Introduction
The first prebiotic model – a coacervate – was suggested and explored by Oparin (Reference Oparin1957). According to his opinion, coacervate drops composed of biologically important organic compounds were predecessors of primary cells. It was proved that they possess some signs of internal and external activity characterizing a living organism. For instance, a coacervate is able to grow and selectively extract substance from the environment. Nevertheless, plenty of experimental attempts to transform coacervates into really living units failed. In the long run the initial signs of activity faded away in the explored coacervates and they came to passive existence in the experimental chamber. Meanwhile, the transition from chemical evolution into biological evolution evidently assumes strengthening of the activity signs following their transition into a self-maintaining regime. Therefore, a coacervate could not be transformed into a microsystem with the self-maintaining dynamic processes evolving to life.
Later some other prebiotic models were suggested by various researchers: proteinoide microspheres (Fox & Dose Reference Fox and Dose1977; Fox et al. Reference Fox, Balin, Pappelis, Yu, Chela-Flores and Raulin1996), RNA-World macromolecules (Gilbert Reference Gilbert1986; Joice & Orgel Reference Joice and Orgel1993), liposomes (Deamer Reference Deamer1986, Reference Deamer and Seckbach2004; Luisi Reference Luisi2000), aromatic hydrocarbons (Ehrenrfreund et al. Reference Ehrenfreund, Rasmussen, Cleaves and Chen2006). The models also demonstrate some signs of activity relevant to the vital processes. For instance, in a catalytic activity peculiar to proteinoide microspheres, some RNA macromolecules are able to self-replicate. However, intensive dynamic reorganization of proteinoide microspheres during the self-assembly process in the long run ends with the transition to passive existence in the medium. Chemical complication of lipid micelles and vesicles does not lead to internal self-maintaining dynamic processes as well. In some cases the synthesis of RNA macromolecules completes with self-replication. However, this complex multi-step process is governed hourly by the experimenter. It stops as soon as the researcher ceases to guide the experiment. So, the listed organic microsystems cannot develop to the living state by themselves, like a coacervate. The inability to self-develop seems to be a common property of all explored prebiotic models. The tendency to self-evolve to life implies that the initial signs of activity strengthen and become self-maintaining after a certain critical level, giving rise to metabolism, regular self-replication and other vital dynamic processes. In fact, the mentioned common property emphasizes the principal gap separating non-living organic microsystems and living cells. Lack of understanding of the nature of this gap can be considered as an obstacle to further advancement in the origin of life science. How is it possible to clarify a permissible way of transition from a still unknown kind of organic microsystem to primary living units?
The real gap between non-living prebiotic microsystems and living microorganisms means that they are fundamentally different types of natural systems. Any system is characterized by its own properties, which make it different from others. A set of the most fundamental properties expresses the essence of a system. So, the essence of this gap can become clearer through investigation of the set of properties, which are peculiar to living systems and not non-living ones, including prebiotic models. This thesis was a starting point for the original version of the systemic approach to the origin of life (Kompanichenko Reference Kompanichenko2004). From the chemical point of view, the origin-of-life theory should be based on the succession of chemical transformations, which are intermediate between organic matter and primary cells. From the point of view of the systemic approach, the direction of proper chemical transformations depends on the specific order of the macroprocesses occurring in a prebiotic organic aggregation for the period of its transition to the living state. This order can be traced by means of investigation of the difference between the fundamental properties of non-living systems (including organic microsystems) and living ones (beginning with the primary life forms). According to the author's conception, the transition can be conditionally divided into three successive stages that are considered in this paper.
Properties of biological systems: a basis for the elaborated approach
The systemic conception is elaborated on the basis of systematization and analysis of the distinguished properties of biological systems, and their all-round comparison with the properties of non-biological systems (Kompanichenko Reference Kompanichenko, Palyi, Zucci and Caglioti2002, Reference Kompanichenko2003, Reference Kompanichenko2004). The initial set was composed of 230 biological properties distinguished by 73 competent world scientists. They made contributions into the book Fundamentals of Life (Palyi et al. Reference Palyi, Zucci and Caglioti2002), which is the latest summary in this field of fundamental biology. The properties very often repeat each other. Their juxtaposition and integration allowed the author to formulate 31 fundamental biological properties. They are subdivided into two groups. According to the elaborated generalization, 19 properties can be considered as unique fundamental properties of biological systems, which are not peculiar to any other natural system (Table 1, left column). According to the generalization, the unique fundamental biological properties can be narrowed down to the following four integrated properties (Table 1, central column): the ability to concentrate free energy and information (by means of their extraction from the environment); the ability to exhibit intensified counteraction to external influences; expedient behaviour (or the expedient character of interactions with the environment); regular self-renovation at different hierarchical levels (molecular, tissue, genome, organism, species, biosphere). The remaining 12 out of 31 are attributed to the non-unique fundamental biological properties (Table 2). The same or similar features characterize some non-biological systems as well, although they are devoid of any biological specificity.
