Introduction
In amphibians, as in almost all vertebrates, oocytes are arrested in meiotic prophase I. Just before ovulation a surge in luteinizing hormone (LH) stimulates ovarian follicles to unleash multiple signals that ultimately trigger oocytes to re-enter the cell cycle in a process called maturation (Albertini & Carabatsos, Reference Albertini and Carabatsos1998). Oocytes progress through meiosis to metaphase II, where they are again arrested until after fertilization, when meiosis is completed. Oocytes arrested at prophase I have a nucleus with an intact nuclear envelope or germinal vesicle (GV). Germinal vesicle breakdown (GVBD) is the first clearly visible marker of meiosis resumption (Brunet & Maro, Reference Brunet and Maro2005).
For oocyte maturation to occur in amphibians, a surge in LH secretion must stimulate a follicular production of progesterone (P4). This hormone acts non-transcriptionally to cause GVBD by interaction with a receptor located at the cell membrane, and that was demonstrated in Xenopus oocytes (Maller, Reference Maller2001). P4 starts a cascade of transmembrane signaling events (Zelarayán et al., Reference Zelarayán, Ajmat, Unías, Bonilla, Sánchez Toranzo and Bühler2007; Reference Zelarayán, Ajmat, Bonilla and Bühler2012), leading to the activation of a key regulator of the G2–M phase transition (Liu et al., Reference Liu, Ma, Hamam and Liu2005), the cytoplasmic maturation promoting factor (MPF), a complex of the cyclin-dependent kinase p34cdc2 and cyclin B that induces GVBD (Sánchez Toranzo et al., Reference Sánchez Toranzo, Bonilla, Zelarayán, Oterino and Bühler2006). However, the mechanisms involved in this process have not been fully elucidated to date.
Large numbers of signaling mechanisms and second messengers have been implicated in P4-induced oocyte maturation. There is a general consensus that a transient decrease in cAMP intracellular levels, resulting at least partly from the inhibition of membrane adenylyl cyclase (AC) activity, is an obligatory step in the mechanism by which P4 induces oocyte maturation (Kwon et al., Reference Kwon, Lin, Choi and Ahn1989; Zelarayán et al., Reference Zelarayán, Ajmat, Bonilla and Bühler2012). In addition to cAMP, other second messengers have been studied in the P4-induced maturation of Rhinella arenarum (ex Bufo arenarum) oocytes (Zelarayán et al., Reference Zelarayán, Oterino, Sánchez Toranzo and Bühler2000). However, the role of arachidonic acid (AA) metabolites in P4-induced oocyte maturation in amphibians has not been clarified.
Prostaglandins (PGs), produced in almost all cells, are important mediators of functions in the female reproductive tract. They result from the hydrolysis of polyunsaturated fatty acids (PUFAs), which are natural constituents of cell membranes. They may derive from 8,11,14-eicosatrienoic acid (PGE1) or from 5,8,11,14-eicosatetraenoic acid or AA (PGF2α and PGE2). Cyclo-oxygenases (COX) catalyze their conversion to PGs (Grosser et al., Reference Grosser, Yusuff, Cheskis, Pack and FitzGerald2002; Sirois et al., Reference Sirois, Sayasith, Brown, Stock, Bouchard and Doré2004), considered to be important physiological regulators of ovarian functions. Other eicosanoids, such as leukotrienes, can be formed from PUFAs by the action of lipoxygenases (LOX).
Although PGs have been associated with ovulation and their relevance has been acknowledged in many vertebrates, studies conducted in zebrafish oocytes have demonstrated their role during maturation (Lister & Van Der Kraak, Reference Lister and Van Der Kraak2008). However, their participation during meiosis resumption and their role as paracrine regulation agents are not yet definitely known.
Arachidonic acid and its metabolites may induce meiosis resumption in oocytes of some species of starfish (Meijer et al., Reference Meijer, Maclouf and Bryant1986), fish (Sorbera et al., Reference Sorbera, Asturiano and Zanuy1998), mice (Downs & Longo, Reference Downs and Longo1983) and sheep (Murdoch, Reference Murdoch1988). Patiño et al. (Reference Patiño, Yoshizaki, Bolamba and Thomas2003) demonstrated that AA metabolites may induce ovulation in ovarian follicles of Atlantic croaker and may have a permissive role in oocyte maturation. In this sense, AA seems to have an important role in signal transduction leading to ovulation but its role regarding meiosis resumption in certain teleosts is unclear.
In amphibians, in R. arenarum in particular, the role of fatty acids and their metabolites during oocyte maturation has been scarcely analyzed. In R. arenarum we showed that AA metabolites produced through COX and LOX pathways play an important role in P4-induced oocyte maturation. In agreement with this finding, we demonstrated that phospholipase A2 (PLA2) activation and the consequent AA release induced maturation in R. arenarum oocytes in a dose-dependent manner, but PLA2 inactivation did not affect P4-induced maturation. It seems probable that other phospholipases are involved in P4-induced maturation in this species. Our results suggest that AA metabolites resulting from the COX or LOX pathways would be involved in P4-induced R. arenarum oocyte maturation (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013).
