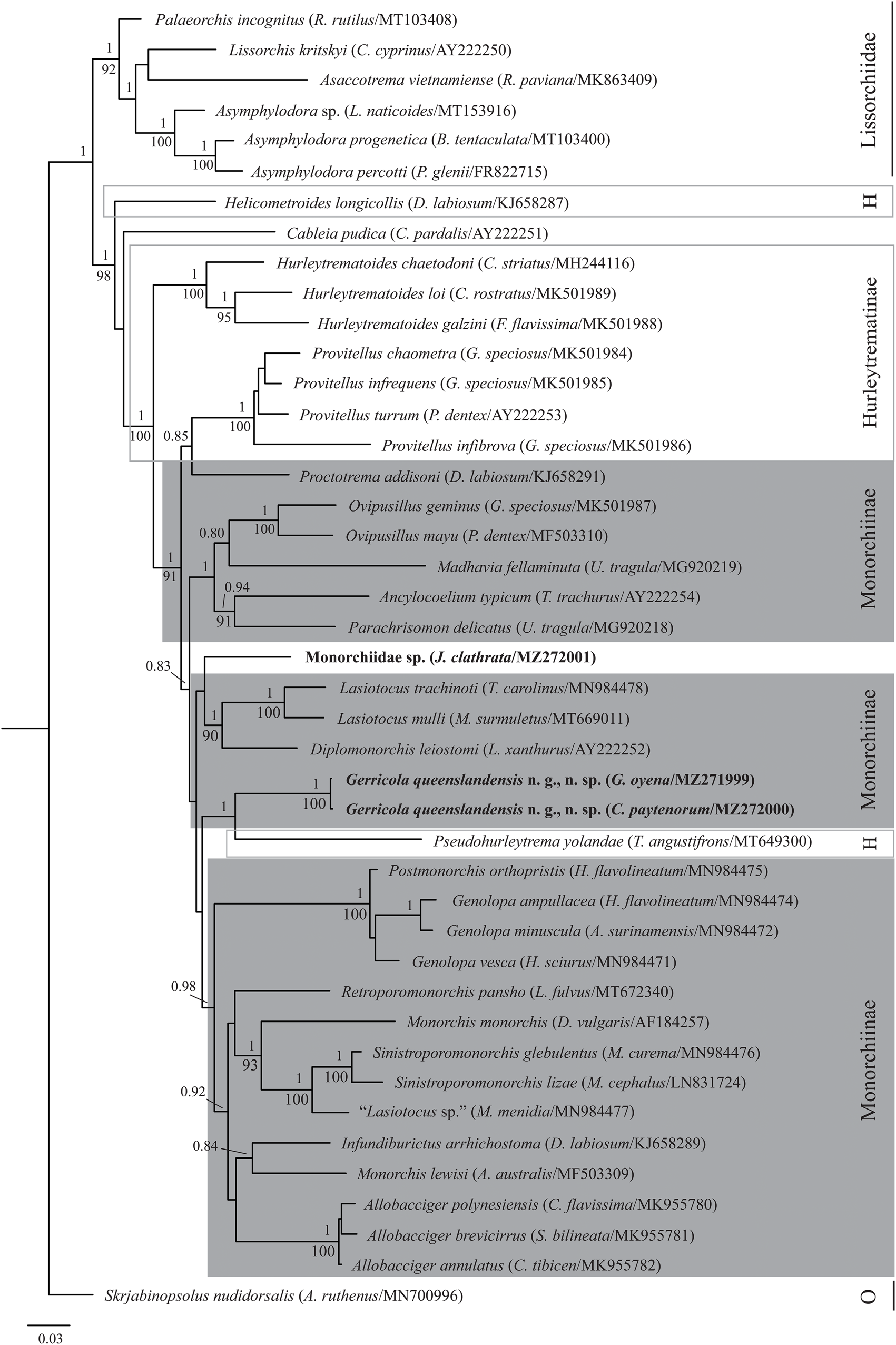
Fig. 2. Relationships of monorchiid taxa based on Bayesian inference analyses of 28S rDNA. Novel sequences for Gerricola queenslandensis n. g., n. sp. and the infection from Jactellina clathrata are indicated in bold. Host species and GenBank accession numbers are provided in parentheses. Monorchiid subfamilies are marked; the Hurleytrematinae is polyphyletic, the Monorchiinae is paraphyletic and Cableia pudica and the infection from J. clathrata are unassigned. Posterior probabilities are shown above the nodes, and where relationships were replicated in the maximum likelihood analysis, bootstrap values are shown below. Nodal support below 0.80/80 not shown. The scale bar represents the expected number of substitutions per site. Abbreviations: H, Hurleytrematinae; O, outgroup.
