Introduction
Tetrahedrite-group minerals are the most common sulfosalts in many hydrothermal ore deposits. These chalcogenides form a complex isotypic series, with the general structural formula M (2)A6M (1)(B4C2)X (3)D4 S(1)Y12 S(2)Z, where A = Cu+, Ag+, □ (vacancy) and (Ag6)4+ cluster; B = Cu+ and Ag+; C = Zn2+, Fe2+, Hg2+, Cd2+, Ni2+, Mn2+, Cu2+, Cu+ and Fe3+; D = Sb3+, As3+, Bi3+ and Te4+; Y = S2– and Se2–; and Z = S2–, Se2– and □ (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a). Thus, tetrahedrite-group minerals are characterised by different homo- and heterovalent substitutions and represent an interesting link between mineralogy and ore geochemistry. The classification and nomenclature of the tetrahedrite-group minerals, in keeping with the current rules from the International Mineralogical Association (IMA), was recently published by Biagioni et al. (Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a).
Silver-rich members with 3 to 6 Ag atoms per formula unit (apfu) (A-constituent, freibergite/arsenofreibergite series) have been known for a long time. Indeed, ‘freibergite’ was first described from the Hab Acht Mine near Freiberg, Saxony, Germany by Weissenbach (Reference Weissenbach1831) and named by Kenngott (Reference Kenngott1853). Currently IMA-accepted minerals belonging to the freibergite series are argentotetrahedrite-(Fe) (Welch et al., Reference Welch, Stanley, Spratt and Mills2018), argentotetrahedrite-(Hg) (Wu et al., Reference Wu, Gu, Qu, Yang and Wang2021), argentotetrahedrite-(Zn) (this paper), kenoargentotetrahedrite-(Fe) (the former freibergite – Welch et al., Reference Welch, Stanley, Spratt and Mills2018; Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a) and kenoargentotetrahedrite-(Zn) (Qu et al., Reference Qu, Sima, Gu, Sun, Fan, Hou, Ni, Wang, Yang and Wang2021). The arsenofreibergite series comprises fewer species, being currently represented only by argentotennantite-(Zn) (Spiridonov et al., Reference Spiridonov, Sokolova, Gapeev, Dashevskaya, Evstigneeva, Chvileva, Demidov, Balashov and Shulga1986; Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a) and kenoargentotennantite-(Fe) (Biagioni et al., Reference Biagioni, Sejkora, Moëlo, Makovicky, Pasero and Dolníček2020b).
The most common C-constituent in minerals of the freibergite series is Fe (Riley, Reference Riley1974; Pattrick and Hall, Reference Pattrick and Hall1983; Peterson and Miller, Reference Peterson and Miller1986; Johnson et al., Reference Johnson, Craig and Rimstidt1986; Welch et al., Reference Welch, Stanley, Spratt and Mills2018; Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a) but some Zn-dominant chemical compositions have been reported previously (see a brief review below). Pattrick and Hall (Reference Pattrick and Hall1983) studied Ag substitution into synthetic Zn-, Cd-, and Fe-bearing tetrahedrites and for Zn members found that the maximum Ag content was 4.7 apfu.
The recent findings of mineral specimens corresponding to argentotetrahedrite-(Zn) at the Kremnica deposit, Slovak Republic, in the Lengenbach quarry, Switzerland, and the Zvěstov deposit, Czech Republic, allowed the submission of a formal proposal to the IMA Commission on New Minerals, Nomenclature and Classification, in order to give an official definition of argentotetrahedrite-(Zn). The mineral and its name (symbol Attr-Zn) have been approved (IMA2020-069, Sejkora et al., Reference Sejkora, Biagioni, Števko, Raber and Roth2021a). Holotype material of argentotetrahedrite-(Zn) from Kremnica and type material from Zvěstov are deposited in the collections of the Department of Mineralogy and Petrology, National Museum in Prague, Cirkusová 1740, 19300 Praha 9, Czech Republic, under the catalogue number P1P 51/2020 and 70/2021, respectively. The crystals used for the single-crystal X-ray diffraction study, along with cotype material from Lengenbach, are kept in the mineralogical collection of the Museo di Storia Naturale of the Università di Pisa, Via Roma 79, Calci (PI), under catalogue number 19922 (Kremnica), 19923 (Lengenbach) and 19939 (Zvĕstov).
Occurrence and physical properties
Argentotetrahedrite-(Zn) was found at three different localities: the Kremnica Au–Ag epithermal deposit, Žiar nad Hronom Co., Banská Bystrica Region, Slovak Republic (type locality; 48°42′44″N, 18°54′7″E); the Lengenbach quarry, Imfeld, Binn Valley, Canton Valais, Switzerland (co-type locality; 46°21′54″N, 8°13′15″E); and the small deposit of Zvěstov (Stříbrnice), central Bohemia region, Czech Republic (co-type locality; 49°38′39.069"N, 14°47′51.993"E).
