Introduction
Obsessive–compulsive disorder (OCD) is characterized by the presence of recurrent unwanted and intrusive thoughts (obsessions) and repetitive, ritualistic behaviors (compulsions). Along with these core features, patients with OCD also experience strong feelings of distress accompanying obsessions, and cognitive models emphasize the importance of dysfunctional appraisals (Salkovskis, Reference Salkovskis1985). However, the neurobiological correlate of these affective symptoms and its relevance for OCD pathophysiology remain to be elucidated. Recent neuroanatomical models propose alterations in an affective cortico-striatal circuit involving the anterior cingulate cortex (ACC) and the ventromedial prefrontal cortex (VMPFC) as cortical nodes (Milad & Rauch, Reference Milad and Rauch2012). The ACC is implicated in the regulation of emotional and cognitive processes (Bush et al. Reference Bush, Luu and Posner2000), and the VMPFC plays a central role in the consolidation of fear extinction learning (Milad et al. Reference Milad2005). These brain areas are also involved in automatic emotion regulation (Phillips et al. Reference Phillips, Ladouceur and Drevets2008). Thus, abnormalities in this circuit might relate to distress and dysfunctional appraisal in OCD. Importantly, the amygdala, which has been shown to be hyperactive during symptom provocation (van den Heuvel et al. Reference van den Heuvel2004; Simon et al. Reference Simon2010, Reference Simon2014) may also play a role in mediating affective symptoms in OCD, but conclusive studies are still pending (Milad & Rauch, Reference Milad and Rauch2012).
Previous studies suggest that instructions to elaborate on symptom-provoking stimuli (v. distraction) are crucial for the occurrence of amygdala hyperactivity in OCD. More precisely, we recently demonstrated increased electrocortical responsivity when OCD patients were instructed to reappraise unpleasant pictures as less negative, a strategy that requires elaborate stimulus processing, while distraction successfully reduced electrocortical responses (Paul et al. Reference Paul2016). Similarly, amygdala hyperactivation in patients with OCD could be attenuated by task distraction (Simon et al. Reference Simon2014). Thus, deficient top–down control of emotion and distress when focusing on OCD-relevant stimuli might contribute to affective symptoms in OCD.
Top–down regulation of affective responses depends on amygdala–prefrontal interactions (Ochsner & Gross, Reference Ochsner, Gross and Gross2007). Importantly, dense reciprocal connections exist between the amygdala and ACC and VMPFC, respectively (Öngür & Price, Reference Öngür and Price2000; Timbie & Barbas, Reference Timbie and Barbas2014) – regions that are known to be inversely activated during emotion regulation (Urry et al. Reference Urry2006; Kanske et al. Reference Kanske2011). Moreover, the strength of their functional coupling predicts emotion regulation success (Banks et al. Reference Banks2007). Given the striking overlap between cortical areas involved in automatic emotion regulation and the proposed affective circuit in OCD, this study sought to investigate amygdala connectivity with these cortical areas in three independent samples of unmedicated OCD patients obtained from previous studies (sample 1: Simon et al. Reference Simon2014; sample 2: Simon et al. Reference Simon2010; sample 3: Beucke et al. Reference Beucke2013). The main goal was to test for altered amygdala–prefrontal coupling during appraisal of OCD symptom triggers (sample 1). Subsequent analyses were performed to determine whether connectivity alterations selectively occur during active appraisal, or rather globally, thus occurring during symptom provocation without explicit appraisal instructions (i.e. during a passive viewing task, sample 2) or even in the absence of stimulus presentation during the resting state (sample 3).
To test the hypothesis of abnormal amygdala–prefrontal coupling during appraisal of OCD symptom triggers (sample 1), neutral and disorder-relevant as well as generally aversive stimuli were presented in two conditions, i.e. instructed appraisal (appraisal task) and automatic attentional control (distraction task). Results of the corresponding conventional general linear model (GLM) analyses have already been reported in a previous publication and will hence not be described here in detail (Simon et al. Reference Simon2014). In brief, OCD patients showed increased amygdala–orbitofrontal co-activation during symptom provocation, which might point to abnormalities in functional connectivity between prefrontal cortices and the amygdala, resulting in insufficient downregulation. During distraction, amygdala hyperactivation in response to OCD-relevant pictures was significantly reduced, suggesting unimpaired inhibitory control of amygdala activation. To test these assumptions, in the current study, we re-analyzed these data using functional connectivity analyses. For the appraisal task, we predicted reduced connectivity between amygdala and prefrontal areas in OCD patients compared with healthy control participants during appraisal of OCD-relevant as compared with generally aversive pictures. We expected that when OCD-related stimulus appraisals were hindered by distraction, prefrontal areas should work properly, which would be reflected by normal amygdala–prefrontal coupling. Consequently, we hypothesized to find no connectivity differences between patients and controls in the distraction task. Results may contribute to further understanding of the influence of stimulus material, task instructions, and global connectivity alterations on abnormal amygdala–prefrontal connectivity during symptom provocation in OCD and elucidate the neural underpinnings of affective symptoms in this disorder.
