Introduction
Many aspects of bovine innate and acquired host defenses are suboptimal during distinct periods of the lactation cycle, particularly around the time of calving. Most notably, the three weeks prior to calving through the first three weeks of lactation have long been recognized as a period when key host defense mechanisms are altered dramatically. As a consequence, dairy cattle are more susceptible to metabolic and infectious diseases during this periparturient period (Drackley et al., Reference Drackley, Overton and Douglas2001; Goff, Reference Goff2006). Health disorders occurring during this time may greatly impact the productive efficiency of dairy cattle in the ensuing lactation. Therefore, it is not surprising that considerable research efforts have focused on defining how host defenses change as a consequence of the lactation cycle and understanding those factors that may contribute to immune dysfunction during this critical period. Several recent reviews have examined dairy cattle immunity in considerable detail (Sordillo, Reference Sordillo2005; Rainard and Riollet, Reference Rainard and Riollet2006; Lippolis, Reference Lippolis2008; Sordillo and Aitken, Reference Sordillo and Aitken2009). This review, however, will focus on how increased metabolic demands of the periparturient cow may contribute to compromised host defenses, particularly as related to the regulation of inappropriate inflammatory responses.
Bovine immunobiology
Dairy cattle are protected against disease by a dynamic immune system that consists of a complex network of cells and soluble mediators. The immune system can be conveniently separated into two categories: innate and acquired immunity. Innate immunity, also known as non-specific responsiveness, includes a set of resistance mechanisms that are not specific to a particular antigen. Innate defense mechanisms provide the initial protection when the host is first exposed to infectious pathogens and before the adaptive immune system is activated. The generalized responses of innate immunity can be localized within affected tissues or activated for quick mobilization to the site of infection by numerous stimuli, but they are not augmented by repeated exposure to the same insult. Components of innate defense are diverse and include the physical barrier of the skin, leukocytes (macrophages, neutrophils and natural killer cells), non-immune cells (epithelial and endothelial cells), certain soluble mediators (cytokines and eicosanoids) and other physiological factors (Sordillo, Reference Sordillo2005; Rainard and Riollet, Reference Rainard and Riollet2006; Lippolis, Reference Lippolis2008). Acquired or specific immunity is triggered if a pathogen is able to evade or is not completely eliminated by the innate defense system. Specific immune responses are elicited to particular antigenic challenges associated with infectious agents or any other foreign bodies for elective elimination. If a host should encounter the same antigen more than once, a heightened state of immune reactivity would occur as a consequence of immunological memory. In comparison with the first exposure to a particular antigen, a memory response will be much faster, considerably stronger, last longer and often be more effective in clearing the pathogen (Sordillo, Reference Sordillo2005). This feature of the specific immune response is the foundation for vaccination protocols. Whereas it is convenient to discuss the highly complex nature of the immune system in terms of non-specific and specific responses, it should be emphasized that innate and acquired immunity do not operate independently of each other. Effective host defense requires that both innate and acquired protective factors be highly interactive and coordinated to provide optimal resistance to disease.
The immune system as a whole must maintain a delicate balance between sufficient activity needed to eliminate the insult and controlling the response to avoid bystander damage to host tissues. Therefore, efficient regulation of the bovine immune system is related to the overall susceptibility of dairy cows to diseases. For example, the inflammatory response is a hallmark of innate immunity. Inflammation is a complex biological response to invading pathogens or other harmful stimuli that has two main functions: to remove the injurious agent and to initiate the tissue healing process. Acute inflammation can be characterized by a distinct series of physiological responses including the release of soluble mediators, vasodilatation, increased blood flow, extravasation of fluid (increased endothelial cell permeability), cellular influx (chemotaxis) and elevated cellular metabolism. Each of these physiological responses contributes to the clinical symptoms associated with inflammation including heat, redness, swelling and pain. An effective inflammatory response will result in the rapid elimination of microbial pathogens or other insults, and often will not result in any detrimental changes to host tissues. When not regulated properly, however, an overzealous inflammatory response is often associated with the pathophysiology of several inflammatory-based diseases of dairy cattle such as coliform mastitis and septic shock (Hill, Reference Hill1981; Burvenich et al., Reference Burvenich, Bannerman, Lippolis, Peelman, Nonnecke, Kehrli and Paape2007).
Metabolic and nutritional challenges of periparturient dairy cows
Research in both human and veterinary medicine shows a clear relationship among nutrition, inflammation and disease susceptibility (Calder, Reference Calder2008; Wood et al., Reference Wood, Scott, Garg and Gibson2009). Several physiological changes that occur in cows during the transition period can impact nutritional status and likely contribute to increased disease susceptibility. The periparturient period is characterized by a sudden increase in energy requirements imposed by the onset of lactation and by a decrease in voluntary dry matter intake. The resulting negative energy balance (NEB) is further aggravated by fetal metabolic demands and the nutrient prioritization toward the mammary gland (Leroy et al., Reference Leroy, Vanholder, Van Knegsel, Garcia-Ispierto and Bols2008). Homeostasis of all the energy substrates is altered during this time period. Approximately 85% of whole-body glucose, for example, is directed to the mammary gland for milk synthesis and secretion. Amino acid supply also is altered. A dairy cow during early lactation producing over 30 liters of milk needs at least 2320 g/day of protein, which is three times the requirement of a cow during late pregnancy (Bell, Reference Bell1995). Furthermore, amino acid requirements could increase based on the fact that these compounds are used as gluconeogenic substrates by the liver (Herdt, Reference Herdt2000). As a result, dairy cows must rely in part on protein mobilization from skeletal muscle reserves to fulfill amino acid requirements (Bell et al., Reference Bell, Burhans and Overton2000; Jafari et al., Reference Jafari, Emmanuel, Christopherson, Thompson, Murdoch, Woodward, Field and Ametaj2006).