The Kremnica (Slovak Republic) occurrence
The Kremnica Au–Ag epithermal deposit is located close to Kremnica town, in the northern part of the Central Slovakian Volcanic Field, which is represented by the remnants of a large Miocene andesite stratovolcano (Lexa et al., Reference Lexa, Halouzka, Havrila, Hanzel, Kubeš, Liščák and Hojstričová1998). The deposit is characterised by a low-sulfidation epithermal vein system with Au–Ag–Sb mineralisation developed on marginal faults in the eastern part of the Kremnica resurgent horst. Two principal vein systems can be distinguished: (1) 1st vein system, which is located NW of Kremnica town, is dominated by a first-order listric fault, intruded by rhyolite dykes and (2) 2nd vein system, which extends underneath the town of Kremnica and is represented by a large-scale complementary vein system with 40 veins in the hanging wall of the listric fault (Böhmer, Reference Böhmer1966; Kraus et al., Reference Kraus, Chernishev, Šucha, Kovalenker, Lebedev and Šamajová1999; Lexa and Bartalský, Reference Lexa, Bartalský, Molnár, Lexa and Hedenquist1999; Koděra et al., Reference Koděra, Šucha, Lexa, Fallick and Andrew2007). The sample with argentotetrahedrite-(Zn) was collected from the hydrothermal quartz–dolomite vein, situated at the hanging wall of the 1st stibnite vein in the crosscut P-102, Václav-south adit, in the central part of the 1st vein system (area of Šturec). This thin (from 3 to 20 cm wide) NE–SW trending vein exhibits a brecciated to drusy structure, with white to grey cavernous or drusy quartz and yellow dolomite as principal gangue minerals. Macroscopic aggregates of older common sulfides (sphalerite, pyrite and minor chalcopyrite) up to 5 mm as well as late-stage Ag-sulfosalts (mainly miargyrite, pyrargyrite and proustite) up to 2 mm are scattered locally in both quartz and dolomite. A Se-enriched association of ore minerals from this vein was studied in detail by Števko et al. (Reference Števko, Sejkora, Dolníček and Škácha2018) with Au–Ag alloys, uytenbogaardtite, minerals of the galena–clausthalite and of the acanthite–naumannite series, diaphorite, miargyrite, pyrargyrite–proustite, members of the polybasite group, various minerals of the tetrahedrite group and andorite branch (quatrandorite, senandorite, Ag-excess fizélyite), freieslebenite as well as Pb–Sb sulfosalts (scainiite, robinsonite and plagionite) being identified.
At Kremnica, argentotetrahedrite-(Zn) occurs as anhedral grains, up to 0.1 mm in size (Fig. 1), steel-grey in colour, tarnished to black and with a black streak. Lustre is metallic. The Mohs hardness may be close to 3½–4, in agreement with other members of the tetrahedrite group. Argentotetrahedrite-(Zn) is brittle, with an indistinct cleavage and a conchoidal fracture. Density was not measured, owing to the small amount of available material; on the basis of the empirical formula and the single-crystal unit-cell parameters, the calculated density is 5.089 g/cm3. In reflected light, argentotetrahedrite-(Zn) is isotropic. It is grey, with blue-greenish tints. Internal reflections were not observed. Reflectance values, measured in air using a spectrophotometer MSP400 Tidas at Leica microscope, with a 50 × objective, are given in Table 1 and shown in Fig. 2.

Fig. 1. Argentotetrahedrite-(Zn), anhedral grains up to 0.1 mm in length as seen in reflected light microscopy (a) and back-scattered electrons (b). The darker rims in (b) have a composition corresponding to the potential end-member argentotennantite-(Fe). The red box indicates the area where the grain used for single-crystal X-ray diffraction was extracted. Holotype material from Kremnica, Slovak Republic, catalogue number P1P 51/2020.

Fig. 2. Reflectance curves for argentotetrahedrite-(Zn) from Kremnica, Lengenbach and Zvěstov compared with the curve for Ag-bearing tetrahedrite-(Zn) from Lengenbach.
Table 1. Reflectance data for argentotetrahedrite-(Zn) from Kremnica, Lengenbach, and Zvěstov.*

*The reference wavelengths required by the Commission on Ore Mineralogy (COM) are given in bold.
Argentotetrahedrite-(Zn) is directly associated with thin earlier rims of the not-yet approved end-member ‘argentotennantite-(Fe)’ and tiny chalcopyrite grains. Its crystallisation is related to the activity of hydrothermal fluids during an early stage of the formation of the Kremnica Au–Ag epithermal deposit.
The Lengenbach (Switzerland) occurrence
The Lengenbach quarry exploits a Triassic metadolostone overlying the gneiss basement, at the northern front of the Monte Leone Nappe, in the Penninic domain of the Alps. The rocks were metamorphosed up to upper greenschist – lower amphibolite-facies conditions. The mineralisation occurs in the uppermost part of a 240 metre thick metadolostone sequence, 180 to 200 metres above its base, close to the contact with the overlying Jurassic to Lower Cretaceous Bündnerschiefer. Graeser et al. (Reference Graeser, Cannon, Drechsler, Raber and Roth2008) distinguished five major, bedding-parallel zones. Argentotetrahedrite-(Zn) occurs in the Ag-rich, realgar-poor ‘Zone 0’, which also hosts the so-called jordanite–galena paragenesis with other rare Ag-minerals (e.g. marrite, quadratite, proustite, xanthoconite and hatchite). The Lengenbach quarry is the type locality for 46 new mineral species; a review of its mineralogy is given in Roth et al. (Reference Roth, Raber, Drechsler and Cannon2014).
At Lengenbach, argentotetrahedrite-(Zn) forms domains within a tristetrahedral crystal of Ag-bearing tetrahedrite-(Zn), with composition (Cu3.12Ag2.89)Σ6.01(Cu4.03Zn1.56Cd0.22Fe0.17Hg0.02)Σ6.00(Sb2.64As1.36)Σ4.00S12.97, 1 mm in size associated with dolomite (Fig. 3). Its optical properties are similar to those reported for the Kremnica type material and the reflectance values are given in Table 1 and shown in Fig. 2. The crystallisation of argentotetrahedrite-(Zn) is related to the activity of hydrothermal fluids during the tectono-metamorphic Alpine events. Probably, it formed as a consequence of increasing Ag and Sb activities in the hydrothermal fluids during the late-stage evolution of the Lengenbach ore deposit and the consequent partial replacement of earlier Ag-bearing tetrahedrite-(Zn).