Table 1. Unique fundamental properties of biological systems and their generalization (the list of researchers who distinguished the fundamental properties is given below)

Note to Tables 1 and 2. The list of authors whose contributions to the book Fundamental of Life (2002) were used to distinguish 31 fundamental properties of biological systems is as follows: 1- D.L. Abel, 2- A.D. Alstein, 3- M. Anbar, 4- G.O. Arrhenius, 5- H. Baltscheffsky, A. Schultz & M. Baltscheffsky, 6- L. Boiteau, 7- A. Brack, 8- D. Brin, 9- R. Buick, 10- M. Colin-Garcia & A. Guzman-Marmolejo, 11- D.W. Deamer, 12- A.H. Delsemme, 13- K. Dose, 14- C. de Duve, 15- F.R. Eirich, 16- A. Elitzur, 17- A.S. Erokhin, 18- J. Farmer, 19- R. Guerrero & L. Margulis, 20- R. C. Guimaraes, 21- V.K. Gupta, 22- V.A. Gusev, 23- R.M. Hazen, 24- R.J.-C. Hennet, 25- R.D. Hill, 26- N. Horowitz, 27- H.P. Yockey, 28- G.F. Joice, 29- L. Keszthelyi, 30- G. von Kiedrowski, 31- E.I. Klabunovsky, 32- V.M. Kolb, 33- B. Kopperhoefer, 34- W.E. Krumbein, 35- H. Kuhn, 36- I.S. Kulaev, 37- N. Lahav & S. Nir, 38- D.Z. Lippmann, 39- P. Lopez-Garcia, 40- L. Marco, 41- S. Mendez-Alvarez, 42- S.I. Miller, 43- S.J. Mojzsis, 44- Y. Momotani, 45- C.K.K. Nair, 46- K.H. Nealson, 47- S. Nir, 48- H. Noda, 49- T. Owen, 50- G. Palyi, C. Zucci & L. Cagliati, 51- B.F. Poglazov, 52- R.F. Polyshchuk, 53- M. Rizotti, 54- M. Russell, 55- X. Sallantin, 56- D. Schulze-Makuch & L.N. Irvine, 57- R.I. Scorei, 58- J. Siefert, 59- A.A. Spirin, 60- E. Szathmary (& T. Ganti), 61- C.Y. Valenzuela, 62- T.G. Waddell, 63- J.T-F. Wong.
Table 2. Non-unique fundamental properties of biological systems and their correlation with similar properties of some non-biological systems (on the basis of the contribution of the researchers whose list is given in the note below Table 1)

In fact, the unique fundamental properties emphasize the strict barrier separating animate and inanimate parts of nature, while the non-unique ones can be considered as the connecting thread between them. During the origin-of-life process on the ancient Earth the unique properties appeared for the first time, unlike the non-unique ones that transited from the maternal geological medium and acquired biological specificity. Therefore, the non-unique properties may serve as a ‘load-star’ to characterize conditions in the geological Cradle of Life. From this point of view, the 10th, 11th and 12th non-unique fundamental properties of biological systems are remarkable. The properties concern the specific peculiarities of processes in living organisms and communities: thermodynamic and chemical non-equilibrium; integrity through cooperative events; and capability for self-organization. The requirement of non-equilibrium conditions relates the origin-of-life process with the wide range of specific events related with non-equilibrium bifurcate transitions and formation of dissipative structures, which are being explored in the framework of non-equilibrium thermodynamics.