A significant adaptation to NEB during the transition period, however, is the mobilization of fat from body stores and the release of non-esterified fatty acids (NEFAs) into the blood stream. Fat-derived fuels, such as NEFAs and ketone bodies, are important sources of energy because the majority of available glucose is redirected to the mammary gland for lactose synthesis (Herdt, Reference Herdt2000). Therefore, adequate body fat reserves can promote milk production and health during times of NEB. Continuous lipolysis, however, promotes NEFA transformation into triacylglycerols (TAG) by the liver and excessive accumulation of TAG could result in fatty liver disease (Drackley et al., Reference Drackley, Overton and Douglas2001). Indeed, large amounts of adipose stores during times of energy deficiency are directly linked with adverse health effects on the transition cow. A major factor that may contribute to the development of metabolic and infectious diseases in over-conditioned periparturient dairy cattle is the inappropriate extensive adipose mobilization and the excessive accumulation of plasma NEFA.
Fatty acids and inflammatory responses
Research in humans and various animal models suggest that fatty acids are important modulators of inflammatory reactions (Calder, Reference Calder2008; Serhan et al., Reference Serhan, Chiang and Van Dyke2008). Fatty acids can impact host inflammatory responses in several ways. An adequate supply of fatty acids is essential as a key energy source where they can be oxidized to produce Acyl-CoA and yield ATP. When present in excess, however, increased levels of circulating NEFA concentrations are associated with increased systemic inflammatory conditions in humans (Wood et al., Reference Wood, Scott, Garg and Gibson2009). Ample evidence in human medicine showed that elevated NEFA concentration increases the susceptibility of individuals to several inflammatory-based diseases including diabetes and atherosclerosis (Massaro et al., Reference Massaro, Scoditti, Carluccio and De Caterina2008; Wood et al., Reference Wood, Scott, Garg and Gibson2009). Excessive adipose stores and elevated NEFA concentrations also are positive risk factors for many pro-inflammatory periparturient diseases in dairy cows including mastitis and metritis (Bernabucci et al., Reference Bernabucci, Ronchi, Lacetera and Nardone2005; Goff, Reference Goff2006; Douglas et al., Reference Douglas, Rehage, Beaulieu, Bahaa and Drackley2007). Even though the importance of NEFA in the inflammatory responses has been recognized in recent years, the underlying mechanism for this effect is still subject to speculation.
There are several proposed mechanisms by which elevated NEFA can regulate the inflammatory response to either the benefit or detriment of the host. Fatty acids can be incorporated into membrane phospholipids and influence several important cellular functions by controlling membrane fluidity and lipid-raft protein formation. Evidence also exists to suggest that fatty acids are potent regulators of gene expression by influencing receptor binding, intracellular signaling and transcriptional factor activation (Martins de lima et al., Reference Martins de lima, Gorjo, Hatanaka, Cury-boaventura, Portiolisilva, Procopio and Curi2007; Yaqoob and Calder, Reference Yaqoob and Calder2007). Activation of the innate immune response occurs through Toll-like receptors (TLR) located on both immune and non-immune cells that can recognize pathogen-associated molecular patterns on bacterial pathogens. For example, TLR4 recognizes lipopoylsaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria. TLR4 activation triggers an intracellular signaling cascade that can result in NF-κB translocation into the nucleus and upregulation of pro-inflammatory genes (Bannerman and Goldblum, Reference Bannerman and Goldblum2003). The typical pro-inflammatory response to LPS includes the expression of several acute phase cytokines (TNF, IL1 and IL8) and adhesion molecules by leukocytes and endothelial cells, followed by the influx and activation of neutrophils into the affected tissues. TLR4 also can be activated by fatty acid agonists including lauric, palmitic and oleic acids through activation of NF-κB. In fact, lauric acid is a major component of the lipid A associated with LPS and therefore, plays a role in ligand recognition and receptor activation through TLR4 (Shi et al., Reference Shi, Kokoeva, Inouye, Tzameli, Yin and Flier2006). Saturated fatty acids, such as palmitate, have a more potent effect on macrophage TLR4 activation than unsaturated fatty acids (Lee et al., Reference Lee, Sohn, Rhee and Hwang2001). Moreover, polyunsaturated FA (PUFA) such as eicosapentaenoic acid (EPA) and docosapentanoic acid (DHA) can actually inhibit LPS-induced NF-κB activation by targeting the TLR4 or its associated molecules (Lee et al., Reference Lee, Plakidas, Lee, Heikkinen, Chanmugam, Bray and Hwang2003). Another important group of transcription factors that are regulated by fatty acids are the peroxisome proliferator-activated receptors (PPARs). These nuclear receptors regulate gene expression by binding DNA sequence elements localized in the promoter region of target genes (Kliewer et al., Reference Kliewer, Sundseth, Jones, Brown, Wisely, Koble, Devchand, Wahli, Willson, Lenhard and Lehmann1997). In monocytes, PPARγ activation by certain PUFAs such as α-linolenic acid and DHA can cause suppression of the inflammatory response (Lee et al., Reference Lee, Plakidas, Lee, Heikkinen, Chanmugam, Bray and Hwang2003).