Although in R. arenarum oocytes P4-induced maturation takes place throughout the year, oocytes showed a different P4 response capacity dependent upon the period in which animals were captured. During the reproduction period, R. arenarum oocytes reinitiate meiosis with no need of an exogenous hormonal stimulus when they are deprived of their enveloping cells (Zelarayán et al., Reference Zelarayán, Oterino and Bühler1995), a process known as spontaneous maturation. Oocytes that undergo spontaneous maturation are considered to be competent. Coincidentally, during this period, oocytes and follicles exhibit a maximum response to P4-induced maturation.
During fertilization, fusion with the fertilizing sperm triggers a series of morphological and biochemical changes in the oocyte that initiate a program that will allow the development of a new individual. This response of the oocyte arrested in metaphase II to make contact with the sperm is known as oocyte activation and includes a series of events such as exocytosis of cortical granules (CG), end of meiosis II, extrusion of the second polar body, pronuclei formation, DNA replication and reorganization of the cytoskeleton. During the process of activation, sperm act as a signal that causes cytoplasmic and nuclear changes in the oocyte. Under experimental conditions, this signal can be replaced by different stimuli (electric shock or puncture with a fine glass needle) that generate the same initial response in the oocyte. However, later in the course of development, the need for genetic information provided by the sperm is required for normal development to occur (Oterino, Reference Oterino2003; Ph.D. Thesis).
The present work aims to investigate the role of AA and PGs during R. arenarum oocyte maturation, with special focus on the potential participation of PGF2α in oocyte activation and pronuclear formation.
Materials and methods
Animals
Adult R. arenarum males and females were caught throughout the year in the surrounding areas of San Miguel de Tucumán (northwestern Argentina). They were kept in captivity at room temperature with natural light–dark cycles until use, up to 15 days after collection.
Reagents
Prostaglandins (PGE1, PGE2 and PGF2α) and AA were tested for their ability to induce maturation. It is important to emphasize that AA is easily oxidized by oxygen, light, and heat; so, a 20 mM stock solution (dissolved in dimethylsulfoxide) was aliquoted in Eppendorf tubes (100 μl), covered with aluminum foil, sealed with parafilm and stored in a N2 atmosphere at –20°C for almost 1 month. During each experiment one fraction was completely used. Indomethacin was used as a COX inhibitor. Amphibian Ringer solution (AR) (6.60 g NaCl/l, 0.15 g CaCl2/l, and 0.15 g KCl/l) with penicillin G-sodium (30 mg/l) and streptomycin sulphate (50 mg/l), pH 7.4, was used as a culture medium in all routine incubations. P4 (1 μM) was used as a positive control for maturation. All reagents were purchased from Sigma or Merck.
In vitro cultures
Fully grown follicles (1.6–1.8 mm in diameter) were isolated from ovarian tissues using watchmaker's forceps. Denuded oocytes were obtained by manually pulling off the follicle epithelium and the theca layer with fine forceps. Follicle cells were removed by incubation of defolliculated oocytes in AR for 5 min with gentle shaking (100 oscillations/min) (Zelarayán et al., Reference Zelarayán, Oterino and Bühler1995). The incubations were performed at 26°C in multiwell culture dishes (Costar 3524, Cambridge, MA, USA) with randomized samples of 20 oocytes or follicles distributed into separate wells that contained 2 ml of AR. Two-well duplicates were run in each experimental group.
Dose–response curves of PGs were performed by incubating denuded oocytes or follicles for 22–24 h in the presence of different doses of the compound (0–200 ng/ml). In the case of AA, dose–response curves were carried out in the dark by incubating samples for only 60 min in the presence of different doses of the substance (0–200 μM). Then the samples were transferred to AR and incubated in the dark. Oocyte maturation was assessed 24 h after reagent addition.
Time–response curves of PGF2α were conducted with oocytes and follicles using the dose that had induced the best response. Samples were incubated for 0, 3, 6, 9, 12, 15, 18, 21 or 24 h.
Inhibition experiments were performed by pre-incubating the samples for 60 min in the presence of different doses of indomethacin (0–100 μM). Then, AA was added at the dose that had induced the highest maturation response. Both substances were kept together during an additional 60 min period. Finally, samples were transferred to AR plus indomethacin and incubated overnight in the dark.
Maturation criteria
After incubation, samples were fixed overnight at room temperature in Ancel & Vintemberger's solution (10% formol, 0.5% acetic acid and 0.5% NaCl). Meiosis resumption was assessed both by the presence of a transient white spot in the animal pole and by GVBD when fixed samples were dissected under a stereoscopic microscope.