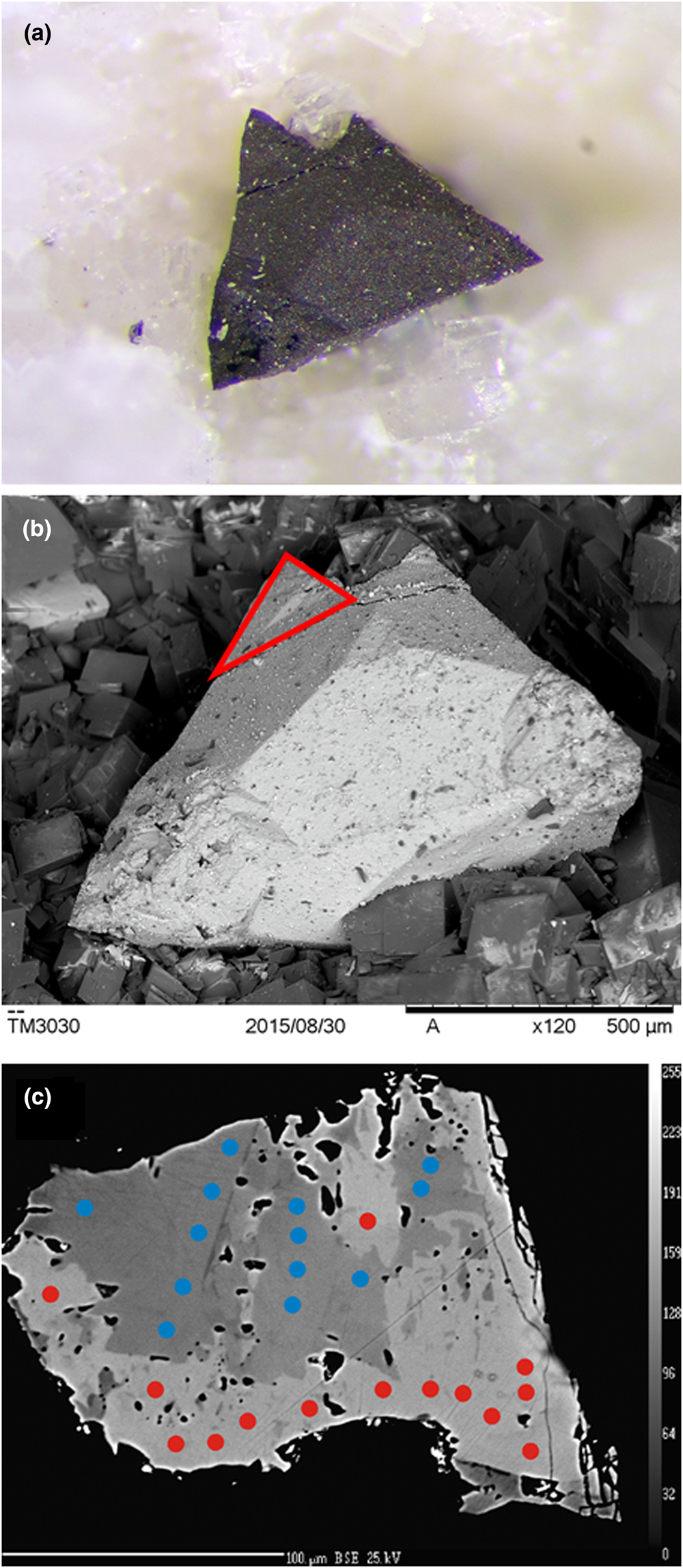
Fig. 3. Argentotetrahedrite-(Zn) from Lengenbach. (a) Tristetrahedral crystal, 1 mm in size, with dolomite. Collection H. Geuer, photo T. Raber. (b) Back-scattered electron (BSE) image of the same crystal. The red line indicates the grain selected for single-crystal X-ray diffraction study. (c) BSE image showing the chemical zoning of the grain used for the structural study. Red and blue circles indicate argentotetrahedrite-(Zn) and tetrahedrite-(Zn) spot analyses, respectively.
The Zvěstov (Czech Republic) occurrence
Argentotetrahedrite-(Zn) was also identified in samples collected from the mine dump in the southern part of the abandoned small deposit of Zvěstov (Stříbrnice), which is located 1200 m NNE from the village of Zvěstov. This deposit located 10 km SW of Vlašim, central Bohemia region, Czech Republic, is one of the small ore occurrences connected with the Blanice Graben, which represents an ~200 km long NNE–SSW trending crustal-scale brittle tectonic zone in the Moldanubian Unit, with minimal sinistral movement of ~17 km (Zachariáš and Hübst, Reference Zachariáš and Hübst2012). The deposit consists of an irregular hydrothermal vein system ~1300 m long, with thickness ~20– 50 cm and a vertical extent (verified by a prospect borehole) of ~60 m (Nouza, Reference Nouza1988). There are no written records about the historical mining of this deposit (Velebil, Reference Velebil2004). A fragment of a ceramic mug found in the studied mine dump was dated to the turn of the 15th and 16th centuries (Velebil et al., Reference Velebil, Macek and Soumar2016). Exploration was last carried-out here in the years 1956–1957 (Nouza, Reference Nouza1988). The quartz + baryte veins of the Zvěstov deposit host grains of galena, sphalerite, chalcopyrite, pyrite, arsenopyrite and minerals of the tetrahedrite group (Nouza, Reference Nouza1988; Velebil et al., Reference Velebil, Macek and Soumar2016); it is also the type locality of the new member of the tetrahedrite group zvěstovite-(Zn), Ag6(Ag4Zn2)As4S13 (Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b).
At Zvěstov, argentotetrahedrite-(Zn) forms irregular aggregates (Fig. 4) up to 200 μm in size, partly rimmed and replaced by younger kenoargentotetrahedrite-(Fe) with average empirical formula (Ag5.36Cu4.60)Σ9.96(Fe1.52Zn0.41)Σ1.93(Sb4.01As0.11)Σ4.12S12.23. Its optical properties are similar to those reported for the Kremnica type material and the reflectance values are given in Table 1 and shown in Fig. 2. Its crystallisation is related to the activity of Variscan hydrothermal fluids; samples are partly supergene altered.

Fig. 4. Argentotetrahedrite-(Zn) from Zvěstov, Czech Republic. Aggregates up to 0.1 mm in in size as seen in the BSE image. The lighter rims have a composition corresponding to kenoargentotetrahedrite-(Fe). The red box indicates the area where the grain used for single-crystal X-ray diffraction was extracted.