Methods
Participants
Sample 1 comprised 21 OCD patients and 21 healthy control participants. Sample 2 comprised 14 OCD patients and 14 healthy control participants. Sample 3 comprised 23 OCD patients and 23 healthy control participants. All patients in these three independent samples were recruited from the outpatient clinic of the Humboldt-Universität zu Berlin, had a DSM-IV diagnosis of OCD, and were medication-free. Control participants were matched case-by-case for gender, age, handedness, and education. Exclusion criteria included the presence of other major psychiatric disorders and neurological diseases. Demographic and clinical characteristics of the study samples are shown in Table 1. A comparison between the three patient samples regarding demographic and clinical variables is provided in the supplement (see online Supplementary Table S1). Participants provided written informed consent approved by the local ethics committees (samples 1 and 2: ethics committee of the Charité-Universitätsmedizin Berlin; sample 3: ethics committee of Humboldt-Universität zu Berlin).
Table 1. Demographic and clinical variables for OCD patients and healthy controls in the emotion regulation task (sample 1), the passive viewing task (sample 2), and during resting state (sample 3)

OCD, obsessive–compulsive disorder; HC, healthy controls; BDI, Beck Depression Inventory; OCI-R, Obsessive–Compulsive Inventory-Revised; MADRS, Montgomery–Asberg Depression Rating Scale; Y-BOCS, Yale–Brown Obsessive Compulsive Scale; STAI-T, Trait version of State-Trait Anxiety Inventory; STAI-S, State version of State-Trait Anxiety Inventory.
a Lifetime co-morbid Axis-I diagnoses included major depressive disorder (n = 10), specific phobia (n = 4), generalized anxiety disorder (n = 1), panic disorder (n = 1), social phobia (n = 2), agoraphobia (n = 1), substance abuse (n = 1), and binge-eating disorder (n = 1).
b The gender ratio was 13 females to eight males.
c Lifetime co-morbid Axis-I diagnoses included major depressive disorder (n = 2), dysthymia (n = 1), specific phobia (n = 1), panic disorder (n = 1), social phobia (n = 1), bulimia (n = 1), anorexia (n = 1), and substance abuse (n = 1).
d The gender ratio was four females to 10 males.
e Lifetime co-morbid Axis-I diagnoses included major depressive disorder (n = 12), dysthymia (n = 1), specific phobia (n = 1), generalized anxiety disorder (n = 1), panic disorder (n = 2), social phobia (n = 6), bulimia (n = 1), anorexia (n = 1), hypochondria (n = 2), substance abuse (n = 2), and somatoform disorder (n = 1).
f The gender ratio was 12 females to 11 males.
g BDI-II was used in samples 1 and 3; BDI-I was used in sample 2
Measures
Patients were diagnosed by a licensed clinical psychologist at the outpatient clinic using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (SCID, First et al. Reference First1996; German version, Wittchen et al. Reference Wittchen1997). The SCID I is a well-validated semistructured interview for making major Axis I diagnoses with fair-to-excellent inter-rater reliability (κ = 0.71; Lobbestael et al. Reference Lobbestael, Leurgans and Arntz2011). Obsessive–compulsive symptom severity was assessed using the clinician-rated Yale–Brown Obsessive Compulsive Scale (Goodman et al. Reference Goodman1989; German version, Hand & Büttner-Westphal, Reference Hand and Büttner-Westphal1991), which has been shown to have good internal consistency (Cronbach's α = 0.8) and inter-rater reliability (r = 0.9; Jacobsen et al. Reference Jacobsen2003). Severity of depressive symptoms was assessed with the German translation of the Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979; German version, Neumann & Schulte, Reference Neumann and Schulte1989). With Cronbach's α ranging from 0.82 to 0.92, the MADRS shows good-to-excellent internal consistency (Maier & Philipp, Reference Maier and Philipp1985). Additionally, all participants were administered well-validated and reliable questionnaires to assess self-reported depressive and obsessive–compulsive symptoms, i.e. the Beck Depression Inventory-II (Beck et al. Reference Beck, Steer and Brown1996; German version, Kühner et al. Reference Kühner2007) and the Obsessive–compulsive Inventory-Revised (Foa et al. Reference Foa2002; German version, Gönner et al. Reference Gönner, Leonhart and Ecker2008), respectively. Verbal intelligence was measured with the German Wortschatztest (Schmidt & Metzler, Reference Schmidt and Metzler1992), and state and trait anxiety was assessed using the State-Trait Anxiety Inventory (Spielberger, Reference Spielberger1983; German version, Laux et al. Reference Laux1981).