Mature cytoplasm transfers
Cytoplasm from mature oocytes was obtained using a modified Hedeimann's method (Bühler & Petrino, Reference Bühler and Petrino1983). Briefly, oocytes matured with PGF2α (200 ng/ml) were suspended at the interface of 60% Ficoll (v/v) in AR and then centrifuged (10,000 g, 20 min) at 4°C (Eppendorf centrifuge 5417C). After this procedure, oocytes displayed four distinct phases: yolk platelets, pigment granules, clear cytoplasm, and a lipid layer. Denuded fully grown immature oocytes were microinjected with 40 nl of cytoplasm obtained from the clear cytoplasm layer, which contains MPF. Microinjection was performed using intracytoplasmic sperm injection (ICSI) micropipettes (Humagen™ Fertility Diagnostics) attached to a micromanipulator (Leitz Wetzlar). After microinjection, oocytes were incubated in AR for 18–20 h, fixed and GVBD was scored. As the negative control, a batch of immature oocytes was microinjected with the same volume of cytoplasm obtained from immature oocytes (immature cytoplasm). Another batch was microinjected with mature cytoplasm obtained from P4-treated oocytes (1 μM) as the positive control.
Oocytes activation techniques
Oocyte activation was carried out in oocytes matured with PGF2α (200 ng/ml) or P4 (1 μM) (control) by using the following techniques:
• Mechanical activation: by puncture in the animal hemisphere with a glass needle.
• Electric activation: by electric shock using an acrylic chamber (25 × 20 × 0.5 mm) with a platinum electrode connected to an electrical stimulator; 20 oocytes were placed in the chamber with 2 ml of AR and stimulated with 10 manual pulses of direct current (DC) (pulse duration: 2 s; intensity: 220 mV) (Oterino Reference Oterino2003; PhD thesis).
The activation parameters used were: disappearance of the white spot, elevation of the vitelline envelope and exocytosis of CG. Twenty minutes after activation, oocytes were fixed and the presence or absence of CG was analyzed by a histochemical technique (Alcian Blue, pH 2.5).
Sperm extract preparation and microinjection
Sperm suspensions were obtained by gently disrupting the testes in 4 ml AR and centrifuging at 1085 g for 10 min. The resulting pellet was resuspended in 2 ml of 10% AR and kept at 4°C until use. Sperm concentration was determined by counting in a Neubauer chamber and adjusted to 2 × 106 sperm/ml (Bonilla et al., Reference Bonilla, Ajmat, Toranzo, Zelarayán, Oterino and Bühler2008).
Sperm microinjections were performed in denuded oocytes matured with PGF2α (200 ng/ml) and P4 (1 μM) as positive control. Oocytes that showed a white spot were transferred to calcium-free Tris-buffered saline. The sperm suspension was injected into the animal pole of matured oocytes (30–40 nl/oocyte) using ICSI micropipettes (Humagen™ Fertility Diagnostics) attached to a micromanipulator as described above. Buffer microinjection did not result in pronuclei formation in the oocytes (negative control). Two hours after microinjection, the oocytes were fixed overnight in Ancel & Vintemberger's solution, subjected to histological technique (Manes & Nieto, Reference Manes and Nieto1983) and stained with Ehrlich's hematoxylin-eosin to visualize pronuclei formation (Bühler et al., Reference Bühler, Petrino and Legname1987).
Statistical analysis
Results are expressed as means ± standard deviation (SD) from experiments carried out in triplicate using different animals in each experiment. Comparisons among different treatments were carried out using one-way analysis of variance (ANOVA) followed by Tukey's honest significant difference (HSD) test. A value of P < 0.05 was considered to be statistically significant.
Results
In vitro incubation of follicles and oocytes in AA
In a previous work we demonstrated the role of PLA2 in meiosis resumption in R. arenarum oocytes (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013). It is possible that their activation may lead to maturation as a result of PUFAs release, which have been involved in maturation in other species (Sorbera et al., Reference Sorbera, Asturiano, Carrillo and Zanuy2001). On the basis of these results, experiments were conducted to analyse the effect of AA on the maturation of oocytes and follicles of R. arenarum.
PUFAs such as AA are extremely labile and undergo rapid oxidation by exposure to light, so that their availability as a source of metabolites is short-lived. In preliminary experiments we observed that continuous exposure of denuded oocytes and follicles to AA at any of the doses assayed was toxic and caused a gradual lysis or an excessive increase in cell volume during the first few hours of incubation. Considering these unexpected effects, the experiments were conducted as indicated in the Materials and methods section.
The results obtained show that incubation in AA stimulates the maturation of denuded oocytes and follicles in a dose-dependent manner, maximum response being obtained with the dose of 150 μM (25 ± 2% and 36 ± 5%, respectively) (Fig. 1). In all cases, the response of the follicles was higher than that obtained in denuded oocytes. Larger doses were less effective.

Figure 1 Effect of arachidonic acid (AA) on maturation. Oocytes and follicles were incubated with increasing doses of AA (12.5–200 μM) in the dark. Samples incubated with progesterone (P4) (1 μM) were used as positive control. Amphibian Ringer (AR) negative control was performed only with oocytes. Germinal vesicle breakdown (GVBD) was assessed after 18–24 h of incubation. Data represent the mean ± standard deviation (SD) (n = 8) of experiments performed in triplicate with different animals. a–dMeans with different letters are significantly different (P < 0.05).
When AA-induced maturation was studied in comparison with maturation induced by P4, a steroid considered the physiological maturation inducer in R. arenarum (Zelarayán et al., Reference Zelarayán, Oterino and Bühler1995), the response of both denuded oocytes and follicles to AA was twice as slow as their response to P4. After 20 h of incubation in the presence of AA, signs of maturation were observed. In contrast, 50% of the oocytes and follicles treated with P4 revealed a white spot at 8–10 h after the hormonal stimulus.