In dairy cows, intense lipomobilization during the periparturient period will cause significant shifts in both the plasma fatty acid profiles and the phospholipid content of cellular membranes of different organs such as adipose tissue and the liver (Douglas et al., Reference Douglas, Rehage, Beaulieu, Bahaa and Drackley2007). For example, palmitic acid concentration in the cellular membrane phospholipid layer of hepatocytes and adipocytes increase significantly with a concomitant decrease in EPA and DHA during the periparturient period (Douglas et al., Reference Douglas, Rehage, Beaulieu, Bahaa and Drackley2007). Similar changes were observed in the membrane phospholipids of circulating white blood cells in lactating women and these alterations resulted in changes of immune cell functions (Otto et al., Reference Otto, van Houwelingen, Badart-Smook and Hornstra2001). Although not demonstrated in bovine leukocytes or endothelial cells, incremental changes in the plasma membrane fatty acid content of key cell types involved in the inflammatory response may affect how cows respond to infectious pathogens during times of increased metabolic stress.
Lipid mediators
Another important way that fatty acids can orchestrate immune and inflammatory responses is through the biosynthesis of lipid mediators including eicosanoids, lysophospholipids, phosphoinositides, sphingolipids, diacylglycerol, phosphatidic acid and ceramide (Serhan et al., Reference Serhan, Chiang and Van Dyke2008). Among these lipid mediators, the family of eicosanoids has long been recognized as key regulators of both acute and chronic inflammatory reactions. Linoleic acid and some PUFA (arachidonic acid, DHA and EPA) can serve as precursors for the biosynthesis of eicosanoids including prostaglandins (PG), prostacyclins, leukotrienes, lipoxins and thromboxanes. Depending on the timing and magnitude of expression, certain eicosanoids can either enhance or resolve the inflammatory response (Fig. 1). Therefore, variations in the fatty acid profiles in plasma and immune cellular membranes could affect the expression of pro-inflammatory mediators through lipid mediator biosynthetic pathways (Calder, Reference Calder2006).
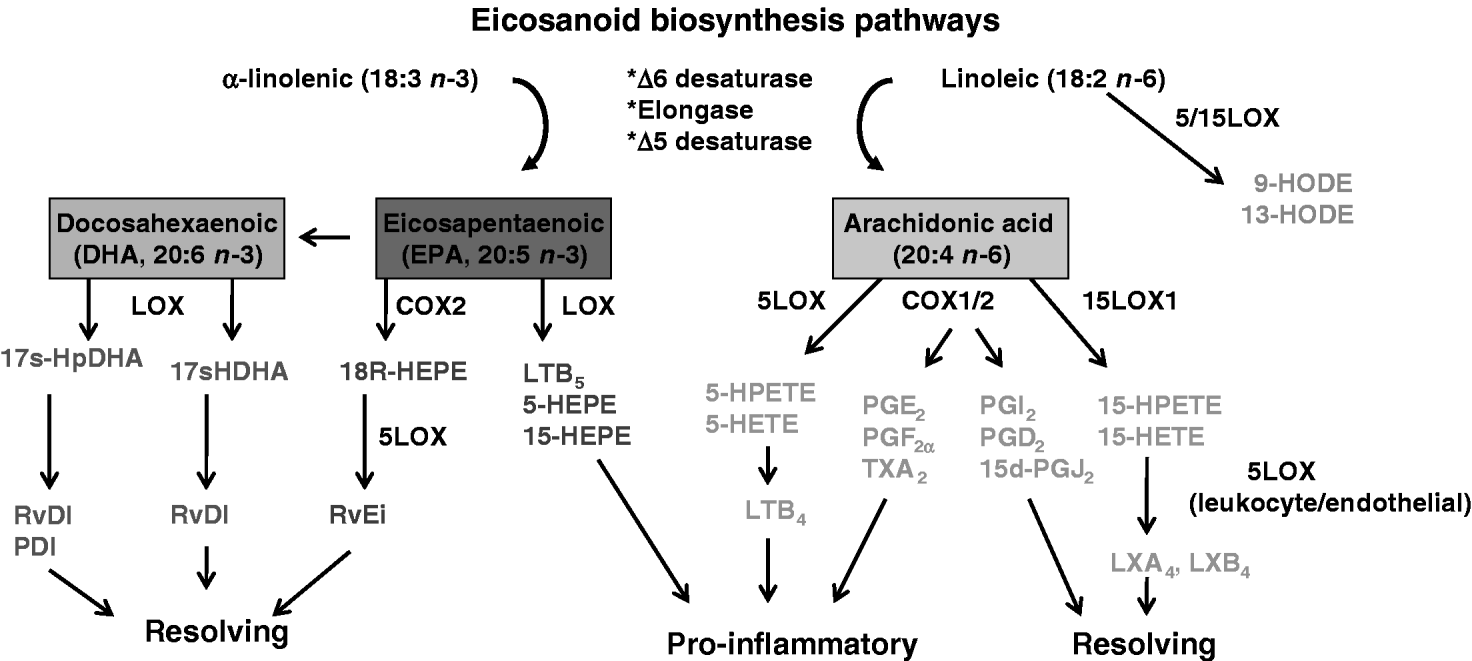
Fig. 1. Enzymatic pathways leading to the production of biologically active lipid mediators. Linolenic acid is the parent compound of the n−6 family of fatty acids and α-linolenic acid is the n−3 fatty acid precursor. These fatty acids compete for a microsomal enzyme system that desaturates (desaturase) and lengthens (elongase) them to form long-chain PUFA including arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid. These PUFAs are incorporated into membrane phospholipids, but serve as important substrates for the biosynthesis of eicosanoids through the cyclooxygenase (COX) and lipoxygenase (LOX) pathways.