Stimuli
Stimuli are described in detail elsewhere (Simon et al. Reference Simon2010; Reference Simon2014). In brief, samples 1 and 2 were presented with OCD-relevant, generally aversive, and neutral pictures during scanning. Due to the heterogeneity of OCD symptoms, OCD-relevant pictures were selected for each patient individually based on patients’ picture evaluations obtained 1 week prior to scanning (Simon et al. Reference Simon2010; Reference Simon2014). During this rating session, all participants evaluated OCD-relevant, generally aversive, and neutral pictures according to unpleasantness, arousal, provoked anxiety, and elicited OCD symptoms (patients only). Based on these ratings, picture sets were created for each patient individually including OCD-relevant pictures that elicited maximal OCD symptoms and generally aversive and neutral pictures that elicited minimal OCD symptoms. Control participants were presented with the same stimuli as the corresponding patient they were matched with. Compared with healthy control participants, OCD patients of samples 1 and 2 evaluated OCD-relevant pictures of the final picture sets as more arousing, more unpleasant, and eliciting stronger feelings of anxiety (all p ⩽ 0.001). Patients reported higher levels of OCD symptoms in response to OCD-relevant pictures compared with both neutral and aversive pictures (all p < 0.001). While patients’ self-reported arousal and unpleasantness of OCD-relevant pictures was at least comparable to (or higher than) aversive pictures, healthy control participants evaluated OCD-relevant pictures as less unpleasant and arousing than aversive ones (all p ⩽ 0.002). More detailed results are reported in Simon et al. (Reference Simon2010) and Simon et al. (Reference Simon2014).
Experimental tasks
Sample 1: emotion regulation task
A detailed task description is provided in Simon et al. (Reference Simon2014). In brief, participants were instructed to either attend to the presented pictures and evaluate their unpleasantness (appraisal task) or to perform a concurrent visual discrimination task that distracted attention from the pictures (distraction task). In the appraisal task, participants appraised stimuli by indicating whether the pictured scene made them feel unpleasant or not. In the distraction task, participants indicated whether two lines, which were superimposed on the pictures in both the appraisal and distraction task, were aligned in parallel or not. Participants responded by pressing one of two buttons. Between-group comparisons of mean accuracy and reaction time are provided as online Supplementary Material S1. Detailed behavioral analyses are further reported in Simon et al. (Reference Simon2014). The experiment consisted of 18 appraisal and 18 distraction blocks, which were interspersed with a 14-s visual baseline. Each block contained six pictures of the same picture type. Block and picture order was pseudorandomized. Pictures were presented for 1 s with a variable intertrial interval (ITI) averaging 3030 ms (see online Supplementary Fig. S1A).
Sample 2: passive viewing task
A detailed task description is provided in Simon et al. (Reference Simon2010). In brief, pictures were presented either for a brief (1 s) or long duration (6 s) and participants were instructed to attend to all stimuli. Pictures were presented in blocks with a variable ITI averaging 3500 ms. Long stimulation blocks (n = 12) contained six pictures of the same picture type and short stimulation blocks (n = 6) contained 12 pictures, resulting in equal numbers of pictures in short and long conditions. Block and picture order was pseudorandomized. Picture blocks were interspersed with a 14-s visual baseline (see online Supplementary Fig. S1B).
Sample 3: resting state
Participants were instructed to keep their eyes closed and relax for a 5-min, 20-s resting-state scan (Beucke et al. Reference Beucke2013).
fMRI acquisition and preprocessing
Stimuli were presented using Presentation (Neurobehavioral Systems, Inc.) and were projected to a mirror that was attached to the head coil. In all three samples, 176 anatomical MDEFT images [spatial resolution = 1 × 1 × 1 mm, repetition time (TR) = 12.24 ms, echo time (TE) = 3.56 ms, flip angle = 23°, 256 × 224 matrix] were acquired on a 1.5 T Siemens Sonata scanner. A total of 353 functional volumes were obtained in sample 1 (in each of the two runs), 344 volumes in sample 2 (in each of the two runs), and 160 volumes in sample 3 using the following parameters: T2*-weighted single-shot gradient EPI sequence, TR = 2120 ms (sample 3, TR = 2000 ms), TE = 40 ms, 38 consecutive axial slices (sample 3, 35 slices), 3 × 3 × 3 mm voxel, flip angle = 90°, field of view = 192 mm, 64 × 64 matrix.