Inhibition of AA-induced maturation with indomethacin
AA is usually metabolized by an oxidation process in which COX and/or LOX participate. In this sense, in a previous work we demonstrated that P4-induced maturation in R. arenarum can be partially inhibited when oocytes are treated with indomethacin (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013). This finding suggests that COX would participate in meiosis reinitiation in R. arenarum oocytes. In order to verify if the AA metabolites resulting from the action of COX were involved in AA-induced maturation in R. arenarum oocytes, we examined the effect of indomethacin, an inhibitor of the enzyme.
The effect of indomethacin on AA-induced maturation was effective as preincubation of follicles and oocytes with indomethacin (0–100 μM) inhibited AA-induced maturation in a dose-dependent manner (Fig. 2). Doses of 100 μM of indomethacin inhibited AA-induced maturation in both oocytes and follicles (7 ± 3% and 6 ± 2% GVBD, respectively). These results agree with those obtained previously with P4 in this species (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013).

Figure 2 Effect of indomethacin on arachidonic acid (AA)-induced maturation. Oocytes and follicles were preincubated for 1 h with different indomethacin doses (0–100 μM). Maturation was induced with AA (150 μM). Incubations were performed in triplicate in the dark. Samples incubated with progesterone (P4) (1 μM) were used as positive control. Amphibian Ringer (AR) negative control was performed only with oocytes. Germinal vesicle breakdown (GVBD) was assessed after 24 h of incubation. Values represent the mean ± standard deviation (SD) (n = 6) of experiments performed on different animals. a–cMeans with different letters are significantly different (P < 0.05).
Involvement of PGs in oocyte maturation
The participation of PGs in the maturation process was studied in denuded oocytes and follicles incubated with PGF2α, PGE1 and PGE2 (25–200 ng/ml). The results indicated that these PUFA metabolites induce maturation in both oocytes and follicles and this response is dose dependent. However, PGF2α is more potent than PGE1 and PGE2 (Fig. 3) and produces results similar to those obtained when maturation is induced by P4. With doses of 200 ng/ml of PGF2α, maximum maturation response was obtained in both oocytes and follicles (94 ± 5% and 89 ± 4%, respectively). Higher doses of PGF2α failed to induce a greater response (data not shown). As PGF2α is the strongest prostaglandin to induce meiosis resumption in oocytes or follicles of R. arenarum and it is also an AA metabolite, the following experiments were designed only in the presence of this prostaglandin.

Figure 3 Dose–response curves of oocytes and follicles treated with prostaglandin F2α (PGF2α), PGE1 and PGE2. Oocytes and follicles were incubated in the presence of increasing doses of PGF2α, PGE1 and PGE2 (25–200 ng/ml). The incubations were performed in triplicate. Germinal vesicle breakdown (GVBD) was assessed after 18–24 h of incubation. Values represent the mean ± standard deviation (SD) (n = 20) of experiments performed on different animals. a–dMeans with different letters are significantly different (P < 0.05).
PGF2α induces maturation in R. arenarum oocytes and follicles in a time-dependent manner, maximum GVBD percentages being reached at 18 h of incubation (90 ± 6% and 45 ± 5% GVBD, respectively) with the dose of 200 ng/ml (Fig. 4). The onset of the white spot as an external sign of maturation was slower with respect to P4-induced maturation.

Figure 4 Time course of maturation induced by prostaglandin F2α (PGF2α) on oocytes and follicles. Maturation was induced with PGF2α (200 ng/ml) in oocytes and follicles. Samples were fixed at 3, 6, 9, 12, 15, 18, 21 and 24 h of incubation and germinal vesicle breakdown (GVBD) was assessed. Data are represented as the mean ± standard deviation (SD) (n = 9) of experiments performed in triplicate on different animals. a,bMeans with different letters are significantly different (P < 0.05).
Annual variation of oocyte response to P4 and PGF2α action
In amphibians, as in other vertebrates, the physiology of the ovary is subject to seasonal variations in the environment. The ability of denuded oocytes of R. arenarum to resume meiosis under the action of P4 varies throughout the year. Thus, previous observations showed that the oocytes of this species present a higher response to the action of the hormone during the reproductive period (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013), coincidentally with their ability for spontaneous maturation (Zelarayán et al., Reference Zelarayán, Oterino and Bühler1995). Our experience during the last 4 years allows us to infer that the response of R. arenarum denuded oocytes and follicles to P4 varies seasonally and agrees with the reports of Valdéz Toledo & Pisanó (Reference Valdéz Toledo and Pisanó1980) and Medina et al. (Reference Medina, Ramos, Crespo, Gonzalez-Calvar and Fernandez2004), which strengthen this idea. Our results in vitro could be grouped into three periods according to the seasonal variations of the ovary (Fig. 5).