Chemical data
Quantitative chemical analyses were carried-out using a Cameca SX 100 electron microprobe at the National Museum of Prague, Czech Republic. Experimental conditions were: wavelength dispersive spectroscopy mode, accelerating voltage = 25 kV, beam current = 20 nA and beam diameter = 1 μm. Standards (element, emission line) were: pyrite (FeKα), chalcopyrite (CuKα and SKα), ZnS (ZnKα), NiAs (AsLβ), Ag metal (AgLα), Sb2S3 (SbLα), PbSe (SeLα), HgTe (HgMα) and Bi2Se3 (BiMα). Peak counting times were 20 s for all elements, and 10 s for each background. Bismuth and Se were found to be below the detection limits (0.02–0.05 wt.%). Matrix correction by PAP software (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) was applied to the data.
There are several different approaches to calculate the chemical formula of the tetrahedrite-group minerals; due to possible vacancies at the S(2) site in the Ag-rich members the best approaches are (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a; Sejkora et al. Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b): (1) normalisation on the basis of ΣMe = 16 apfu, assuming that no vacancies occur at M(2), M(1) and X(3); or (2) normalisation on the basis of (As + Sb) = 4 apfu, taking into account that previous studies (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1986) revealed that negligible variations with respect to the ideal number of X(3) atoms occur. The results of our study indicate that only very minor vacancies possibly occur at M(2), M(1) and X(3) sites and therefore the first approach was selected for calculation.
Chemical data for the holotype grain of argentotetrahedrite-(Zn) from Kremnica are given in Table 2 and give the following formula: Cu6.74Ag3.27Zn1.69Fe0.23Cd0.02Hg0.01(Sb3.86As0.17)Σ4.03S12.73; taking into account the results of the crystal structure refinement (see below), the crystal-chemical formula can be written as (Ag3.27Cu2.69)Σ5.96[Cu4.00(Zn1.69Fe0.23Cu0.05Cd0.02Hg0.01)Σ2.00]Σ6.00(Sb3.86As0.17)Σ4.03S12.73. The end-member formula of argentotetrahedrite-(Zn) is Ag6(Cu4Zn2)Sb4S13 (Z = 2), corresponding to (in wt.%) Cu 13.13, Ag 33.43, Zn 7.65, Sb 25.16, S 21.53, total 100.00.
Table 2. Compositional data for argentotetrahedrite-(Zn) from Kremnica (holotype sample) and Lengenbach (cotype sample).

e.s.d. = estimated standard deviation.
The chemical compositions for other samples (Kremnica grain 2, Lengenbach, Zvěstov) are given in Tables 2 and 3 and presented in Fig. 5. The Ag contents for all samples studied are found to lie in the range 3.10–3.54 apfu and do not correlate with S contents (Fig. 5a), whereas the positive correlation between Sb and Ag is indistinct (Fig. 5b); a similar situation was described for Ag-rich tetrahedrites by Johnson et al. (Reference Johnson, Craig and Rimstidt1986). All samples studied have Zn as the dominant C-constituent (Fig. 5c). Samples from Kremnica and Lengenbach are Fe-poor, with contents in the range 0.11–0.34 apfu Fe. The sample from Zvěstov shows slightly higher Fe contents, in the wider range 0.21–0.74 apfu. Moreover, the Lengenbach sample contains 0.21–0.29 apfu Cd. The extent of SbAs–1 substitution in all samples is limited to 0.42 apfu As (Fig. 5d). The determined S contents in the range 12.57–13.30 apfu indicates only minor possible vacancies at the S(2) site, which is consistent with results of crystal structure refinements (see below). The chemical composition of the studied samples can be expressed by the following empirical formulae: Kremnica (grain 2) (Ag3.50Cu2.44)Σ5.94[Cu4.00(Zn1.66Fe0.27Cu0.04Cd0.02Hg0.01)Σ2.00]Σ6.00(Sb3.88As0.17)Σ4.05S12.70; Lengenbach (Ag3.17Cu2.79)Σ5.96[Cu4.00(Zn1.55Cd0.23Fe0.16Cu0.05Hg0.01)Σ2.00]Σ6.00(Sb3.71As0.32)Σ4.03S12.77; and Zvěstov (Ag3.27Cu2.67)Σ5.94[Cu4.00(Zn1.39Fe0.50Cu0.07Cd0.03Hg0.01)Σ2.00]Σ6.00(Sb4.03As0.04)Σ4.07S13.08.

Fig. 5 Chemical relationships (in apfu) between Ag vs S (a), Sb vs Ag (b), Zn vs Fe (c) and Sb vs As (d) in argentotetrahedrite-(Zn) from Kremnica, Lengenbach and Zvěstov.
Table 3. Compositional data for argentotetrahedrite-(Zn) from Kremnica (grain 2) and Zvěstov.

e.s.d. = estimated standard deviation.
X-ray crystallography
Powder X-ray diffraction data could not be collected, due to the paucity of available material. Consequently, powder X-ray diffraction data, given in Table 4, were calculated through the software PowderCell 2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) using the structural model of holotype material from Kremnica discussed below.
Table 4. Calculated powder X-ray diffraction data for argentotetrahedrite-(Zn).*

*Intensity and d hkl were calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the structural model of the Kremnica sample given in Table 6. Only reflections with I calc > 1 are listed. The five strongest reflections are given in bold.