Imaging data analysis was performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/ software/spm8/). Preprocessing for samples 1 and 2 included removal of the first four volumes, slice time correction, motion correction, spatial normalization, spatial smoothing (8-mm Gaussian kernel), and temporal filtering (sample 1, high-pass filter with a 128 s cutoff period; sample 2, high-pass filter with a 512 s cutoff period). The 512 s high-pass filter was applied after noticing that regressors were not orthogonal when using the 128 s high-pass filter due to a longer experimental period (periods between different psychological conditions) in the design. Preprocessing and regression of nuisance variables for sample 3 were identical to the procedures described previously (Buckner et al. Reference Buckner2009).
fMRI statistical analysis
Sample 1: emotion regulation task
An event-related GLM was specified for each participant's functional run by modeling onsets and durations of each picture. The design matrix for each participant included six regressors according to the experimental conditions (OCD-relevant, aversive, and neutral pictures in the distraction and appraisal task). The six movement parameters estimated during motion correction were included as regressors of no interest. Regressors were modeled as box car functions, convolved with a hemodynamic response function, and regressed against the blood oxygenation level-dependent signal in each voxel.
Psychophysiological interaction analysis
Psychophysiological interaction (PPI) analysis allows to study whether changes in connectivity of a single brain region with the rest of the brain are due to an experimental manipulation (Friston et al. Reference Friston1997). In this study, PPI analysis was used to test whether the presentation of OCD-relevant (as compared with aversive) pictures changes the connectivity between the amygdala and areas that are known to be involved in emotion regulation and have also emerged in recent brain circuit models of OCD. PPI analysis involves three steps: seed voxel extraction, building the PPI interaction term, and testing its effect as a regressor in a GLM, producing individual PPI images (see online Supplementary material S2). In the current study, voxels that were maximally activated in the appraisal task (i.e. across all three conditions) compared with the implicit baseline were extracted from the amygdala seed region. Online Supplementary Table S2 shows peaks of significant regional activation for this contrast, including robust [i.e. significant whole-brain family-wise error (FWE) corrected] amygdala activation, which is shown in Fig. 1. Further brain regions typically involved in emotional processing and regulation including insula, dorsolateral prefrontal cortex (DLPFC), and ACC were also found to be active during the appraisal task (see online Supplementary Table S2). Brain regions that were activated during distraction are provided in online Supplementary Table S3. Overall, the GLM task effects confirm that the amygdala played a crucial role in the appraisal of the pictorial stimuli and to a much lesser extent during distraction.
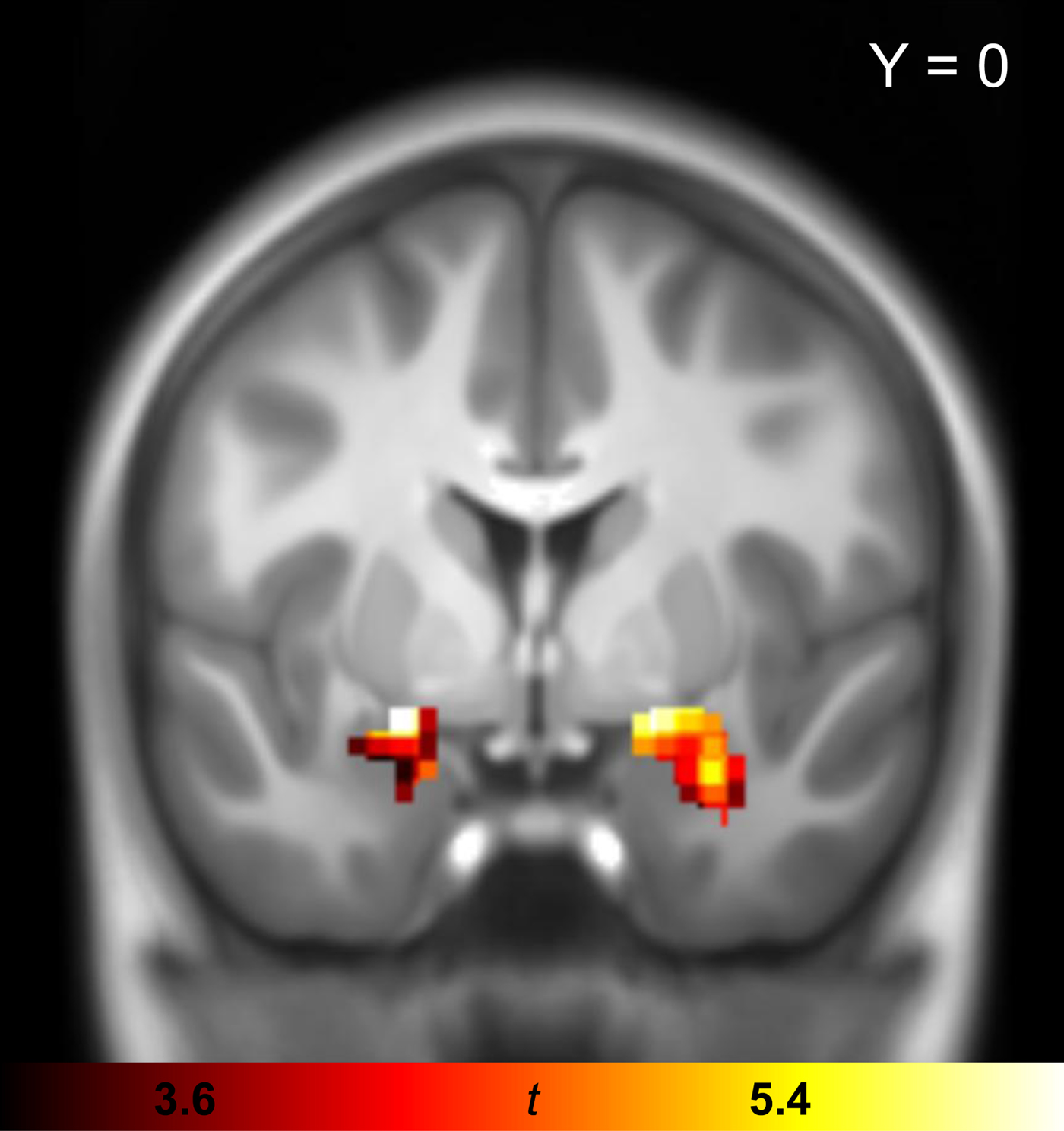
Fig. 1. Univariate GLM task effect for the appraisal (across all picture types) minus implicit baseline contrast in the combined sample of OCD patients and healthy controls (sample 1). The statistical parametric map of the task effect is shown in coronal plane at p FWE < 0.05. Activated voxels are shown exclusively for bilateral amygdala seed region. GLM, general linear model; OCD, obsessive–compulsive disorder; FWE, family-wise error.
PPI group analysis