Figure 5 Seasonal response of oocytes and follicles to progesterone (P4) and prostaglandin F2α (PGF2α). Oocytes and follicles obtained during February to June, July to September and October to January were incubated in the presence of P4 (1 μM) or PGF2α (100 and 200 ng/ml). Values represent the mean ± standard deviation (SD) (n = 9) of experiments in triplicate with different animals. Amphibian Ringer (AR) control was performed only with oocytes. a–fMeans with different letters are significantly different (P < 0.05).
From February to June, which represents a recovery stage after the summer, non-competent oocytes and follicles exhibit a low ability to respond to P4in vitro (56 ± 6% and 32 ± 6% of maturation, respectively), a gradual increase in this response being observed during subsequent periods. During the July to September period, oocytes respond to the hormonal stimulus but do not mature spontaneously (incompetent oocytes). During the October to January period, animals ovulate in response to the photoperiod and to the increase in gonadotropins. During this period, oocytes can mature spontaneously in vitro if they are deprived of their enveloping follicle cells (competent oocytes) (51 ± 5%) and reach maximum GVBD values in response to P4 (97 ± 3%). Similarly, during this period, follicles also reach their maximum response to the hormone (96 ± 4%) (Fig. 5). The ovary shows its highest activity (ovulation and oviposition). This time is a period of high P4 response capacity.
Based on the seasonal variation of the oocyte response to P4in vitro, we investigated the effect of PGF2α on maturation in the three periods in the year. The results obtained in the February to June period indicate that oocytes present low sensitivity to PGF2α, reaching a maturation percentage of 15 ± 5% and 47 ± 7% for doses of 100 and 200 ng/ml, respectively (Fig. 5). In contrast, during the October–January period, maturation indices were highest, no significant differences being observed between the doses used (95 ± 3% and 97 ± 6%). In the July–September period, the response to PGF2α presented differences according to the dose used (36 ± 8% and 94 ± 6% for 100 and 200 ng/ml, respectively). Follicles also exhibited a seasonal response to PGF2α. These results indicate that oocytes and follicles of R. arenarum mature under the action of this prostaglandin, showing a correlation with the response to P4 throughout the year.
Additionally, during the July–September period, experiments were conducted with PGF2α (100 ng/ml) in combination with a suboptimal dose of P4 (0.01 μM) in order to analyze if synergistic effects appeared. Incompetent oocytes and whole follicles were incubated in the presence of both substances for 24 h. Results are shown in Fig. 6. The maturation induced by PGF2α (100 ng/ml) (35 ± 6% in oocytes and 28 ± 4% in follicles) was potentiated by the addition of a suboptimal concentration of P4 (0.01 μM), so that oocytes and follicles reached significantly greater percentages of GVBD (64 ± 5% and 58 ± 5%, respectively) (P < 0.05). The treatment of denuded oocytes with PGF2α in combination with a suboptimal dose of P4 showed a synergistic effect of both substances on the maturation process.

Figure 6 Effect of the combined action of prostaglandin F2α (PGF2α) and progesterone (P4) on meiosis resumption. Oocytes and follicles from animals captured during the July to September period were incubated in the presence of P4 (0.01 μM) and of PGF2α (100 ng/ml). Values represent the mean ± standard deviation (SD) (n = 8) of experiments performed in triplicate with different animals. Germinal vesicle breakdown (GVBD) was assessed after 24 h of incubation. a–cMeans with different letters are significantly different (P < 0.05).
PGF2α-induced maturation and its relationship with MPF activation
In a previous study, our research group demonstrated that microinjection of mature cytoplasm from P4-treated oocytes into R. arenarum immature oocytes (intact GV) is sufficient to activate an amplification loop that induces the conversion of pre-MPF into active MPF, leading to meiosis resumption (Sánchez Toranzo et al., Reference Sánchez Toranzo, Bonilla, Zelarayán, Oterino and Bühler2006). In order to analyze in this species if PGF2α-induced maturation activates MPF, its amplification was analyzed with a microinjection of cytoplasm from mature oocytes.
When immature oocytes were microinjected with cytoplasm from oocytes treated with PGF2α (200 ng/ml), they matured (63 ± 5% of GVBD) after 20 h of incubation (Fig. 7 III). Cytoplasm transfer from PGF2α-matured oocytes produced results statistically similar to those obtained with microinjection of cytoplasm from P4-treated oocytes (65 ± 6%) (P < 0.05) (Fig. 7 II). The oocytes microinjected with immature cytoplasm did not resume meiosis (negative control) (Fig. 7 I). Donor oocytes (those from which cytoplasm was obtained) reached 94 ± 3% and 84 ± 6% GVBD when they were treated with P4 (1 μM) and PGF2α (200 ng/ml), respectively.

Figure 7 MPF amplification in denuded oocytes. Immature incompetent oocytes were microinjected with 40 nl of (I) immature cytoplasm, (II) cytoplasm matured with progesterone (P4) (1 μM) and (III) cytoplasm matured with prostaglandin F2α (PGF2α) (200 ng/ml). After microinjection, oocytes were incubated in Amphibian Ringer (AR) solution for 20 h and germinal vesicle breakdown (GVBD) was assessed. Each bar represents the mean ± standard deviation (SD) (n = 4) of experiments performed in duplicate on different animals. a,bMeans with different letters are significantly different (P < 0.05).