Single-crystal X-ray diffraction studies were performed using a Bruker Apex II diffractometer equipped with an air-cooled CCD detector, and graphite-monochromatised MoKα radiation (Dipartimento di Scienze della Terra, Università di Pisa). The detector-to-crystal distance was 50 mm. The data were corrected for Lorentz and polarisation factors and absorption using the software package Apex3 (Bruker AXS Inc., 2016). The crystal structure of argentotetrahedrite-(Zn) was refined using Shelxl-2018 (Sheldrick, Reference Sheldrick2015) starting from the atomic coordinates of argentotetrahedrite-(Fe) (Welch et al., Reference Welch, Stanley, Spratt and Mills2018). In all three refinements, the modelling of the racemic twin indicated that the structure had to be inverted, and the difference-Fourier maps indicated the splitting of the M(2) site. Since electron microprobe data did not suggest any vacancy or cation excess at the M(2) site, the sum of the site occupancy factors (s.o.f.) at M(2a) and M(2b) was constrained to the full occupancy. The following neutral scattering curves, taken from the International Tables for Crystallography (Wilson, Reference Wilson1992) were initially used: Ag vs □ at M(2a), Cu vs □ at M(2b), Cu at M(1), As vs Sb at X(3), S at S(1) and S(2) sites. Details of the crystal structure refinement of the three samples studied are described below and reported in Table 5. Fractional atomic coordinates and equivalent isotropic displacement parameters are reported in Table 6. Table 7 reports selected bond distances. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 5. Summary of crystal data and parameters describing data collection and refinement for argentotetrahedrite-(Zn).

1 w = 1/[σ2(F o2)+(aP)2+bP], where P = (F o2+2F c2)/3. KR: a = 0, b = 10.6938; LE: a = 0, b = 17.9923; ZV: a = 0, b = 28.1692.
2 Flack (Reference Flack1983)
Table 6. Atoms, Wyckoff positions, site occupancy factors (s.o.f.), atom coordinates, equivalent isotropic displacement parameters (Å2), and refined (obs) and calculated (calc) site scattering for argentotetrahedrite-(Zn).

Note: s.o.f. are used to model the site scattering, giving the MANSREF reported in Table 8.
Table 7. Selected bond distances (Å) for argentotetrahedrite-(Zn).

Kremnica (Slovak Republic)
A prismatic fragment of argentotetrahedrite-(Zn) from Kremnica, 50 μm × 40 μm × 35 μm in size, was extracted from the polished section analysed using electron microprobe (Fig. 1). Data were collected using the φ scan mode, in 0.5° slices, with an exposure time of 45 s per frame. Unit-cell parameters are a = 10.5505(10) Å, V = 1174.4(3) Å3 and space group I $\bar{4}$![]() 3m. Several cycles of isotropic refinement converged to R 1 = 0.0797, confirming the correctness of the structural model. However, a residual maximum of ca. 5 e –/Å3 suggested that the M(2)site was actually split into two sub-positions, namely M(2a) and M(2b). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0410. The anisotropic structural model converged to R 1 = 0.0351 for 327 unique reflections with F o > 4σ(F o) and 22 refined parameters.
3m. Several cycles of isotropic refinement converged to R 1 = 0.0797, confirming the correctness of the structural model. However, a residual maximum of ca. 5 e –/Å3 suggested that the M(2)site was actually split into two sub-positions, namely M(2a) and M(2b). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0410. The anisotropic structural model converged to R 1 = 0.0351 for 327 unique reflections with F o > 4σ(F o) and 22 refined parameters.
Lengenbach (Switzerland)
A fragment, 200 μm × 120 μm × 90 μm, of the tristetrahedral individual shown in Figs 3a and 3b was used for the intensity data collection. As shown in Fig. 3c, argentotetrahedrite-(Zn) from Lengenbach is associated closely with Ag-bearing tetrahedrite-(Zn). As the crystal studied through single-crystal X-ray diffraction was chemically inhomogeneous, the results of the crystal structure refinement are probably a weighted average of the two domains. Unit-cell parameters of the Lengenbach sample are a = 10.5155(13) Å, V = 1162.8(4) Å3 and space group I $\bar{4}$![]() 3m. Several cycles of isotropic refinement converged to R 1 = 0.1085, showing a residual maximum of ca. 6 e –/Å3 around M(2); a second maximum was observed close to M(1). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0516, lowered to 0.0378 refining the s.o.f. at M(1), using the scattering curves of Cu vs Cd. The anisotropic structural model converged to R 1 = 0.0297 for 385 unique reflections with F o > 4σ(F o) and 23 refined parameters.
3m. Several cycles of isotropic refinement converged to R 1 = 0.1085, showing a residual maximum of ca. 6 e –/Å3 around M(2); a second maximum was observed close to M(1). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0516, lowered to 0.0378 refining the s.o.f. at M(1), using the scattering curves of Cu vs Cd. The anisotropic structural model converged to R 1 = 0.0297 for 385 unique reflections with F o > 4σ(F o) and 23 refined parameters.
Zvěstov (Czech Republic)
A tabular fragment of argentotetrahedrite-(Zn), ca. 50 μm × 50 μm × 30 μm, was extracted from the polished section prepared on the sample from Zvěstov. Unit-cell parameters of this sample are a = 10.5663(12) Å, V = 1179.7(4) Å3 and space group I $\bar{4}$![]() 3m. Several cycles of isotropic refinement converged to R 1 = 0.0859, showing a residual maximum of ca. 5 e –/Å3 around M(2). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0477. The X(3) site was found to be occupied by Sb only. The anisotropic structural model converged to R 1 = 0.0381 for 379 reflections with F o > 4σ(F o) and 22 refined parameters.
3m. Several cycles of isotropic refinement converged to R 1 = 0.0859, showing a residual maximum of ca. 5 e –/Å3 around M(2). After the addition of the split positions, the isotropic refinement converged to R 1 = 0.0477. The X(3) site was found to be occupied by Sb only. The anisotropic structural model converged to R 1 = 0.0381 for 379 reflections with F o > 4σ(F o) and 22 refined parameters.
Results and discussion
Crystal structure description
The crystal structure of argentotetrahedrite-(Zn) agrees with the general features of the members of the tetrahedrite isotypic group, i.e. a collapsed sodalite-like framework formed by corner-sharing M(1)S(1)4 tetrahedra with cages hosting S(2)-centred M(2)-octahedra, surrounded by four X(3)S(1)3 trigonal pyramids (e.g. Johnson et al., Reference Johnson, Craig and Rimstidt1988).