Individual statistical PPI images were submitted to second-level random-effects analyses and two-sample t tests were applied to identify between-group differences. Because the ACC and VMPFC are implicated in both the affective cortico-striato-thalamo-cortical circuit (Milad & Rauch, Reference Milad and Rauch2012) and emotion regulation (Ochsner & Gross, Reference Ochsner, Gross and Gross2007), analyses were performed within these a priori regions of interest (ROI) using one combined bilateral mask. The ACC was structurally defined using the SPM Anatomy Toolbox (Eickhoff et al. Reference Eickhoff2006) and the VMPFC was included in two clusters comprising medial and post-central subdivisions of the orbitofrontal cortex (OFC) as defined on the basis of connectivity-based parcellation (Kahnt et al. Reference Kahnt2012). Effects within this mask were considered significant at p FWE < 0.0125 (Bonferroni-corrected p value adjusted for four comparisons, i.e. for left and right amygdala seeds in the distraction and appraisal task). To determine whether the PPI group effect was driven by condition differences in the patient or/and control group, additional explanatory PPIs were calculated in order to plot amygdala connectivity effects for single conditions and for both groups separately (see online Supplementary material S3).
Sample 2: passive viewing task
After observing significant group differences in amygdala–prefrontal coupling in sample 1, we sought to test whether these abnormalities could also be observed when patients were passively viewing stimuli. Because pictures in the emotion regulation study were presented for 1 s, we only included trials of brief stimulus duration. The appraisal and passive viewing tasks only differed regarding the presence of the instruction to appraise stimuli. Therefore, we tested for abnormal coupling between regions that showed significant group differences in the appraisal task (sample 1), namely between right amygdala and left OFC. Event-related time series from right amygdala seed voxels that were maximally activated during visual stimulation (across all picture types) compared with the implicit baseline were extracted. The OFC ROI was anatomically defined using the IBASPM 71 atlas (http://www.thomaskoenig.ch/Lester/ibaspm.htm). Effects were considered significant at p FWE < 0.05.
Sample 3: resting state
Functional connectivity analysis was performed for left and right amygdala seed regions. Connectivity strength was computed by correlating the averaged voxel time series of the seed region with every voxel in the brain and converting obtained correlation coefficients to z-scores. Resulting connectivity maps were entered into random-effects analyses and two-sample t tests were applied to test for between-group differences, which were considered significant at p FWE < 0.025 (Bonferroni-corrected p value adjusted for two comparisons, i.e. for left and right amygdala seeds) for both the whole brain and for ROIs applying the same mask that was used for the PPI analysis in sample 1.
Results
Sample 1: emotion regulation task
All significant between- and within-group PPI effects are shown in Table 2. Two-sample t tests revealed significant connectivity group differences in both the appraisal and distraction tasks for the right amygdala seed. In the appraisal task, the contrast healthy control participants > OCD patients revealed a significant group difference in the left OFC (see Fig. 2, left column) due to a significant stimulus-related connectivity change in the healthy control group. In control participants, the relationship between activity in the right amygdala and left OFC was more positive for OCD-related compared with aversive pictures, while no such differentiation was observed in OCD patients. In the distraction task, significant group differences were found in the VMPFC for OCD patients > healthy control participants (see Fig. 2, middle column) due to a significant stimulus-related connectivity change in the OCD group. In patients, the relationship between activity in the right amygdala and VMPFC was more positive for OCD-related compared with aversive pictures, while no significant connectivity change was observed for control participants. Post-hoc correlation analysis (provided as online Supplementary material S4) showed no significant relationship with symptom severity.