First stage of the origin of life: a prebiotic organic microsystem at the state of bifurcate transition under non-equilibrium conditions
Behaviour of a chemical system under conditions far from equilibrium radically differs from its behaviour under conditions near equilibrium. Non-equilibrium conditions are responsible for transformations that radically change a system's structure. The mechanism of radical transformations of natural systems under conditions far from equilibrium is well explored. This mechanism is a bifurcation. A bifurcation occurs when a system cannot exist further in the current conditions due to changes that have occurred in the surroundings or inner mutations. The universal scheme of bifurcate transition of a system is the following: stable existence of a system→rise of instability through powerful fluctuations→the highest point of instability (bifurcation, or critical point), radical change of the system's structure→choice of a new path of development→the next period of its stable existence (Fig. 1(a)). At the bifurcation point a system undergoes many accidental changes that may influence the choice of its future development path. This is the reason why potential paths of a system's development bifurcate at the moment of its highest instability. Finally, the system chooses one of many permissible ways, which can be united into two principal trends: (1) complication through self-organization (Trend A); (2) simplification and degradation (Trend B), up to full destruction (Trend B').

Fig. 1. Principal scheme of bifurcate transition of a natural system under non-equilibrium conditions: (a) direct transition from the initial stable state into one of permissible advanced stable states due to changed conditions in the outside world; (b) direct and reverse transitions (in the case of oscillating conditions in the outside world). A – trend to advanced higher-organized state; B – trend to advanced lower-organized state; B' – trend to complete destruction; C – reverse trend to the approximately initial state.
Investigation of diverse bifurcate transitions is a major aspect of the theory of dissipative structures (the founder, I. Prigogine) and synergetics (the founder, H. Haken). These transitions were described in detail in their basic works with their co-authors (Nicolis & Prigogine Reference Nicolis and Prigogine1977; Haken Reference Haken1978; Prigogine & Stengers Reference Prigogine and Stengers1984). The following brief description of the transitions can be composed on the basis of extraction of the most essential notions from these books.
Let us image a certain chemical system that persistently exists in the conditions near equilibrium. Each molecule of such a system is bound together only with nearby molecules by short-range links. A significant change of conditions in the outside world compels the system to react and reorganize its own structure. In the case when a value of the change exceeds some critical level, the system must transit to a new state through the point of bifurcation. The transition begins with strengthening of the inner tension and nonlinearity of the processes. Fluctuations amplify throughout the system; correspondingly the radius of correlation between the system's particles expands. The current structure of the system becomes unstable. Closer to the critical point there appears a sharp spatial-temporal heterogeneity that is manifested in plentiful thermodynamic and chemical gradients. Although the gradients continuously decrease due to inevitable spontaneous processes (diffusion, heat conductivity, etc.), they are being restored through self-organization. The competing fluctuations become abnormally high. They organize the molecules by means of a cooperative (synergy) effect and latently arrange the new structure of the system. At the same time many accidental changes influence the competition between the macrofluctuations. Just before the bifurcation point the system is on the threshold of the choice of new path of development. In the long run, one of the competing macrofluctuations embraces the whole system and determines its future path of development. All particles are organized by the prepotent macrofluctuation, and their radius of correlation enlarges up to the limits of the system. The particles perceive each other at macroscopic distances due to the newly appeared long-range links that keep the integrity of the system. The echo of any event spreads over the entire system. The organization that correlates to the dominating macrofluctuation gives rise to the new system's structure; the rest of the macrofluctuations remain dirigible subsystems. The system becomes open: there arises a continuous exchange of energy and matter with the surroundings. As a result, it acquires an extraordinary sensitivity to external influences, in addition to the inner sensitivity based on long-range links between the particles. As soon as the changed system has chosen its new path of development, the internal tension falls. The scale of fluctuations gradually decreases. The changed system transits to the next period of stable development.
Structural aspects of the bifurcate transition consist of replacement of the old structure by new structure that is in congruence with the changed conditions in the outside world (Fig. 2). During the initial period of the transition the new structure latently grows within the old structure and becomes predominant right after the critical point. The change of the structures proceeds with the highest internal tension because of their opposition: the old structure keeps the stability of the system, while the potential new one strives to disturb it. Just at the critical point the structure of a system is biforked because of the relative balance between the old and new structures, which strive to develop in opposite directions (Fig. 2, centre). Transition over the bifurcation point leads to change of the dominant state (from the initial to the advanced one) and dominant structure (from the old to the new one). The critical point is in principle unstable: the opposite forces cannot be absolutely equal to each other for a long time due to many accidental changes happening in this thermodynamic area. The accidental changes inevitably turn the balance between these forces, either to the initial state or to the advanced one. The bifurcate period lasts until the counteracting forces develop and the internal tension is maintained. A reverse change of the external conditions initiates a back transition of the system into the initial state (Fig. 1(b), Trend C).