In the three samples studied, the tetrahedrally coordinated M(1) site shows average distances ranging from 2.339 to 2.348 Å, the larger value being observed in argentotetrahedrite-(Zn) from the Lengenbach quarry, where minor Cd replaces Zn and Fe. Indeed, the crystal radius of Cd2+ in tetrahedral coordination is, according to Johnson et al. (Reference Johnson, Craig and Rimstidt1988), 0.84 Å, similar to that of Hg2+. In the samples from Kremnica and Zvěstov, the average bond distance agrees with those reported for other mixed (Cu,Zn,Fe) tetrahedral sites in tetrahedrite-group minerals (e.g. 2.342 Å – Wuensch, Reference Wuensch1964). Taking into account the results of electron microprobe analyses and the mean atomic numbers (MAN) obtained during the crystal structure refinements (Table 8), the following site populations can be proposed for the M(1) site: (Cu4.00Zn1.70Fe0.25Cu2+0.05) (KR), (Cu4.00Zn1.55Cd0.25Fe0.15Cu2+0.05) (LE), and (Cu4.00Zn1.40Fe0.50Cu2+0.05Cd0.05) (ZV). There is a good agreement between refined MAN and those calculated on the basis of the proposed site populations. Weighted bond-valence sums (Table 9) are 1.44 valence unit (vu) for all the studied samples, compared with a theoretical value of 1.33 vu. Such slight overbonding is a common feature for tetrahedrally coordinated cations in tetrahedrite-group minerals (Welch et al., Reference Welch, Stanley, Spratt and Mills2018).
Table 8. Refined mean atomic numbers (MAN – in electrons) and proposed site populations for cation sites in argentotetrahedrite-(Zn).

Table 9. Weighted bond-valence sums (in vu) in argentotetrahedrite-(Zn).

Note: bond-valence sums are calculated using the bond parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991).
The M(2) site is split in all the three specimens studied, in agreement with previous results (Welch et al., Reference Welch, Stanley, Spratt and Mills2018 and Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b) for rozhdestvenskayaite-(Zn) and zvěstovite-(Zn), respectively. Both sub-positions have a triangular coordination, with average bond distances ranging between 2.339 and 2.408 Å. These distances are shorter than those reported in rozhdestvenskayaite-(Zn) (2.43 and 2.53 Å for the two split positions – Welch et al., Reference Welch, Stanley, Spratt and Mills2018) and zvěstovite-(Zn) (2.39 and 2.57 Å – Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b), in agreement with the lower Ag content occurring in argentotetrahedrite-(Zn).
Shorter distances have been observed for the M(2a) positions (range of average distances: 2.339–2.367 Å), whereas M(2b) shows longer average distances, varying between 2.393 and 2.408 Å. For this reason, it is probable that Cu is preferentially hosted at M(2a), whereas the larger Ag+ cation may be hosted at both sub-positions. Table 8 reports the mean atomic number for M(2) sites [= M(2a) + M(2b)]. The agreement between refined and calculated MAN is usually good, the largest deviation being observed for the sample from Zvěstov. If Cu is assumed to be preferentially hosted at M(2a), the following site populations may be proposed, taking into account the refined mean atomic numbers: M(2a) = Cu0.46Ag0.24, M(2b) = Ag0.15 (KR); M(2a) = Cu0.47Ag0.25, M(2b) = Ag0.14 (LE); and M(2a) = Cu0.46Ag0.39, M(2b) = Ag0.075 (ZV). Using these hypothetical populations, weighted bond-valence sums have been calculated (Table 9), resulting in a general overbonding, as observed in other Ag-bearing tetrahedrite-group minerals (e.g. Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b). This is mainly due to the short M(2)–S(2) distance, definitely shorter than the ideal <Ag–S> distance in three-fold coordination, i.e. 2.56 Å.
The X(3) site shows the typical trigonal pyramidal coordination. Average bond distances range between 2.397 and 2.438 Å. Whereas the bond distance in samples from Kremnica and Zvěstov are very similar, due to similar Sb/(As+Sb) atomic ratios, the distance observed in the sample from Lengenbach is distinctly shorter, owing to its larger As content. However, the results obtained for the Swiss samples agree with its inhomogeneous nature. As described above, the sample from this locality actually comprises two phases, i.e. argentotetrahedrite-(Zn) and Ag-rich tetrahedrite-(Zn). The latter is As-richer than the former, whereas the site populations at the M(1) site are similar in both phases. Whereas such an inhomogeneity was not observed in the refinement of the MAN at the M(2) site, it can be observed considering the MAN at the X(3) site (Table 8). Indeed, whereas electron microprobe analysis suggests an Sb/(As+Sb) atomic ratio close to 0.92, the refined s.o.f. indicates a lower Sb/(As+Sb) atomic ratio, i.e. 0.76. Such a value is in accordance with the ratio calculated on the basis of the observed bond distance, corresponding to a site having an ideal occupancy (Sb0.72As0.28). Taking into account the chemistry of argentotetrahedrite-(Zn) and Ag-rich tetrahedrite-(Zn), it is possible to estimate a ratio of 0.40/0.60 for the two phases in the grain studied. This ratio can help in explaining why such an inhomogeneity cannot be detected at the M(2) site: indeed, the two phases have the ideal occupancy (Ag0.53Cu0.47) and (Cu0.52Ag0.48), respectively. The phase ratio indicated above results in the average composition (Cu0.50Ag0.50), corresponding to a MAN of 38.0 electrons, not dramatically different from the observed value, i.e., 38.72 electrons. Weighted bond-valence sums (Table 9) agree with the theoretical value. The largest discrepancy is observed for the Lengenbach sample, probably due to the inhomogeneity just described.