Activation and formation of pronuclei in PGF2α-matured oocytes
To further the study of the role of PGs in meiosis resumption, we analyzed if PGF2α-induced maturation is a physiological phenomenon that allows the later activation of R. arenarum oocytes. For that purpose, the maturation of denuded oocytes was induced with PGF2α (200 ng/ml) or P4 (1 μM) as control. After 20–22 h of incubation, the oocytes were stimulated mechanically or with electric pulses. The data obtained indicate that at 20 min post-activation 79 ± 4% of the oocytes stimulated with electric pulses and 64 ± 3% of those stimulated mechanically showed signs of activation (Fig. 8). Higher results were observed in oocytes treated with P4 (87 ± 3% and 90 ± 3 % of activation, respectively). The oocytes showed the external signs characteristic of activation: flattening of the animal pole and disappearance of the white spot (Fig. 9A). Conventional histological technique and staining with Alcian Blue, pH 2.5, verified the complete exocytosis of CG (Fig. 9B). Non-activated oocyte is shown in Fig. 9C.

Figure 8 Activation capacity of oocytes matured with prostaglandin F2α (PGF2α). Maturation was induced with PGF2α (200 ng/ml) or progesterone (P4) (1 μM) (control). Oocytes were stimulated mechanically or with electric pulses. After 20 min external signs of activation were assessed. The samples were subjected to conventional histological technique and staining with Alcian Blue dye, pH 2.5, to assess cortical granules (CG) exocytosis. Values represent the mean ± standard deviation (SD) (n = 4) of experiments performed in triplicate on different animals. a–cMeans with different letters are significantly different (P < 0.05).

Figure 9 Activation of oocytes matured with prostaglandin F2α (PGF2α). (A) External signs of activation can be observed in the oocytes: flattening of the animal pole, separation of the vitelline envelope and disappearance of the white spot. Stereoscopic microscope observations, ×30 magnification. (B) Cortical granule (CG) exocytosis. Activated oocyte stained with Alcian Blue dye. Absence of CG can be observed, ×400 magnification. (C) Non-activated oocyte stained with Alcian Blue dye. The presence of stained CG can be observed, ×400 magnification.
In order to verify if PGF2α-induced maturation capacitates R. arenarum oocytes to form pronuclei, homologous sperm were microinjected into denuded oocytes previously matured in vitro with PGF2α (200 ng/ml) or P4 (1 μM) (control). PGF2α-matured oocytes were able to form pronuclei in similar time periods (2 h) to control treated with P4 (1 μM). The cytological analysis at 120 min of the microinjected and fixed, oocytes revealed the presence of female and male pronuclei (Fig. 10A and 10B, respectively). In the sperm microinjection experiments an incubation time of 2 h is required to visualize the pronuclei formation. We did not perform assays for longer periods of time as the development of the embryos was abortive after 3–4h of incubation when oocyte maturation was induced by P4 (Oterino et al, Reference Oterino, Toranzo, Zelarayán and Bühler1997).
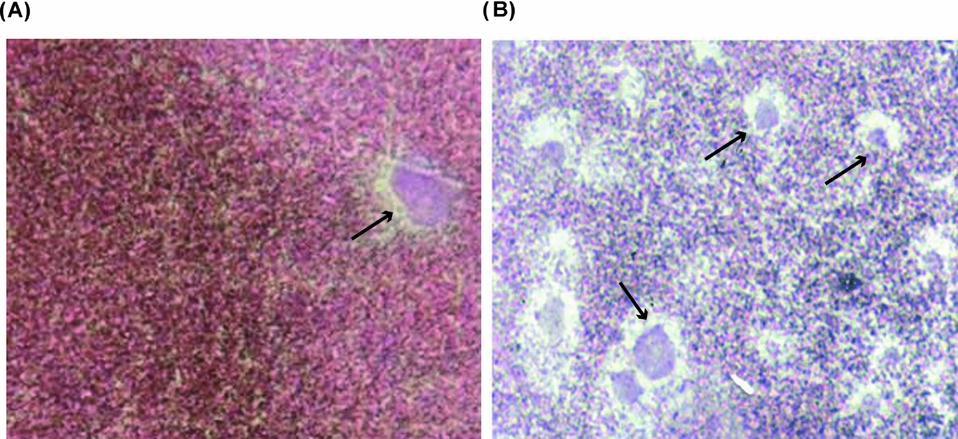
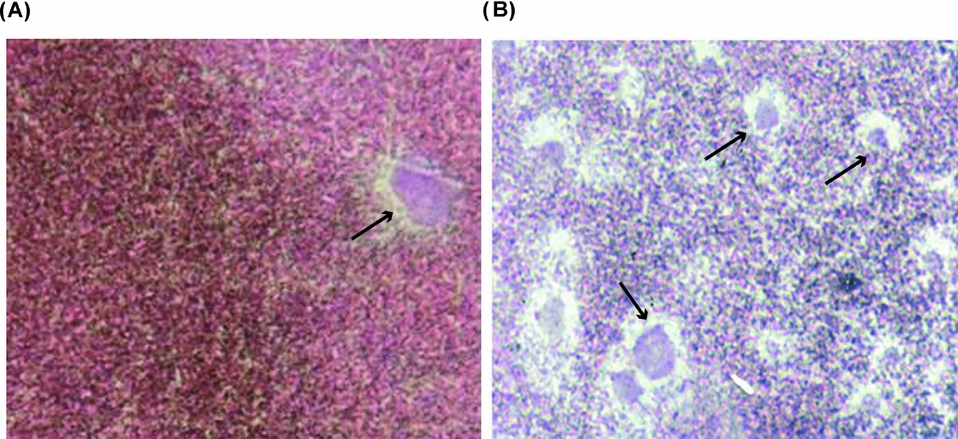
Figure 10 Fully grown denuded oocytes matured with PGF2α were injected with a homologous sperm suspension and fixed 2 h later. Photographs show: (A) female pronucleus (arrow); (B) numerous male pronuclei (arrows) ×600 magnification.