Discussion
Morphology and taxonomy of G. queenslandensis n. g., n. sp.
In the possession of a single testis, unfilamented eggs and a genital pore in the forebody, our specimens clearly belong to the subfamily Monorchiinae Odhner, 1911, as recognized by Madhavi (Reference Madhavi, Bray, Gibson and Jones2008). According to the key to monorchiine genera of Wee et al. (Reference Wee, Cutmore, Pérez-del-Olmo and Cribb2020c), the present species most closely conforms to the newly restricted concept of Lasiotocus Looss, 1907 in the possession of an unspined genital atrium, bipartite terminal organ, round oral sucker and unlobed ovary. However, as demonstrated by analysis of the 28S rDNA dataset, our new specimens are only distantly related to the two sequenced species of Lasiotocus, Lasiotocus mulli (Stossich, 1883) Odhner, 1911 (the type species) and Lasiotocus trachinoti Overstreet & Brown, 1970, and thus require a separate genus. Gerricola queenslandensis n. g., n. sp. is morphologically similar to these two Lasiotocus species, differing significantly only in the posterior extent of the caeca (terminating in the post-testicular region in G. queenslandensis n. g., n. sp. vs. at level of the testis in both Lasiotocus species) and spination of the terminal organ (no gap in terminal organ spines in G. queenslandensis n. g., n. sp. vs. distinct gap in terminal organ spines in both Lasiotocus species). The gap in terminal organ spines was not mentioned as an important taxonomic feature in the most recent revision of Lasiotocus (see Wee et al., Reference Wee, Cutmore, Pérez-del-Olmo and Cribb2020c); however, Panyi (Reference Panyi2020) speculated that the feature might be of generic level importance and our findings here support that view.
The remaining six species of Lasiotocus are morphologically similar to G. queenslandensis n. g., n. sp. in the possession of caeca that terminate in the post-testicular region, which might ultimately warrant their transfer to Gerricola n. g. However, in the recent revision of Lasiotocus, Wee et al. (Reference Wee, Cutmore, Pérez-del-Olmo and Cribb2020c) cast doubt on the monophyly of these six species due to a combination of significant morphological variation, a lack of information on some features and their exploitation of fishes from a wide range of families. These issues will persist even if they are transferred to Gerricola n. g., and we think the conservative course of action is to leave these six species in Lasiotocus until molecular sequence data are available to better inform on their phylogenetic positions.
Phylogeny
Although G. queenslandensis n. g., n. sp. resolves deep within a large clade predominantly comprising other monorchiine taxa, it is surprising that it resolves sister to the hurleytrematine species P. yolandae, with strong nodal support. Although the position of P. yolandae within this large clade consisting primarily of monorchiine taxa was demonstrated by Wee et al. (Reference Wee, Crouch, Cutmore and Cribb2020b), the nodal support for its position at the time (sister to Proctotrema addisoni Searle, Cutmore & Cribb, 2014) was poor. Overall, the phylogenetic position of P. yolandae and species of Provitellus Dove & Cribb, 1998 relative to G. queenslandensis n. g., n. sp. and P. addisoni, respectively, in which a hurleytrematine taxon/taxa (G. queenslandensis n. g., n. sp. and Provitellus spp.) forms a clade with a monorchiine taxon, is evidence that both subfamilies require restructuring. However, given that so many genera are not yet represented by molecular sequence data, it remains premature to propose a major revision of either subfamilial structure.