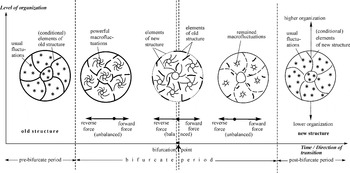
Fig. 2. Conditional scheme of the change of a system's structure during its bifurcate transition. Thick lines – conditional elements of old structure; thin lines – conditional elements of new structure.
The above description can be expressed in terms of several principal characteristics of a system whilst it is in the state of the bifurcate transition. The most essential of these bifurcate or critical properties are the following: (1) sharp heterogeneity with the intensive counter processes along and against the gradients; (2) continuous fluctuations (waves) and re-arrangement of molecules; (3) integrity through cooperative processes; (4) incessant exchange by matter and energy with the surroundings (open system). Let us consider the striking analogies between these properties and the corresponding characteristics of living organisms. A comparison is drawn in Table 3. Each of four critical properties is distinctly reflected in the vital processes. Although the corresponding characteristics of living cells do not express the unique essence of life, they are in its background. Loss of even one of these characteristics would make life impossible. Meanwhile, the critical properties are temporal characteristics of a chemical system: they appear at the beginning of the bifurcate transformation and disappear with the completed transition to the advanced stable state. On the basis of these facts the following thesis is formulated: life on Earth arose from the bifurcate state that was inherent to still indeterminate kinds of organic microsystems. This threshold of the origin-of-life process can be designated as its first stage.
Table 3. Correlation between the principal properties of a chemical system at the bifurcate state and the corresponding characteristics of a living organism.

The first stage ends with a paradoxical situation: the bifurcate state should be considered as a starting point of living systems, but the bifurcation point is in principle unstable. A system cannot be at the bifurcation point for a long time, because the occurrence of many accidental changes inevitably turns its development either into the initial state or into the advanced one. However, nature on Earth apparently found a way to prolong the bifurcate state of the initial prebiotic microsystems about four billion years ago. The permissible opportunities for this to occur are investigated in the next section.
Second stage of the origin of life: oscillating prebiotic microsystem at the balanced bifurcate state
The period of a bifurcate transition is characterized by the appearance of two opposite forces in a system. They strive to turn the development of the system into the opposed states. Action of these forces dualizes the system's structure, maintains the internal tension and keeps the considered bifurcate properties. The bifurcation point designates the peak of the bifurcate period (Fig. 2). As soon as a system has overcome the point of bifurcation, it may either develop further to the completed advanced state with loss of the critical properties, or evolve back to the point of bifurcation staying inside the bifurcate area (Fig. 3). The reverse transition over the point of bifurcation leads to the same alternative: either the path to the stable initial state beyond the bifurcate area or the return to the point of bifurcation again. Therefore, the only permissible way for a system to be inside the bifurcate area for a ling time consists of balanced oscillations around the bifurcation point. Regular advanced and reverse transitions over the critical point might really occur under oscillating conditions in the surroundings, because a system at the critical state is open and very sensitive to external changes. It seems that there is no alternative way for a chemical system to continuously maintain the critical properties. On this basis, the following thesis can be formulated as a hypothesis: the critical properties in a prebiotic microsystem can be maintained for a long time through regular oscillations around the bifurcation point. This principal opportunity to maintain the critical properties allowed the original prebiotic microsystems to evolve to life on the early Earth, in concordance with the trend shown in Fig. 3. This thesis will be theoretically corroborated in the further sections of this paper; the way to its future experimental proof will be outlined as well.

Fig. 3. Oscillations of a prebiotic (micro)system around the bifurcation point (inside the bifurcate area). Arrows with dotted lines: trends to an advanced state (on the right-hand side from the bifurcate area) or close to the initial state (on the left-hand side from the bifurcate area). Arrows with dotted lines inside the bifurcate area: balanced oscillations of the system between the initial and advanced stable states. Thick arrow: trend to life through accumulation of accidental changes during the oscillations.