Discussion
The results of this work constitute the first study that examines the effect of AA and its metabolites, PGs, on R. arenarum oocyte maturation, their potential role in the regulation of P4-induced maturation and their effect on oocyte activation and pronuclei formation.
In this study, induction of in vitro oocyte maturation by AA treatment was observed. Although AA induces maturation of oocytes and follicles in a dose- and time-dependent manner, the maturation rate is lower than in the presence of P4. AA-induced meiosis resumption is a slower process. These results agree with the ones obtained in starfish where AA and eicosapentaenoic acid were found to induce maturation in the same way as the natural hormone, 1-MeAde (Meijer et al., Reference Meijer, Guerrier and Maclouf1984). In fish oocytes, AA mobilization and PGs formation are required to induce GVBD (Sorbera et al., Reference Sorbera, Asturiano, Carrillo and Zanuy2001; Lister & Van Der Kraak, Reference Lister and Van Der Kraak2008). However, when oocytes and follicles of R. arenarum were exposed to AA in doses higher than 150 μM, the maturation values reached were lower. Our results are similar to those obtained in fish oocytes, in which the low response could be attributed to different causes, such as the rapid oxidation of high AA doses, AA transformation into other compounds (Mercure & Van Der Kraak, Reference Mercure and Van Der Kraak1995) or its low availability to be transformed into PGs. In this paper we only analysed the role of AA in oocyte maturation. However, other PUFAs have been investigated. In fish, their importance in ovulation has been demonstrated when specimens were treated with diets enriched with PUFAs. In that case, the effect of high AA doses (300 μM) was analysed during oocyte maturation (Sorbera et al., Reference Sorbera, Asturiano, Carrillo and Zanuy2001). In our experiments, lower doses were used. Our results indicate that AA-induced maturation can be inhibited by indomethacin, a COX inhibitor, suggesting that PGs could be implicated in meiosis resumption in R. arenarum. In a similar way to the results found by Van Der Kraak & Chang (Reference Van Der Kraak and Chang1990) for goldfish oocytes, the effect of PGF2α was blocked by drugs that inhibit COX activation, preventing the formation of PGs from AA. In the ovary of gold fish, the mechanism by which AA affects oocyte maturation is still poorly understood. In other tissues, many experiments indicate that calcium is a mediator of AA action (Törnquist et al., Reference Törnquist, Ekokoski and Forse1994). We may tentatively assume that AA causes the mobilization of Ca2+ and such mobilization would be related to the maturation of R. arenarum oocytes. In this respect, our research group has previously demonstrated the participation of this cation in spontaneous and P4-induced maturation (Zelarayán et al., Reference Zelarayán, Oterino, Sánchez Toranzo and Bühler2004). However, AA-induced oocyte maturation requires further experiments to clarify it.
In Rana pipiens oocytes the participation of fatty acids and different types of 1,2-diacylglycerol (1,2-DAG) has been demonstrated in P4-induced maturation. In the oocytes of this species the increase of DAGs in their plasmatic membranes is sufficient to trigger meiosis. In fact, P4 acts on cell surface receptors to initiate the sequential release of fatty acids from the different isoforms of DAGs, among them AA (Morrill et al., Reference Morrill, Ma and Kostellow2000). These authors propose that this mobilization could be associated with the maturation process. In this respect, Buschiazzo & Alonso (Reference Buschiazzo and Alonso2005) demonstrated that the reinitiation of meiosis induced by P4 in R. arenarum is accompanied by an increase in PUFAs such as AA with no significant variations in DAGs in the vitelline platelets. In this sense, it is possible that during R. arenarum oocyte maturation a fatty acids mobilization occurs, among which AA would be preponderant, in a similar way to teleosts and starfish. In our species, such mobilization could result from the activation of PLA2, since its inhibition with quinacrine prevents meiosis resumption (Ortiz et al., Reference Ortiz, Bühler and Zelarayán2013).