The S(1) site is coordinated tetrahedrally by two M(1) sites, one M(2) site [i.e. M(2a) or one of the two mutually-exclusive M(2b)] and one X(3) site. Its bond-valence sum ranges between 2.10 and 2.17 vu, in agreement with the occurrence of S2–. On the contrary, the S(2) site, octahedrally coordinated by the M(2) site, is severely overbonded, with values ranging between 3.00 and 3.30 vu, as observed in other Ag-rich tetrahedrite-group minerals (e.g. Sejkora et al., Reference Sejkora, Biagioni, Vrtiška and Moëlo2021b). This is due to the short distance between M(2) and S(2); this kind of behaviour was described previously by Johnson et al. (Reference Johnson, Craig and Rimstidt1988), who stressed the aspherical nature of three-fold Ag. This feature, along with the high U eq value shown by S(2) and the overbonding of the M(2) site, will be further discussed below.
Comparison between argentotetrahedrite-(Zn) and other members of the freibergite series
Argentotetrahedrite-(Zn) belongs to the freibergite series within the tetrahedrite group (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a). It is the Zn-isotype of argentotetrahedrite-(Fe) (Welch et al., Reference Welch, Stanley, Spratt and Mills2018) and the Sb-isotype of argentotennantite-(Zn) (Spiridonov et al., Reference Spiridonov, Sokolova, Gapeev, Dashevskaya, Evstigneeva, Chvileva, Demidov, Balashov and Shulga1986).
Selected data for argentotetrahedrite-(Zn) samples and valid (Zn/Fe)-members of the freibergite series are compared in Table 10. The Ag/(Ag+Cu) atomic ratios range between 0.53 and 0.73 and these show a linear relationship with the unit-cell parameter a, the deviation from the linearity being due to different Sb/(Sb+As) ratios. For instance, the specimen from Lengenbach displays a shorter unit-cell parameter owing to the higher As content with respect to the specimens from Kremnica and Zvěstov with very similar Ag contents. In all the samples studied currently, Sb/(Sb+As) is > 0.90. Different Zn/(Zn+Fe+Me2+) ratios, where Me2+ are other divalent metals (e.g. Hg2+, Cd2+), do not significantly affect the unit-cell parameter. The same is true for the M(1)–S(1) bond distance, that varies between 2.339 and 2.348 Å, the longer distance probably being due to the partial replacement of Zn and Fe by Cd in the Lengenbach sample. In the M(2) polyhedron, the M(2)–S(1) distance is definitely longer and more sensitive to the Ag content than the M(2)–S(2) distance, in agreement with previous studies (e.g. Peterson and Miller, Reference Peterson and Miller1986; Welch et al., Reference Welch, Stanley, Spratt and Mills2018). The M(2)–S(2) distance is usually short, being in the range 2.25–2.30 Å; this short Ag–S contact results in the exceptional overbonding of the S atoms hosted at S(2), as shown in Table 9. This physically unrealistic value is probably the effect of S(2) disorder, in agreement with the high U eq values, varying between 0.11 and 0.14 Å2. A high U eq value may be related to the incorrect refinement of the s.o.f., that is, more S than that actually present is assumed at the S(2) site, or to positional disorder. As electron microprobe data do not support the occurrence of significant vacancies (S contents ranging between 12.68 and 13.08 apfu in specimens of argentotetrahedrite-(Fe/Zn) reported in Table 10), the second hypothesis could be the correct one. This is in agreement with Peterson and Miller (Reference Peterson and Miller1986), who suggested that in order to achieve longer Ag–S bonds, S should be located off site 2a. Indeed, in the crystal structure refinement of the three samples of argentotetrahedrite-(Zn) studied in this work, a residual maximum at position 8c (x, x, x), with x = 0.0384, 0.0284 and 0.0321 for samples from Kremnica, Lengenbach and Zvěstov, respectively, was found. The displacement of S(2), coupled with minor shifts in the positions of Ag and Cu atoms hosted at M(2), agreeing with the high U eq value and the split nature of this latter site, will probably be able to achieve a physically sound coordination for both Ag and S in the argentotetrahedrite structure.
Table 10. Comparison of argentotetrahedrite-(Zn) samples and other members of the freibergite series.

Me2+ = e.g. Cd, Hg. n.p. = not published.
[1] this work; [2] Rozhdestvenskaya et al. (Reference Rozhdestvenskaya, Zayakina and Samusikov1993); [3] Welch et al. (Reference Welch, Stanley, Spratt and Mills2018); [4] Qu et al. (Reference Qu, Sima, Gu, Sun, Fan, Hou, Ni, Wang, Yang and Wang2021).
The range of Ag/(Ag+Cu) atomic ratios for argentotetrahedrite-(Zn) overlaps partially with that of kenoargentotetrahedrite-(Fe) and kenoargentotetrahedrite-(Zn), 0.63–0.90 (Table 10). Actually, few crystallographic data are available for the keno-members of the freibergite series. What is clear is that a detectable contraction of the unit-cell parameter can be observed for these keno-phases. For instance, kenoargentotetrahedrite-(Zn), having an Ag/(Ag+Cu) atomic ratio of 0.63, has a unit-cell parameter a = 10.4624(4) Å (Qu et al., Reference Qu, Sima, Gu, Sun, Fan, Hou, Ni, Wang, Yang and Wang2021), significantly shorter than that given by Rozhdestvenskaya et al. (Reference Rozhdestvenskaya, Zayakina and Samusikov1993) for argentotetrahedrite-(Zn) with Ag/(Ag+Cu) = 0.60, i.e. a = 10.576(3) Å. This probably reflects the contraction of the S(2)-centred M(2)-octahedron. In the two keno-members of the freibergite series reported in Table 10, S ranges between 11.93 and 12.01 apfu, agreeing with the empty nature of S(2). Indeed, the volume of the M(2)-octahedron is 10.8 Å3 in kenoargentotetrahedrite-(Fe) (Welch et al., Reference Welch, Stanley, Spratt and Mills2018), to be compared with 15.2 and 16.2 Å3 (+ 40–50%) in argentotetrahedrite-(Zn/Fe). This seems to be a clear structural feature allowing for the distinction between argentotetrahedrite and kenoargentotetrahedrite.