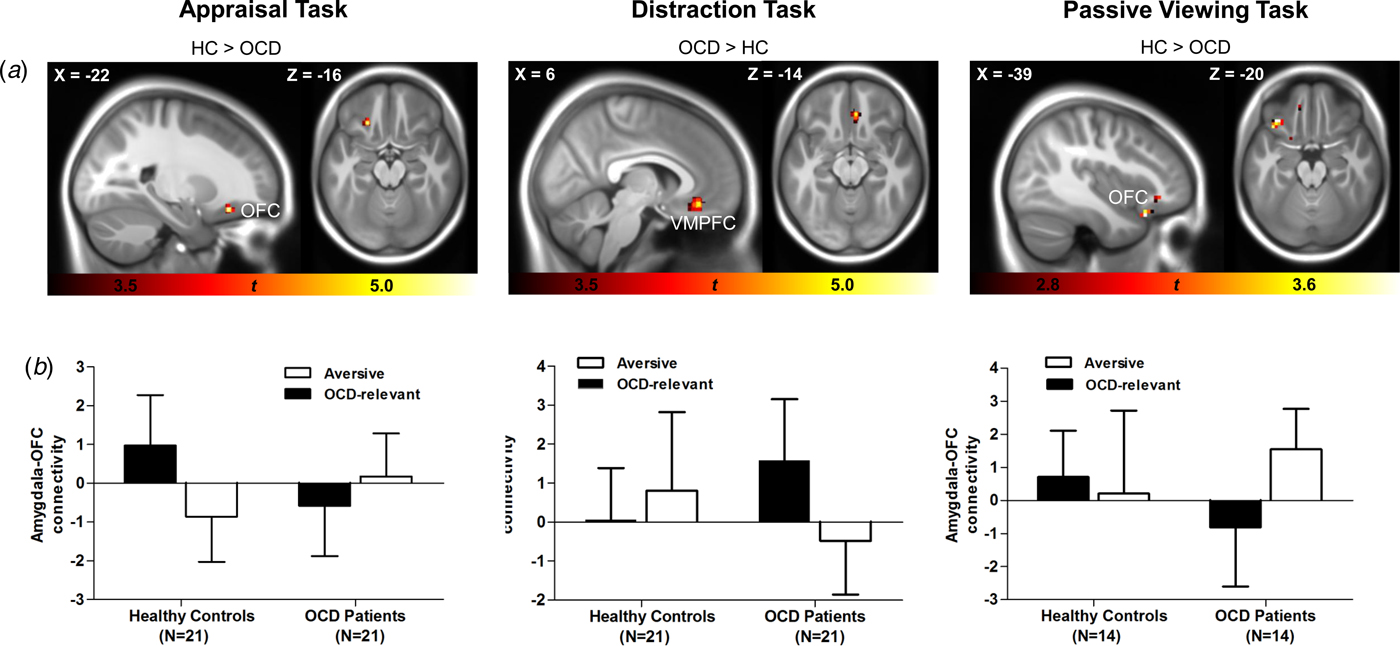
Fig. 2. PPI group effects in the appraisal, distraction, and passive viewing tasks. Statistical parametric maps for the appraisal (sample 1, left column), the distraction (sample 1, middle column), and the passive viewing task (sample 2, right column) are displayed in sagittal and axial planes, thresholded at p < 0.001, uncorrected (sample 1) and p < 0.01, uncorrected (sample 2) for visualization purposes (A); bar graphs showing mean (SD) PPI β estimates for connectivity between the right amygdala seed and prefrontal areas for aversive and OCD-relevant pictures for healthy controls and OCD patients (B). HC, healthy controls; OCD, obsessive–compulsive disorder; PPI, psychophysiological interaction; OFC, orbitofrontal cortex; VMPFC, ventromedial prefrontal cortex; SD, standard deviation.
Table 2. Brain regions showing significant PPI effects for OCD patients and healthy controls with the right amygdala seed region during appraisal and distraction for OCD-relevant (minus aversive) pictures and between-group differences (sample 1)

PPI, psychophysiological interaction; OCD, obsessive–compulsive disorder; HC, healthy controls; MNI, Montreal Neurological Institute; OFC, orbitofrontal cortex; dACC, dorsal anterior cingulate cortex; VMPFC, ventromedial prefrontal cortex; k e, cluster extent; FWE, family-wise error; CI, confidence interval.
a Significant within-group effects in a priori-defined regions of interest applying a corrected threshold of p FWE < 0.05 with a minimum cluster size of three contiguous voxels.
b Significant between-group differences in a priori-defined regions of interest applying a corrected threshold of p FWE < 0.0125 (corresponding to a Bonferroni-corrected p value adjusted for four comparisons) with a minimum cluster size of three contiguous voxels
c Direction of the PPI effect: positive indicating a more positive relationship between right amygdala seed region and PPI effect region for OCD-related compared with aversive pictures.