To date, there are no studies that document the production of PGs in the ovary of R. arenarum. However, our in vitro studies demonstrated the role of AA and its metabolites in the maturation process. In this work we demonstrated that PGs from the hydrolysis of AA such as PGF2α, and to a lesser extent PGE2, induce meiosis resumption in R. arenarum oocytes. In this species, oocyte response to PGE1 is scarce, in a similar way to the response observed in fish oocytes (Sorbera et al., Reference Sorbera, Asturiano, Carrillo and Zanuy2001). In agreement with this situation, previous studies have demonstrated the participation of PGs in the maturation of Xenopus oocytes, in which a gradual increase in the levels of PGF2α was observed when follicles were treated with hCG, this effect being lower for PGE2 (Sena & Liu, Reference Sena and Liu2008). In R. arenarum, the effects of PGF2α on the maturation of oocytes and follicles are very similar to those produced by P4, its natural inducer. This fact would allow us to suggest that PGF2α is a metabolite of AA that would mediate its stimulatory effect on meiosis resumption in this species.
The interaction of PGF2α with its receptor is not well defined. In hepatocytes it has been suggested that this interaction could be coupled to a Gi protein as the activation of mitogen activated protein (MAP) kinase by PGF2α can be inhibited by pertussis toxin (Melien et al., Reference Melien, Thoresen, Sandnes, Ostby and Christoffersen1998). In a previous study, our research group proposed the participation of a Gi protein in P4-induced maturation in R. arenarum oocytes (Zelarayán et al., Reference Zelarayán, Ajmat, Bonilla and Bühler2012). In this species, Toranzo et al. (Reference Toranzo, Bonilla, Bühler and Bühler2011) demonstrated the role of MAP kinases in oocyte maturation.
In amphibians, ovarian response is influenced by seasonal changes, R. arenarum being no exception, as shown by our in vitro studies with recently captured animals. In fact, the maturation of oocytes and follicles shows a seasonal response to PGF2α in a similar way to P4, its natural inducer. Thus, during the reproductive period, oocytes and fully grown follicles of R. arenarum respond to favourable environmental conditions and to PGF2α, showing elevated maturation percentages. In denuded oocytes, AA metabolites could promote maturation by a direct effect, potentiating their competence to mature spontaneously when they are deprived of their follicle cells during the October to January period. At this time, the oocytes surrounded by their enveloping follicle cells do not mature spontaneously, but do so under the influence of PGF2α and PGE2. In the case of our species, it is possible that AA metabolites could promote follicle maturation in an indirect, synergistic way, as observed in the follicles of teleost fish where PGs have been associated with ovarian steroidogenesis (Van Der Kraak & Chang, Reference Van Der Kraak and Chang1990). Additionally, treatment of R. arenarum denuded oocytes with PGF2α and low doses of P4 showed a synergistic effect on the maturation process.
Taking into account that there are no previous studies on the role of AA metabolites in the maturation of R. arenarum oocytes, the effect of PGF2α on maturation was confirmed by analysing the capacity of the treated oocytes to activate and form pronuclei after being injected with homologous sperm. It is probable that the action of PGF2α only leads to the dissolution of the nuclear envelope due to an unknown cause and not to physiological maturation. The cytological analysis of the oocytes activated with mechanical or electric stimuli allowed us to rule out that possibility and demonstrate the absence of CG in the oocyte cortex, suggesting that PGF2α induces GVBD and the continuation of meiosis until metaphase II. In turn, oocytes matured by the action of PGF2α are capable of forming pronuclei after fertilization in a similar way to P4 for this species (Oterino et al., Reference Oterino, Toranzo, Zelarayán and Bühler1997).
The existence of pre-MPF in immature oocytes of R. arenarum was demonstrated by the microinjection of mature cytoplasm assays. Small amounts of cytoplasm from oocytes matured under the action of P4 are sufficient to induce maturation in immature oocytes (Sánchez Toranzo et al., Reference Sánchez Toranzo, Bonilla, Zelarayán, Oterino and Bühler2006). In a similar way, our MPF amplification experiments with oocytes treated with PGF2α led to the transformation of pre-MPF into active MPF. These data suggest that injection of a small amount of mature cytoplasm was able to induce MPF amplification in immature oocytes and promote its maturation.
This work focuses on the role of AA and PGs, especially PGF2α, during the process of R. arenarum oocytes maturation. Our results show that AA and its metabolite PGF2α induced oocyte maturation and increased maturation induced by low doses of P4in vitro. Once maturation was reached, the oocytes treated with PGF2α were competent to activate and form pronuclei. This fact demonstrates the capacity of PGF2α to induce physiological maturation and represents a novel contribution to the scarce data on the participation of the AA cascade in the process of amphibian oocyte maturation. Although the mechanisms involved in PGF2α-induced maturation leading to MPF activation in amphibian oocytes have not yet been clarified, the results of this work represent an original contribution to the study of the maturation process. Further studies would be necessary to elucidate the cellular and molecular mechanisms of AA metabolites involved in meiosis resumption.
Acknowledgements
This work was supported by a grant from the Science Council of the National University of Tucuman (CIUNT), Argentina. The authors are grateful for the suggestions and comments of Dr Marta I. Bühler and for her invaluable help in the revision of the manuscript, and for Dr Federico Bonilla's kind assistance in performing the microinjection experiments.