Previous findings of argentotetrahedrite-(Zn): a brief review
Tetrahedrite-group minerals having chemical compositions corresponding to that of argentotetrahedrite-(Zn) have been reported previously. To the best of our knowledge, the first chemical data corresponding to argentotetrahedrite-(Zn) were reported by Ixer and Stanley (Reference Ixer and Stanley1983), who gave two analyses on samples from the Sark's Hope mine, Sark, Channel Islands, showing 3.6–3.9 Ag apfu and 1.4–1.7 Zn apfu. Later, in the 1990s, Li and Wang (Reference Li and Wang1990) reported four analyses from the Dachang ore field, Guangxi, China, with 3.1–3.7 Ag apfu and 1.0–1.8 Zn apfu, whereas Rozhdestvenskaya et al. (Reference Rozhdestvenskaya, Zayakina and Samusikov1993) described the crystal structure investigation of ‘freibergite’ from eastern Yakutia, Russia. Electron microprobe data gave the formula (Ag3.62Cu6.37)Σ9.99(Zn1.33Fe0.72)Σ2.05(Sb3.89As0.07)Σ3.96S12.85, and their structural data [a = 10.576(3) Å, R 1 = 0.041] represent the first determination of the crystal structure of argentotetrahedrite-(Zn). Filimonov and Spiridonov (Reference Filimonov and Spiridonov2005) described members of the freibergite series from the Kvartsitovye Gorki deposit, central Kazakhstan, characterised by an unusual wide range of Ag contents (3.1–6.7 apfu) with 1.5–1.7 Zn apfu and S in the range 12.6–13.3 apfu. Recently, argentotetrahedrite-(Zn) was reported also from the Vyhne deposit, Slovak Republic (3.1–3.2 Ag, 1.6–1.9 Zn and 12.7–13.0 S apfu) by Vlasáč et al. (Reference Vlasáč, Chovan, Vojtko, Žitňan and Mikuš2021) and from the San Genaro mine, Peru (3.2–3.3 Ag, 1.5–1.9 Zn and 12.9–13.1 S apfu) by Velebil et al. (Reference Velebil, Hyršl, Sejkora and Dolníček2021).
In addition to these occurrences, other Zn-dominant members of the freibergite series were reported from Silvermines, County Tipperary, Ireland (Zakrzewski, Reference Zakrzewski1989), the Keno Hill district, Yukon, Canada (Lynch, Reference Lynch1989), and the Xinhua Pb–Zn deposit, China (Wang et al., Reference Wang, Zhang, Guo, Pi and Yang2018). However, the chemical data given by these authors do not allow for an accurate classification of these samples. For instance, Zakrzewski (Reference Zakrzewski1989) reported seven chemical analyses of tetrahedrite-group minerals from Silvermines; among these, five have Ag contents ranging between 20.6 and 23.9 wt.%. This author recalculated the chemical data on the basis of 29 apfu, obtaining ΣMe in the range 16.29–16.49 apfu, with (Sb+As) varying between 4.09 and 4.32 apfu. Normalising these analyses on the basis of ΣMe = 16 apfu or (Sb + As) = 4 apfu leads to chemical formulae that are intermediate between argentotetrahedrite-(Zn) and kenoargentotetrahedrite-(Zn). The lack of structural data, coupled with the uncertainty on the actual S content, do not allow for a straightforward classification at a species level, and thus these samples must be labelled as ‘Zn-rich freibergite’, using the series name.
Finally, synthetic analogues of argentotetrahedrite-(Zn) were studied by Pattrick and Hall (Reference Pattrick and Hall1983), who reported a unit-cell parameter a = 10.565 Å for a sample with 3.6 Ag apfu, whereas samples with 4.49 and 4.70 Ag apfu showed unit-cell parameters a = 10.620 and 10.625 Å, respectively.
Conclusions
Following the approval of the new nomenclature of the tetrahedrite group (Biagioni et al., Reference Biagioni, George, Cook, Makovicky, Moëlo, Pasero, Sejkora, Stanley, Welch and Bosi2020a), a renewed interest in this group of common sulfosalts has allowed for the definition of several new mineral species. Argentotetrahedrite-(Zn) is one of the latest additions to this group. In addition to the new mineral, new structural data have been collected, thereby improving our knowledge on the crystal chemistry of these chalcogenides. This is particularly important for Ag-bearing tetrahedrite-group minerals, whose actual definition was unclear up to the paper by Welch et al. (Reference Welch, Stanley, Spratt and Mills2018), who confirmed the results of Rozhdestvenskaya et al. (Reference Rozhdestvenskaya, Zayakina and Samusikov1989, Reference Rozhdestvenskaya, Zayakina and Samusikov1993), pointing out the difference between ‘freibergite’ [now kenoargentotetrahedrite-(Fe)] and argentotetrahedrite-(Fe). Even if the distinction between argentotetrahedrites and their keno-counterpart is not always an easy task using only electron microprobe data, due to uncertainties in S determination, single-crystal X-ray diffraction data seem to allow for a distinction between them, with implications on the knowledge of ore-forming processes. Further studies on Ag-bearing members of the tetrahedrite group will be useful, not only for depicting a better picture of the chemical variability of tetrahedrite-group minerals, but also for achieving a better understanding of the crystal chemistry of Ag in these compounds.
Acknowledgements
The authors thank Zdeněk Dolníček (National Museum, Praha) for his help with the analytical work, Dalibor Velebil (National Museum, Praha) for useful information about the history and geology of the Zvěstov occurrence and Kai Qu (Tianjin Centre, China Geological Survey, Tianjin) for unpublished information on kenoargentotetrahedrite-(Zn). The helpful comments of an anonymous reviewer, Peter Leverett, Yves Moëlo, Associate Editor Ian Graham and Principal Editor Stuart Mills are greatly appreciated. The study was financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019–2023/1.II.d; National Museum, 00023272) for JS, MŠ and LV. CB acknowledges financial support from the Ministero dell'Istruzione, dell'Università e della Ricerca through the project PRIN 2017 “TEOREM – deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.21


