So, the object for further theoretical investigation is a (prebiotic) chemical system that oscillates around the bifurcation point and, therefore, keeps the critical properties. Its relative stability is supported by the balanced oscillations between two polar equilibrium (stable) states. The opposite forces are approximately equal; as a result, the advanced force blocks its return into the initial state and the reverse force opposes its transition into one of the advanced states. The system cannot get absolute equilibrium between the forces as well, because many accidental changes at the bifurcation point persistently generate internal instability. The biforked system in this way exists simultaneously in two autonomous and interrelated states that become dominating in turn due to oscillations around the central bifurcation point. This rare and unusual type of natural system has been called a bistate system or, in short, a bisystem (Kompanichenko Reference Kompanichenko2004). It may appear in oscillating conditions in the maternal medium, when the regular inversions of the responsible parameters maintain the balance between the competing non-equilibrium states. Once formed, a bistate system resists its destruction through adequate response to external actions. This opportunity follows the Le Chatelier principle: any action to a chemical system due to change of conditions in the outside world initiates its counteraction.
From the structural point of view, a bisystem oscillates between the stages ‘b’ and ‘d’ of the process of the bifurcate transition regularly passing over the stage ‘c’ (Fig. 2). It follows that the conservative and advanced co-structures, as well as the corresponding initial and forward non-equilibrium co-states, become prepotent and ‘recessive’ in turn. The point of instability repulses the co-structures from opposite sides of the axis of smooth subsymmetry (Fig. 4(a)). Therefore, the core of a bistate system is latently forked. This is the area where the co-structures have maximum interpenetration. They do not fuse because the inner instability maintains their striving to develop in different directions by means of change of the dominant states. In this way, incessant oscillations are generated in the core maintaining internal circulative processes and providing an unlimited potential to self-evolve. A bisystem simultaneously strives to evolve in accordance with three main potential trends peculiar to a system during the bifurcate transition: to the conservative state (trend C), to the advanced higher-organized state (A) and to the advanced lower-organized (B) state (Fig. 1).

Fig. 4. Principal scheme of a bistate system (A) and its dichotomy (B).
Let us emphasize a paradoxical way for the organization of a bistate system. On the one hand, the bifurcation point, which is a point of principal instability, is preserved inside a bisystem. It persistently generates the processes resulting in disintegration of this kind of system. On the other hand, the latent biforked structure maintains the stability of a bisystem if the co-structures and opposite forces are in balance. They serve as a counterbalance to each other. Thereby, the instability supports the tendency towards separation of the co-structures. This process proceeds in opposite directions just from the core. Maintenance of the relative equilibrium between the co-structures leads to the balanced separation that is finally displayed in the organic division of a bisystem into two sub-identical components (Fig. 4(b)). In this case, it is as if the instability point is caught in a trap between two equal forces. Significant disbalance between the non-equilibrium co-states and co-structures may initiate the irreversible transition of a bistate system into one of stable equilibrium states. In this way, a bisystem loses integrity and also the source of persistent internal transformations, with a following transition to a passive existence in the medium or destruction. In summary, the paradoxical way a bisystem is organized consists of a contradictive combination of stability and instability that results in its endless capability to modify. Concerning the origin of life, the instability appears in a prebiotic chemical system for the first stage of this process (it is one of the critical properties), while the relative stabilization of the instability is achieved during the second stage, when the system acquires the bistate status.
Based on the above, the following set of most essential properties inherent to a bistate system is theoretically argued. Acquisition of these properties is achieved by a bistate prebiotic microsystem for the second stage of the origin-of-life process. These properties are added (‘overbuilt’) to the four critical properties, which arose during the first stage of the origin of life.
1. A latent biforked structure consisting of two autonomous co-structures divided by the plane of smooth sub-symmetry. The plane is maintained by relative equilibrium between the opposite forces. Because the co-structures develop in opposite directions they are not absolutely identical. This means a bistate system is characterized with the paradoxical combination of the tendency to symmetry and asymmetry that results in its specific dissymmetric organization.
2. Dichotomy at the end of the normal cycle of existence. This property follows from the paradoxical method of organization of a bisystem, i.e. simultaneous repulsion of the non-equilibrium co-structures from the central point of instability and integration through maintenance of relative equilibrium between them. It seems that the dichotomy is the obligate result of continuous balance between these contradictory tendencies. Maintenance of the relative equilibrium on the background of repulsive forces should inevitably lead to the final division into two sub-identical components. Figuratively speaking, the normal cycle of existence of a bisystem represents its gradual ‘sawing’ combined with the tendency to form sub-identical dichotomous units.