PUFAs released from membrane phospholipids through the action of phospholipases can be metabolized by either the cycloxygenase (COX) or lipoxygenase (LOX) pathway to yield biologically active eicosanoids. The COX family is composed of two major isoforms. COX1 is constitutively expressed in most tissues and synthesizes low levels of PG, such as prostacyclin (PGI2), that are thought to function in the maintenance of normal physiological functions. Conversely, COX2 is highly inucible in response to pro-inflammatory stimuli and it traditionally has been associated with the biosynthesis of pro-inflammatory mediators such as PGE2, PGF2α and thromboxane A2 (TXA2). Non-steroidal anti-inflammatory drugs (NSAIDs) can inhibit PG biosynthesis by targeting COX activity and are used widely to treat a variety of inflammatory-based diseases including coliform mastitis in dairy cows (Erskine et al., Reference Erskine, Wagner and DeGraves2003). Suppression of these enzymes, however, also can cause undesirable side effects. The most common consequence of prolonged COX1 inhibition is the development of abomasal ulcers (Anderson and Muir, Reference Anderson and Muir2005). Although selective COX2 inhibitors minimize the risk of gastrointestinal events such as stomach ulcers, these drugs have been related to fatal cardiovascular reactions in humans, possibly by decreasing vascular PGI2 production (Rainsford, Reference Rainsford2007). Previous assumption that all COX2 metabolites are solely responsible for propagating the inflammatory response is not supported by current literature (Serhan et al., Reference Serhan, Chiang and Van Dyke2008). Indeed, studies in COX1 and COX2 knockout mice indicate that both isoforms can contribute to agonist-induced inflammatory responses (Langenbach et al., Reference Langenbach, Morham, Tiano, Loftin, Ghanayem, Chulada, Mahler, Lee, Goulding, Kluckman, Kim and Smithies1995, Reference Langenbach, Loftin, Lee and Tiano1999; Morham et al., Reference Morham, Langenbach, Loftin, Tiano, Vouloumanos, Jennette, Mahler, Kluckman, Ledford, Lee and Smithies1995). Conversely, there is now compelling evidence that some COX2 metabolites may be critical in mediating the resolution of acute and chronic inflammation (Rajakariar et al., Reference Rajakariar, Yaqoob and Gilroy2006, Reference Rajakariar, Hilliard, Lawrence, Trivedi, Colville-Nash, Bellingan, Fitzgerald, Yaqoob and Gilroy2007). The ability of various COX2 metabolites to regulate inflammation is largely dependent on the timing of expression. Increased COX2 expression during the onset of inflammation is typified by PGE2 production, whereas enhanced COX2 expression during the resolution of inflammation is associated with the presence of PGD2, 15d-PGJ2 and 15R-HETE. Both PGD2 and its dehydration end product 15d-PGJ2 can inhibit leukocyte adhesion to endothelial cells and decrease cytokine expression by blocking NF-κB activation (Prasad et al., Reference Prasad, Giri, Singh and Singh2008). The 15R-HETE can be further metabolized by leukocyte 5LOX to produce the potent pro-resolving lipoxins (LX), LXA4 or LXB4 (Serhan et al., Reference Serhan, Chiang and Van Dyke2008).