Intramolluscan stages and their bivalve hosts
The sporocysts of both infections reported here are consistent with previously reported monorchiid sporocysts (Cremonte et al., Reference Cremonte, Kroeck and Martorelli2001; Gilardoni et al., Reference Gilardoni, Carballo and Cremonte2013; Bagnato et al., Reference Bagnato, Gilardoni, Pina, Rodrigues and Cremonte2016) in being unspecialized, immobile, thick-walled sacs containing cercariae of varying developmental stages. Cercariae from both infections exhibit typical monorchiid cercarial features – two suckers, a pharynx, eye-spots, a thick-walled excretory vesicle, a long, thin tail and lacking a stylet (Cable, Reference Cable1956). However, as our specimens were under-developed, tegumental spines exhibited by other monorchiid cercariae were not observed in either species. As per the classification of monorchiid cercariae proposed by Cremonte et al. (Reference Cremonte, Kroeck and Martorelli2001), both new cercariae fall into Group 1, cercariae that possess eye-spots and a well-developed tail. The present infection in C. paytenorum is the second record of a monorchiid infection in a lucinid bivalve. The other record is of an unidentified monorchiid, Cercaria caribbea LXIV (and its sporocysts) from Parvilucina pectinella (C. B. Adams) from Jamaica (Cable, Reference Cable1963), which also conforms to the concept of Group 1 cercariae. The unidentified infection from J. clathrata is the fourth record of monorchiids from tellinid bivalves, and the Tellinidae is the second most infected bivalve family, with more reports from only the Veneridae (five records). Two of the other three monorchiid infections from tellinids are unidentified, Cercaria caribbea XXXV Cable, 1956 infects Macoma cerina C.B. Adams from Puerto Rico (Cable, Reference Cable1956), and Cercaria caribbea LXIII Cable, 1963 infects Serratina martinicensis (d'Orbigny) from Jamaica (Cable, Reference Cable1963). The third, Monorcheides cumingiae (Martin, 1938) Martin, 1939, infects Macoploma tenta (Say) from the USA. These cercariae also conform to the concept of Group 1 cercariae.
Our study is the second report of bivalves as monorchiid intermediate hosts in Australia, following Bott et al. (Reference Bott, Healy and Cribb2005). Bott et al. (Reference Bott, Healy and Cribb2005) examined 47 bivalve species from 17 families off Queensland, including from off Heron Island. They reported monorchiids in five bivalve species, four from Heron Island: C. paytenorum (Lucinidae), J. clathrata (Tellinidae), Scissulina dispar (Conrad) (Tellinidae) and Pinguitellina robusta (Hanley) (Tellinidae). However, the study did not describe nor sequence the intramolluscan stages and the monorchiids were not formally identified. The infections in C. paytenorum and J. clathrata in the present study likely correspond to the same species reported by Bott et al. (Reference Bott, Healy and Cribb2005).
Bivalves reported as the first intermediate hosts of monorchiids are relatively diverse, comprising 22 species from ten families from two superorders, the Anomalodesmata and Imparidentia, both of the infraclass Heteroconchia. Following the present study, these bivalves harbour 11 species of monorchiids matched to adults, as well as an additional 11 unidentified cercariae. Of the 11 matched monorchiids, only G. queenslandensis n. g., n. sp. is represented in our phylogenetic analysis. While there is molecular representation for four other genera that have had elucidated life cycles – Lasiotocus, Monorchis, Postmonorchis Hopkins, 1941 and Proctotrema Odhner, 1911 – the specific species for which the first intermediate host is known are not represented. It is noteworthy that the monophyly of some of these genera (particularly Lasiotocus and Monorchis) is doubtful; thus, it is uncertain if the species matched to adults will prove convincing congeners of the species in our phylogenetic analyses.
Acknowledgements
We sincerely thank all members of the Marine Parasitology Laboratory for their support and assistance in collecting host and trematode specimens. We also thank Moreton Bay and Heron Island Research Stations for their support of our work.
Financial support
This project was supported by the PADI Foundation, the Joyce W. Vickery Research Fund administered by the Linnean Society of NSW and the Holsworth Wildlife Research Endowment & The Ecological Society of Australia, through funding awarded to NW. This project was also supported by the Australian Biological Resources Study (ABRS National Taxonomy Research Grant RG19-37) awarded to SCC. NW is supported by a PhD scholarship from the University of Queensland (Research Training Program Scholarship).
Conflicts of interest
None.
Ethical standards
This study was conducted in compliance with all institutional, national and international guidelines on the care and use of animals.