Additional post-hoc PPI analyses were performed for aversive (v. neutral) pictures to test for right amygdala–OFC/VMPFC connectivity alterations unrelated to OCD-relevant stimuli. No significant between-group differences were observed at a Bonferroni-corrected threshold of p FWE < 0.025 (corresponding to a Bonferroni-corrected p value adjusted for two comparisons, i.e. appraisal and distraction). At a Bonferroni-uncorrected threshold of p FWE < 0.05, OCD patients showed greater coupling between right amygdala and right OFC in the distraction task (peak voxel, MNIxyz = 12, 56, −12; z = 3.94; p FWE = 0.03).
In both tasks, no significant group differences were found for the left amygdala seed region.
Sample 2: passive viewing task
Similar to the appraisal task, two-sample t tests (healthy control participants > OCD patients, OCD-relevant minus aversive pictures) revealed significant connectivity group differences between the right amygdala seed and left OFC (peak voxel, MNIxyz = −39, 23, −23; z = 4.06; p FWE = 0.006). While control participants showed a more positive relationship between amygdala and OFC activity for OCD-relevant compared with aversive pictures, decreased amygdala–OFC coupling was observed in OCD patients (see Fig. 2, right column). Post-hoc correlation analysis (provided as online Supplementary material S4) showed no significant relationship with symptom severity.
Sample 3: resting state
In both groups, activity in left and right amygdala seed regions was positively correlated with activity in the contralateral amygdala, the insula, limbic and paralimbic regions as well as the medial prefrontal cortex (see online Supplementary Table S4). No significant group differences in connectivity were found during resting state.
Discussion
The main goal of the study was to investigate whether cognitive appraisal of symptom-provoking stimuli leads to abnormalities in amygdala–prefrontal pathways that are crucial for affective appraisal and successful emotion regulation in patients with OCD. Furthermore, the study sought to examine whether abnormal amygdala–prefrontal coupling includes prefrontal targets that have been highlighted in recent neuroanatomical models of OCD. Abnormal amygdala–prefrontal coupling was observed in all three experimental contexts involving symptom provocation (i.e. during stimulus appraisal, passive viewing, and distraction), whereas no abnormalities were found during the resting state. Moreover, reductions in positive amygdala–OFC coupling were observed during appraisal and passive viewing of symptom-provoking stimuli, whereas higher connectivity between amygdala and the VMPFC was observed when performance of a simple task interfered with stimulus processing. Taken together, these amygdala–prefrontal connectivity alterations, which selectively occurred during symptom provocation, demonstrate context-dependent affective circuit abnormalities in patients with OCD, and further suggest that the extent to which OCD-relevant stimuli can be actively processed and appraised has a significant effect on amygdala–prefrontal coupling in this patient group.
In the appraisal and passive viewing task, relative to healthy control participants, OCD patients showed reduced coupling between the amygdala and the medial OFC when they processed OCD-relevant compared with aversive stimuli. As suggested by explanatory PPIs, control participants demonstrated increased connectivity during appraisal of OCD-relevant compared with aversive stimuli. Amygdala–OFC coupling has been shown to be related to emotion regulation success (Banks et al. Reference Banks2007). Because for healthy individuals OCD-relevant pictures are less emotional than aversive ones, increased amygdala–OFC coupling in healthy control participants may reflect the appraisal of OCD-relevant stimuli as non-threatening. In contrast, reduced amygdala–OFC connectivity in patients during appraisal of OCD-relevant stimuli presumably reflects impaired automatic emotion regulation resulting in affective symptoms such as elevated emotional distress. Thus, in addition to our original GLM analyses that showed amygdala hyperactivity in response to symptom triggers (Simon et al. Reference Simon2010; Reference Simon2014), the present analyses suggest that emotional dysregulation during appraisal and passive viewing of OCD-relevant stimuli is not solely due to elevated emotional reactivity, but also to impaired prefrontal downregulation of the amygdala response, as demonstrated by altered amygdala–OFC coupling. The modulatory role of the prefrontal cortex on amygdala activation during processing of symptom-provoking v. neutral stimuli in OCD patients has been demonstrated before by Banca et al. using effective connectivity analysis (Banca et al. Reference Banca2015). However, this study could neither differentiate between specific effects during obsessive–compulsive stimulation and general emotional hyperarousal, nor rule out confounding effects of medication. We complement these findings by showing connectivity differences between unmedicated patients and control participants (i.e. reduced amygdala–OFC connectivity in patients) that were specific to symptom-related (v. generally aversive) stimuli.