LOX is a heterogeneous family of non-heme enzyme dioxygenases with the ability to oxidize PUFA. There are several different LOX isoforms including 5LOX and 15LOX, where the nomenclature is defined by the capability of each enzyme to introduce molecular oxygen on a specific carbon of the fatty acid structure (Kühn and O'Donnell, Reference Kühn and O'Donnell2006). Metabolism of arachidonic acid by the 5LOX pathway gives rise to hydroxyl and hydroperoxy derivatives (5-HETE and 5-HPETE, respectively), and LTA4, LTB4, LTC4 and LTD4 that are often elevated in acute and chronic conditions. The 15LOX1 isoform is characterized as an inducible enzyme expressed in endothelial cells, epithelial cells, reticulocytes, monocytes and macrophages with the ability to oxygenate PUFA during inflammation. The initial oxygenated product formed during arachidonic acid metabolism by 15LOX is 15-HPETE, which is the biosynthetic precursor of 15-HETE and other leukotrienes (Reilly et al., Reference Reilly, Srinivasan, Hatley, Patricia, Lannigan, Bolick, Vandenhoff, Pei, Natarajan, Nadler and Hedrick2004). Increased expression of 15LOX1 is observed in diseases where oxidative stress plays important roles such as atherosclerosis, Alzheimer's disease and prostate cancer (Kühn and O'Donnell, Reference Kühn and O'Donnell2006). Previous in vitro studies showed that both 15-HPETE and 15-HETE can enhance intercellular adhesion molecule-1 (ICAM-1) expression and monocyte recruitment (Bolick et al., Reference Bolick, Orr, Whetzel, Srinivasan, Hatley, Schwartz and Hedrick2005, Reference Bolick, Srinivasan, Whetzel, Fuller and Hedrick2006). The overexpression of 15LOX1 in vascular endothelium can accelerate early atherosclerosis in mice, while 15LOX1 knock-out mice develop less pronounced atherosclerosis (Harats et al., Reference Harats, Shaish, George, Mulkins, Kurihara, Levkovitz and Sigal2000; Lee et al., Reference Lee, Plakidas, Lee, Heikkinen, Chanmugam, Bray and Hwang2003). Nanomolar concentrations of 15LOX1 hydroperoxy products can be found in early atherosclerotic lesions and increased 15LOX1 activity was associated with enhanced ICAM-1 expression and monocyte adhesion in vessel walls during disease progression (Reilly et al., Reference Reilly, Srinivasan, Hatley, Patricia, Lannigan, Bolick, Vandenhoff, Pei, Natarajan, Nadler and Hedrick2004). These data suggest that 15LOX1 may facilitate the development of inflammation-based diseases, at least in part, by enhancing the pro-inflammatory phenotype of endothelial cells. Recent studies showed that 15-HPETE induces expression of ICAM-1, inhibits PGI2 function, alters platelet activating factor production and induces apoptosis in bovine endothelial cells (Cao et al., Reference Cao, Cohen, Weaver and Sordillo2001; Weaver et al., Reference Weaver, Maddox, Cao, Mullarky and Sordillo2001; Sordillo et al., Reference Sordillo, Weaver, Cao, Corl, Sylte and Mullarky2005, Reference Sordillo, Streicher, Mullarky, Gandy, Trigona and Corl2008; Corl et al., Reference Corl, Gandy and Sordillo2008). Aitken et al. (Reference Aitken, Karcher, Rezamand, Gandy, VandeHaar, Capuco and Sordillo2009) also demonstrated for the first time that 15LOX1 gene expression increases markedly in mammary tissue during early lactation, but its impact on bovine health is unknown.
Despite the ongoing emphasis on the pro-inflammatory properties of arachidonic acid metabolites of the LOX pathways, recent evidence suggests that the LOX pathways play a significant role in the biosynthesis of LX that are a unique class of lipid mediators with dual anti-inflammatory and pro-resolving functions. The LXs are generated by a process of transcellular biosynthesis involving the sequential actions of LOX from at least two different cell types. For example, the initial oxygenation of arachidonic acid through the 15LOX1 pathway in human epithelial cells generates a 15-HETE precursor that is then metabolized through the 5LOX pathway in macrophages to produce LXA4 and LXB4. Conversely, arachidonic acid metabolism by 5LOX in leukocytes and the release of LTA4 can be converted by 15LOX1 in platelets for LX biosynthesis (Serhan et al., Reference Serhan, Chiang and Van Dyke2008). The presence of LX within inflammatory foci can contribute to the resolution of the inflammatory response. LX functions as pro-resolving and anti-inflammatory lipid mediators by inhibiting leukocyte chemotaxis, transmigration, acute phase cytokine production and NF-κB activation (Serhan et al., Reference Serhan, Chiang and Van Dyke2008). Considerable evidence in experimental animal systems also demonstrates the profound anti-inflammatory and pro-resolving actions of lipoxins by reducing inflammation and disease (Serhan et al., Reference Serhan, Chiang and Van Dyke2008). There is no information to date as to how LX may affect the resolution of inflammation in dairy cattle during disease pathogenesis.
During the periparturient period, the expression of genes that encode eicosanoid-producing enzymes in peripheral blood mononuclear cells, uterus and mammary tissue is altered (Silva et al., Reference Silva, Gaivão, Leitão, Amaro, Lopes da Costa and Mateus2008; Aitken et al., Reference Aitken, Karcher, Rezamand, Gandy, VandeHaar, Capuco and Sordillo2009). Whereas, the significance of eicosanoids and other lipid mediators has long been recognized for their ability to influence bovine inflammatory responses and disease progression, the underlying mechanisms responsible for their biosynthesis are largely unknown. The way in which increases in circulating NEFA concentrations and changes in fatty acid profiles during times of enhanced metabolic stress may influence lipid mediator biosynthesis in the bovine requires further investigation.