3. Oscillating character of existence related to periodic change of the dominant states. Oscillations of all inner processes support stability of a bisystem. Ceasing of the oscillations constrains a bisystem to leave the bifurcate area with a following irreversible transition into one of the stable equilibrium states. Oscillations between the polar forces sustain the internal tension that is a source of incessant dynamic processes and intensive rearrangement of molecules in a bistate system.
4. Display of mutability and heredity maintaining the ability to self-evolve. According to the theory of dissipative structures, each forward transition over the bifurcation point (from the initial into the advanced state) brings some accidental changes in the chemical system. During the back transition the system may return only into the altered initial state due to the changes that have occurred. So, the reverse transition can be considered as a way towards conservation of the previous state of a chemical system. In this context, regular oscillations around the bifurcation point occurring in a bistate chemical system work step by step towards accumulation of the accidental changes that provide its ability to evolve. The forward transitions initiate new transformations in a bisystem (mutability), while the reverse ones retain its state (heredity).
5. Ability to choice and correct own behaviour (i.e. to change the character of interaction with the surroundings). A bistate system simultaneously strives to develop in accordance with the different trends A, B and C (Fig. 1); these main trends are branched as well. So, the current way of development of a bisystem depends on the balance between the forces acting in different directions. Because of the oscillating character of the inner processes the balance is changeable. From the other side, a bistate chemical system under external influences generates the processes directional to maintenance of its stability. This is a consequence of the Le Chatelier principle. As a bisystem and its surroundings are in continual dynamic interaction, the path to its higher stability can be executed not only in the direct way (various internal reorganizations), but by a mediate way as well. The mediate way lies through the execution of a specific influence on the surroundings resulting via feedback in the rise of the bisystem's stability. The generating counteraction to external actions may regulate the balance between the A–B–C trends and turn the interaction with the outside world, i.e. the behaviour of the bisystem, onto a more profitable course. In this way, a bistate system may make a choice and later on correct its own behaviour.
Although the listed bistate properties do not strictly belong to the unique properties of biological systems, they are present in the foundation of biological organization and pertain to any living organism. The correlation between these theoretically distinguished properties of a bistate system with the real characteristics of a living organism is given in Table 4. The table shows that the intermediate position of a bistate system between ‘life and non-life’ allows us in principle to explain the fission of a cell, the inner biophysical and biochemical rhythms, the motion of unicellular organisms by means of oscillations of flagellums, accidental mutations in the genetic structures, the morphological subsymmetry (dissymmetry) of living beings, their ability to correct their own behaviour and modify functions, etc. The theoretically substantiated process of the dichotomy of a bistate system (Fig. 4) looks very similar to the real methods of dichotomy of simple and complex cells (amitosis, mitosis and meiosis) shown in Fig. 5. The axes of smooth subsymmetry are substantial structural characteristics of living organisms as well (Fig. 6).

Fig. 5. Scheme of dichotomous division of a cell (A – amitosis, B – mitosis and meiosis).

Fig. 6. Display of the plane of smooth subsymmetry in the theoretically based bistate system (A) and the real biological structures: DNA double spiral (B), embryo of a man (C) and spinal cord of primates (D).