When stimulus processing was disturbed by a distraction task, contrary to our expectation that both groups would not differ in amygdala connectivity, increased amygdala–VMPFC connectivity was found in OCD patients for OCD-relevant compared with aversive pictures. Explanatory PPIs suggested that control participants showed an inverse PPI connectivity pattern, that is, stronger connectivity for aversive compared with OCD-relevant pictures. While for healthy individuals aversive pictures are associated with negative emotional responses that may trigger regulatory attempts, OCD-relevant stimuli are highly relevant for individuals suffering from OCD. Hence, this reverse connectivity pattern may reflect the effort of downregulating the effect of the group-specific, emotionally most-disturbing stimulus category to successfully perform the concurrent task. Accordingly, threat-induced anxiety in a computer-gaming style task increased connectivity between amygdala and prefrontal brain regions including VMPFC and OFC in healthy participants while performance was unimpaired (Gold et al. Reference Gold, Morey and McCarthy2015). Thus, increased amygdala–VMPFC connectivity in OCD patients might reflect increased regulatory attempts to overcome anxiety in order to maintain goal-directed behavior.
Impaired emotion regulation in response to symptom triggers might result from negative stimulus appraisals that require attention. Consistent with this assumption, prefrontal inhibitory control of the amygdala was reinstated in OCD patients when attentional resources were limited due to a concurrent task. In line with this finding, we recently demonstrated that distraction but not reappraisal reduced electrocortical correlates of emotional processing in OCD (Paul et al. Reference Paul2016).
Notably, intact amygdala–prefrontal coupling was observed in the absence of stimulus presentation during rest, with no difference between patients and healthy control participants. Thus, alterations in amygdala–medial prefrontal connectivity in patients exclusively emerged during exposition to symptom-related stimuli. This is in line with OCD phenomenology of elevated fear or distress in response to very specific, symptom-relevant situations and suggests that affective circuit abnormalities, which have been proposed by recent models in OCD (Milad & Rauch, Reference Milad and Rauch2012), include abnormal amygdala–prefrontal coupling, an observation that deserves to be integrated into functional neuroanatomical models of OCD.
In concert with our findings, alterations in the limbic-medial prefrontal cortex circuit are assumed to be implicated in psychiatric disorders characterized by emotional dysregulation (Kim et al. Reference Kim2011). Thus, altered amygdala–OFC/VMPFC connectivity has been demonstrated, for example, in social anxiety disorder (Sladky et al. Reference Sladky2015) and bipolar disorder (Kanske et al. Reference Kanske2015). Further, abnormal interactions between the amygdala and VMPFC are thought to be involved in fear extinction impairments in anxiety disorders and OCD (Milad et al. Reference Milad, Rosenbaum and Simon2014). Thus, the obtained findings suggest that impaired amygdala–OFC connectivity mediates affective symptoms in OCD. It remains to be elucidated if these deficits constitute a vulnerability factor for OCD, as demonstrated for patients with bipolar disorder and their first-degree relatives (Kanske et al. Reference Kanske2015). Because adolescence is a key period for the remodeling of prefrontal areas implicated in emotion regulation (Spear, Reference Spear2000), this may especially be relevant for early-onset OCD.
When interpreting the findings, one has to bear in mind that PPI analysis precludes drawing causal inferences regarding the interplay of brain areas. Although there is evidence of prefrontal inhibitory control of amygdala activation (Motzkin et al. Reference Motzkin2015), it is possible that the obtained findings are due to the amygdala modulating OFC/VMPFC activity or to a third brain area influencing both amygdala and OFC/VMPFC. Future research investigating the causal architecture of neural systems, such as dynamic causal modeling, might clarify this relationship.
In conclusion, we demonstrated abnormal connectivity between the amygdala and prefrontal regions of the affective circuit in patients with OCD during cognitive appraisal of symptom-provoking stimuli. Importantly, these results were obtained from two independent samples of unmedicated patients, providing converging evidence for prefrontal–amygdala connectivity alterations during symptom provocation. Reduced amygdala–prefrontal coupling was observed only when attentional resources were available for elaborate stimulus processing, pointing to impaired downregulation of cognitively learned fear and anxiety in OCD. The obtained findings may advance our understanding of neural mechanisms underlying elevated fear and distress by considering the role of amygdala–prefrontal coupling during appraisal and processing of OCD-relevant stimuli that might also relate to fear extinction deficits. Taken together, we propose to integrate altered amygdala–prefrontal connectivity in the affective circuit model of OCD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171800079X
Acknowledgements
This work was supported by the German Research Foundation (grant numbers: SI 1131/2-1 and SI 1131/2-3) and the Federal Ministry of Education and Research of Germany (grant number: BMBF-01GW0724). Data storage and resting-state analyses were supported by funding from the National Institutes of Health Shared Instrumentation Grants S10RR023401 and S10RR023043.
Declaration of interest
None
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