Oxidative stress in periparturient dairy cows
Oxidative stress is another important factor that may contribute to dysfunctional inflammatory responses in metabolically stressed cows during the periparturient period (Miller et al., Reference Miller, Brzezinska-Slebodzinska and Madsen1993; Sordillo and Aitken, Reference Sordillo and Aitken2009). Aerobic cellular metabolism requires oxygen for the efficient production of energy and consequently produces reactive oxygen species (ROS) including oxygen ions, free radicals and lipid hydroperoxides. The levels of cellular ROS are maintained within a narrow physiological range to optimize cell performance and prevent cellular damage by a network of antioxidant defense mechanisms. Increased oxygen metabolism during the periparturient period, however, augments the rate of ROS production and the subsequent depletion of important antioxidant defenses (Bell, Reference Bell1995; Gitto et al., Reference Gitto, Reiter, Karbownik, Tan, Gitto, Barberi and Barberi2002; Sordillo et al., Reference Sordillo, O'Boyle, Gandy, Corl and Hamilton2007; Sordillo and Aitken, Reference Sordillo and Aitken2009). As a result, excess accumulation of ROS can cause cell and tissue injury and lead to a condition referred to as oxidative stress in periparturient dairy cows. Oxidative stress is thought to be a significant underlying factor leading to dysfunctional host immune and inflammatory responses particularly during times of increased metabolic stress. Indeed, several studies support the concept that oxidative stress can increase the susceptibility of periparturient dairy cattle to a variety of health disorders (Sordillo and Aitken, Reference Sordillo and Aitken2009).
ROS are primarily formed as end products of the mitochondrial electron transport chain or via activation of NADPH oxidase (Sordillo and Aitken, Reference Sordillo and Aitken2009). The mitochondrial electron transport chain produces the majority of ROS in mammalian cells during normal cellular metabolism through the oxidation of biomolecules (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007). Some of the major ROS formed as a consequence of aerobic metabolism include superoxide anion (·O2−), hydrogen peroxide (H2O2), and the highly reactive hydroxyl radical (OH·). In addition to increased metabolic demands, inflammatory events also may contribute to elevated ROS and the development of oxidative stress. Large volumes of ·O2− and H2O2 are produced during the respiratory burst activity of phagocytic cells by stimulation of NADPH oxidase for the destruction of invading pathogens (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007). This enzyme complex is also activated to a lesser extent in non-immune cells, such as endothelial cells, and participates in cellular signaling processes. Therefore, inflammation can exacerbate oxidative stress during times of high metabolic demand. Women have an influx of inflammatory cells into the uterus and an increase in levels of pro-inflammatory cytokines during parturition (Christiaens et al., Reference Christiaens, Zaragoza, Guilbert, Robertson, Mitchell and Olson2008). A similar phenomenon has not been described in the periparturient dairy cow, and the possibility that localized uterine influx of inflammatory cells and subsequent ROS production may contribute to oxidative stress and uterine health in periparturient cows has not been investigated. However, such a phenomenon may help to explain the increased rates of metritis around the time of calving.
Targets of ROS damage and inflammation
Free radicals are defined as having at least one single unpaired electron in atomic or molecular orbitals (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007). The unpaired electron contributes to molecular instability allowing reactions with surrounding molecules, such as DNA, proteins and lipids. Hydroxyl radicals cause breaks in DNA strands, modifications of purine and pyrimidine bases and deoxyribose sugar molecules. Oxidative DNA damage subsequently results in transcription alterations, signal transduction and permanent genomic alterations. These findings are associated with cancer and aging in humans (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007), however, the specific impact in dairy cattle disease is not known.
There is evidence, however, that oxidative stress in cows is associated with significant increases in lipid peroxidation during the transition period (Bernabucci et al., Reference Bernabucci, Ronchi, Lacetera and Nardone2005; Castillo et al., Reference Castillo, Hernandez, Valverde, Pereira, Sotillo, Alonso and Benedito2006; Sordillo et al., Reference Sordillo, O'Boyle, Gandy, Corl and Hamilton2007). Lipid peroxidation begins when free radicals, including hydroxyl radicals, obtain electrons from lipids in cellular membranes. PUFAs are particularly susceptible to oxidative damage due to the presence of double bonds. Following the initial abstraction of an electron from fatty acids, the hydroxyl radical subsequently generates lipid radicals. Lipid radicals in the presence of oxygen generate lipid peroxy radicals. Accumulation of lipid peroxy radicals can cause an autolytic chain reaction where additional electrons are abstracted from adjacent fatty acids in the membrane generating the reactive lipid hydroperoxide (Sordillo and Aitken, Reference Sordillo and Aitken2009). Lipid peroxidation products can damage cellular membranes and organelles affecting cellular function and altering signal transduction. Several studies in bovine endothelial cells provide direct evidence that increased levels of lipid hydroperoxides resulting from oxidative stress could increase the pro-inflammatory phenotype of these cells (Cao et al., Reference Cao, Reddy and Sordillo2000; Weaver et al., Reference Weaver, Maddox, Cao, Mullarky and Sordillo2001; Sordillo et al., Reference Sordillo, Weaver, Cao, Corl, Sylte and Mullarky2005, Reference Sordillo, Streicher, Mullarky, Gandy, Trigona and Corl2008).