Table 4. Correlation between the theoretically based properties of a bistate system and the corresponding real characteristics of a living organism

Third stage of the origin of life: inversion of the balance ‘free energy/entropy contribution’ and the rise of the key biological properties
As it was based in the previous Section, a prebiotic bistate microsystem possesses many properties that are essential for life. However, the bistate microsystem is still not characterized with four key unique biological properties (distinguished in Section 1), although some dawn of the living state are present already – for instance, the existence of dichotomy. The essence of these key properties could be boiled down to the following thesis: a viable organism concentrates free energy (F) and information (I) through the ability to intensify and expediate reaction to external changes. Rewording, it is able to keep the positive F and I gradients with respect to the environment. By means of the positive free energy gradient a viable living system is able to transform the environment more efficiently than the environment is able to transform it (i.e. the ability for intensified counteraction). Due to the positive information gradient, a viable biological system knows about the environment more than the environment knows about it (this is the basis for its expedient behaviour). The combination of the intensified and expedient actions of a living being can be expressed as the criterion of the ‘growing ability to control the environment and the lessening dependence on it’ that was recognized by J. Huxley as being most important in progressive biological evolution (Huxley Reference Huxley1942). To keep this path of existence, the inflow of free energy F (providing the ability to do work) into a biological system must be higher than the inflow of entropy S (devaluing the ability to carry out work). The entropy contribution is connected with the total input of the spontaneous (basic) processes in a natural system, while the free energy contribution is related with the total input of non-spontaneous (coupled) processes. That means the ratio ‘free energy contribution to entropy contribution’ can be used to fix the approximate balance between these counter universal processes. The universal basic and coupled processes embrace all processes that proceed in a system. The coupled processes generate free energy and result in an increase of the inner energetic gradients (including concentrations, pressure, temperature, bioelectric gradients), while the basic ones lead to entropy production and decrease of these gradients. The prevalence of the F contribution over the S contribution means the following: (a) non-spontaneous processes proceed in a viable biological system faster than spontaneous ones; (b) constructive transformations in a living organism are more efficient than destructive ones. In particular, these notions have been considered in detail in a previous work of the current author (Kompanichenko Reference Kompanichenko2003). Thus, the character of re-arrangement of molecules in a living organism is dependent on the balance between the active transport (coupled process) and diffusion (basic process). The prevalence of the diffusion over the active transport gradually works towards a decrease in the concentration gradients with a tendency towards degradation of the entire organism.
The unifying of all biological systems with some inanimate non-equilibrium systems into the wide class of dissipative structures is based on certain analogies between them. According to the common definition, a dissipative structure is a system that continuously dissipates energy, but nevertheless keeps its own existence by means of matter and energy exchange with the surroundings. However, the difference between living and non-living dissipative structures is still not strictly drawn. In accordance with the elaborated conception, the ratio F/S contribution is negative in a non-living dissipative structure and positive in a living one (Kompanichenko Reference Kompanichenko2003). Although this difference is not exhaustive, the ratio allows us to draw the distinct separating line between these types of dissipative structures. Non-living dissipative structures are unable to concentrate F and I by means of the efficient extraction from the outside world that does not permit them to self-evolve. Thus, an oscillating chemical reaction is characterized by continuous oscillations between concentrations of the reagents (waves of concentrations), but in the long run the oscillations inevitably cease (a special case is artificial prolongation of the reaction through supplementation of new portions of the reagents). In this context, the final step to life consists of inversion of the ratio of the F/S contribution from negative to positive. A bistate prebiotic system has additional opportunities to take the plunge into life, in comparison to a usual non-living dissipative structure. First, it is composed of organic compounds which is the most suitable matter for boundless complication; second, its paradoxical method of organization provides it with balanced internal tension – the source of further evolution; third, accumulation of accidental changes provides regular inner transformations (from very destructive to very constructive), etc. How do these opportunities cause a change of the ratio of the F/S contribution, signifying the beginning of the inverse processes in a bistate prebiotic microsystem and its drastic transformation into a primary living unit on the Earth? The permissible method of such a transformation is theoretically outlined below.
As it was argued in the previous section, the complex circulative physical and chemical processes proceed in a bistate system. The results of the local processes contribute to the common F/S ratio. Being a non-living system, a prebiotic bistate microsystem is characterized by a negative total F/S ratio. However, because of the oscillating character of the existence, the F/S ratio in a bistate prebiotic system changes all the time. Moreover, sometimes the amplitude of the swing of the ratio may rise extraordinarily due to a radical constructive or destructive transformation initiated by the accumulation of accidental changes. In the case of high amplitude, from time to time the free energy contribution exceeds the entropy contribution, although the average long-term F/S balance is still negative (Fig. 7-I). During these short periods, the physico-chemical processes in the bistate prebiotic microsystem proceed in inverse: internal energetic gradients rise, and free energy and information accumulate through the changed interaction with the surroundings. This change can be considered as a step towards life through appearance of the initial unique biological properties. The return back to the negative F/S balance gradually resolves the gradients and devalues the surplus of free energy and information. So, periodic inversions occur in a bistate prebiotic microsystem having inner oscillations of high amplitude. Interchange of the steps to ‘life and back’ in the bisystem that oscillates in such a manner means that the universal spontaneous and non-spontaneous processes become dominating by turns. ‘Flashing’ and fading away of the initial sparks of life periodically occur. Therefore, such a ‘pulsing’ bistate microsystem possesses an excess of free energy (in respect to the entropy input) for a short time and a deficit for a long time.