There are several human inflammatory-based diseases that occur as a consequence of oxidative stress including cardiovascular disorders, diabetes and cancer (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007; Bonomini et al., Reference Bonomini, Tengattini, Fabiano, Bianchi and Rezzani2008). The pathologies of these diseases are thought to result from the enhanced expression of redox-regulated pro-inflammatory factors such as eicosanoids, cytokines and adhesion molecules. The accumulation of ROS, including fatty acid hydroperoxides, can affect inflammatory response by acting as secondary messengers in cellular signaling. ROS can participate in the activation of several pathways, but it is the redox-sensitive transcription factor, NF-κB, that specifically contributes to inflammation during infection. During a Gram-negative bacterial infection, for example, the interaction of endotoxin with the TLR4 results in the increased production of ROS. The activation of NF-κB by ROS results in expression of several acute phase cytokines as well as vascular adhesion molecules (VACM) that contribute to the pathogenesis of Gram-negative infections including mastitis (Bannerman et al., Reference Bannerman and Goldblum2003). Pro-inflammatory cytokines are thought to play an important role in the mammary gland's response to a variety of mastitis-causing organisms including Staphylococcus aureus, Streptococcus uberis and Escherichia coli (Oviedo-Boyso et al., Reference Oviedo-Boyso, Valdez-Alarcon, Cajero-Juarez, Ochoa-Zarzosa, Lopez-Meza, Bravo-Patino and Baizabal-Aguirre2007). Indeed, numerous studies showed that TNF-α, IL-1β, IL-6 and IL-8 were linked with the severity of coliform mastitis during the periparturient period when dairy cattle experience oxidative stress (Sordillo and Peel, Reference Sordillo and Peel1992; Shuster et al., Reference Shuster, Kehrli and Stevens1993; Oviedo-Boyso et al., Reference Oviedo-Boyso, Valdez-Alarcon, Cajero-Juarez, Ochoa-Zarzosa, Lopez-Meza, Bravo-Patino and Baizabal-Aguirre2007). Expression of TNF-α from isolated mononuclear cells in either peripheral blood or supramammary lymph nodes was greater in the periparturient period compared to mid-late lactation (Sordillo et al., Reference Sordillo, Pighetti and Davis1995). A reverse relationship between antioxidant activity and TNF-α production by peripheral blood mononuclear cells obtained from cows experiencing oxidative stress also was recently reported (O'Boyle et al., Reference O'Boyle, Corl, Gandy and Sordillo2006).
Increased ROS concentrations in tissue and cells also are associated with enhanced expression of certain pro-inflammatory genes, such as ICAM-1 and VCAM-1 (Bonomini et al., Reference Bonomini, Tengattini, Fabiano, Bianchi and Rezzani2008). VACM are essential for transendothelial leukocyte migration to the site of infection. Enhanced expression of either ICAM-1 or VCAM-1, however, can lead to pathologic pro-inflammatory reactions. Relative to dairy cattle health, oxidative stress enhanced ROS production in bovine endothelial cells and caused a significant increase in ICAM-1 expression (Maddox et al., Reference Maddox, Aherne, Reddy and Sordillo1999; Sordillo et al., Reference Sordillo, Streicher, Mullarky, Gandy, Trigona and Corl2008). The expression of VCAM-1 protein in bovine mammary tissues also was reported to increase significantly during colostrogenesis when dairy cattle are known to experience oxidative stress (Hodgkinson et al., Reference Hodgkinson, Carpenter, Smith, Molan and Prosser2007). Recent studies by our group showed that changes in the expression of several antioxidant factors were correlated with changes in the expression of adhesion molecules and some pro-inflammatory cytokines during the transition from involution to early lactation (Aitken et al., Reference Aitken, Karcher, Rezamand, Gandy, VandeHaar, Capuco and Sordillo2009). Collectively, these data support the contention that reduced antioxidant capacity and enhanced pro-inflammatory status may be related and that this relationship may play a role in dairy cattle disease susceptibility during the periparturient period. Ability to control oxidative stress through manipulation of key antioxidant defenses in the future may modify the pro-inflammatory state of periparturient cows and reduce the incidence and severity of some diseases, such as mastitis.
Antioxidants in dairy cattle health
Host antioxidant defenses prevent oxidative damage either by direct ROS scavenging or by reducing oxidized biomolecules. Main sources of antioxidants include those synthesized endogenously (i.e. superoxide dismutase, catalase, GSH and selenoenzymes) or obtained from the diet (e.g. vitamins A, C and E) (Valko et al., Reference Valko, Leibfritz, Moncol, Cronin, Mazur and Telser2007). There are several micronutrients with antioxidant capabilities that can modify bovine inflammatory responses (Table 1). Vitamin E and selenium, however, are the most widely studied antioxidants in relation to dairy cattle health. Prepartum supplementation of these micronutrients in early studies decreased incidence, duration and severity of mastitis in deficient periparturient dairy cattle compared to those receiving adequate levels (Smith et al., Reference Smith, Harrison, Hancock, Todhunter and Conrad1984; Erskine et al., Reference Erskine, Eberhart, Grasso and Scholz1989). The exact mechanisms of these micronutrients on improving mammary gland health is not entirely known, however, they are probably related to their antioxidant functions involved with cellular signaling and decreasing oxidative damage to mammary tissue.
Table 1. Effects of antioxidant supplementation on inflammation

Vitamin E is a potent chain-breaking antioxidant primarily found in cellular membranes and mainly functions to prevent lipid peroxidation. Activated innate immune cells are particularly susceptible to peroxidative damage due to their ability to produce vast amounts of ROS during respiratory burst activity and the high concentration of PUFAs in the cellular membranes (Spears and Weiss, Reference Spears and Weiss2008). Early studies found benefits of vitamin E supplementation in periparturient mammary health to result from its effects on neutrophils. Additional vitamin E administered to prepartum cows enhanced mastitis resistance by improving phagocytic capacity of blood neutrophils as well as enhancing chemotaxis to the site of infection (Hogan et al., Reference Hogan, Smith, Weiss, Todhunter and Schockey1990, Reference Hogan, Weiss, Todhunter, Smith and Schoenberger1992; Politis et al., Reference Politis, Hidiroglou, White, Gilmore, Williams, Scherf and Frigg1996). In a study evaluating the effects of α-tocopherol, the active form of vitamin E, pre-treatment of mouse neutrophils was shown to prevent endotoxin-induced nuclear translocation of NF-κB with subsequent prevention of pro-inflammatory cytokine production (Asehnoune et al., Reference Asehnoune, Strassheim, Mitra, Kim and Abraham2004). Investigation of several human-inflammatory based diseases, such as atherosclerosis and diabetes, suggest a beneficial role of vitamin E on macrophage function and alleviation of inflammation (Azzi, Reference Azzi2004). In vivo and in vitro bovine studies demonstrated benefits of vitamin E supplementation by enhancing macrophage-derived interleukin 1 and MHC Class II expression and enhanced pokeweed mitogen-induced production of IgM when compared to control cultures (Stabel et al., Reference Stabel, Reinhardt, Stevens, Kehrli and Nonnecke1992; Politis et al., Reference Politis, Hidiroglou, White, Gilmore, Williams, Scherf and Frigg1996). Despite supplementation of vitamin E at recommended levels, periparturient plasma α-tocopherol levels decline and may contribute to the overall loss of antioxidant potential and increased incidence of inflammatory diseases during this time period (Weiss et al., Reference Weiss, Hogan, Smith and Hoblet1990).
Benefits of selenium supplementation to periparturient health have been largely documented in mastitis. Early studies demonstrated decreased clinical mastitis symptoms, decreased rate of new intramammary infections, enhanced rate of milk somatic cell response with fewer bacterial isolates and more rapid clearance of intramammary infections in selenium supplemented cows compared to those with a deficient diet (Smith et al., Reference Smith, Harrison, Hancock, Todhunter and Conrad1984; Erskine et al., Reference Erskine, Eberhart, Grasso and Scholz1989). Vitamin E and selenium supplemented together have a synergistic effect on mastitis resistance that is likely due to their specific antioxidant functions. The main antioxidant effects of selenium are due to the biologic functions of selenium-dependent enzymes. Selenoenzymes are capable of direct ROS scavenging as well as functioning in maintaining redox control. Several selenoenzymes may be important to periparturient mammary gland health. A recent study evaluated several selenoenzymes (i.e. glutathione peroxidase, phospholipid hydroperoxide glutathione peroxidase and thioredoxin reductase) in mammary gland tissue across the transition period (Aitken et al., Reference Aitken, Karcher, Rezamand, Gandy, VandeHaar, Capuco and Sordillo2009). Expression of these selenoenzymes decreased during the transition period, but appeared to rebound in early lactation. Enzyme activities of glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase were low prepartum and increased to maximal levels during early lactation. Adhesion molecule expression followed a similar pattern as the selenoenzymes, some of which were highly correlated, possibly due to a cytoprotective response to inflammation during early lactation (Aitken et al., Reference Aitken, Karcher, Rezamand, Gandy, VandeHaar, Capuco and Sordillo2009). Results from this study agree with findings from others demonstrating a positive correlation between whole blood selenium concentration and neutrophil adhesion in postpartum cows (Cebra et al., Reference Cebra, Heidel, Crisman and Stang2003).
Selenium supplementation is regulated due to potential toxicity, however, values above recommended levels may provide additional benefits to neutrophil function in postparturient cows. Peripheral neutrophils in cows with higher blood selenium levels above the lowest laboratory reference limit, displayed greater neutrophil adhesion and superoxide production (Cebra et al., Reference Cebra, Heidel, Crisman and Stang2003). Despite correction of selenium deficiency in periparturient dairy cows, oxidative stress continues occur during this time period. Current micronutrient recommendations may not be sufficient to counteract oxidative stress during the periparturient period and may lead to increased incidence and severity of infectious diseases. Additional research is necessary to study the mechanisms of antioxidant nutrients as potential preventatives or treatments against periparturient disease.
Conclusions
Inflammation protects dairy cattle against infection and tissue injury, but can have deleterious consequences if it becomes deregulated. The tremendous metabolic burden that dairy cattle experience during the periparturient period can disrupt the precarious balance between the onset and resolution of the inflammatory response. As a consequence, dairy cattle are more susceptible to several economically important diseases such as metritis and mastitis. A better understanding of the interrelationships and pro-inflammatory effects of fatty acids and oxidative stress during the periparturient period may reduce the morbidity associated with uncontrolled inflammatory responses. The possibility of controlling inflammation through targeted nutritional management is an important area for future research.
Acknowledgements
This work was supported in part by a grant from the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2005-01681 and by an endowment from the Matilda R. Wilson Fund (Detroit, MI).