Fig. 7. Oscillation of the balance ‘free energy contribution/entropy contribution’ in a prebiotic bistate (micro)system. Initial sparks of life in the bistate microsystem appear for the periods of positive F/S ratio.
It was considered that oscillations of parameters in the host medium are a necessary condition to sustain the bistate status of prebiotic microsystems. There are main thermodynamic and physico-chemical parameters in a liquid geological medium: pressure, temperature, pH, Eh, concentrations of diverse compounds, including organic molecules. According to the Onsager theorem, which is based on irreversible thermodynamics, thermodynamic and physic-chemical parameters in solutions and melts are interrelated by means of certain interdependencies (Onsager Reference Onsager1931). This means that a change of one parameter initiates changes in others. In the changeable conditions prebiotic bistate microsystems undergo periodic stress. The stress affecting a bistate prebiotic microsystem for the period of negative F/S balance leads to its degradation because of the prevailing entropy contribution (Fig. 7-III). However, in the case of the period of positive F/S ratio a stress stimulates intensive reorganization of the microsystem possessing an excess of free energy. The surplus of free energy can be used for constructive transformations in the bistate microsystem as a respond to stress, with the following rise of free energy production due to more efficient organization of the network of chemical reactions. The enhanced inflow of free energy in the microsystem increases the positive F/S balance further and facilitates new constructive transformations. In this way several successive actions from the outside world having an influence for the favourable period may result in an increasing ‘avalanche-like’ constructive conversion of the prebiotic bisystem which turn the average F/S balance onto the positive branch (Fig. 7-II). This extraordinary radical constructive transformation creates new highly effective energy-giving methods for the chemical reactions. Baltscheffsky (Reference Baltscheffsky1997) investigated such drastic constructive transformations in the process of biological evolution and called them ‘anastrophes’ (the antipode to ‘catastrophes’). As soon as this greatest ‘anastrophe’ occurs, the microsystem is immediately organized in a way which provides faster free energy production than entropy. The excessive free energy in probionts is bound in energy-rich compounds. The first unique biological property appears – the ability to concentrate free energy and information. At this precise moment a prebiotic bistate microsystem is transforming into the simplest living unit – a probiont. The non-spontaneous universal processes already dominate over the spontaneous ones. Imaginatively speaking, a perpetual ‘flame’ of life in a probiont fires off the temporal ‘spark’ of life in a prebiotic bisystem. The process of free energy concentration in a probiont is connected with the continuous increment of the internal energetic gradients. Some important types of energetic gradients exist: concentration, electric field, pressure (osmosis), temperature. The rise of these gradients works to increase the general energetic potential of a probiont. The inversion of the universal processes leads to remarkable changes in the network of chemical reactions. As in a prebiotic bisystem, they proceed in both directions (reducing and enhancing the energetic gradients), but the active transport begins to prevail over diffusion in a probiont. This means the general direction of the chemical processes in a probiont reverses, and particular reactions are involved in the circulation incidental to another method the system is organized already. In this method of organization the aim is to maintain the fixed positive ratio of ‘the constructive processes to destructive processes contributions’. The process of a separate chemical reaction is regulated by the whole organization of a probiont. Fragmentary circulations of the chemical processes in various parts of a probiont integrate into the organized circulative network. This radical transformation gives rise to the metabolism of the initial biological organism and transition of chemical reactions from in vitro into in vivo. Once formed, a probiont has opportunities to support existence because the Le Chatelier principle acts: under regular external actions a probiont persistently develops a counteraction directed to maintain its own structural and functional stability, i.e. the biological way of organization. So, moderate stress to a probiont is a necessary condition for its existence, while too strong a stress (distress) leads to degradation. This thesis is in concordance with the theory of stress by Selye (Reference Selye1974).
Let us consider in detail some aspects of the transition from a prebiotic bisystem to a probiont. The counteraction arising in a simple chemical system due to change of the external conditions is strictly directed to partial compensation of the executed influence, i.e. the reaction is ‘symmetric’ (Fig. 8 (Ia)). Finally, the external action overpowers the counteraction and changes the chemical system (Fig. 8 (Ib)). Because of the universal character of the Le Chatelier principle such a ‘symmetric’ counteraction should also arise in a prebiotic bistate microsystem that is a specific kind of chemical system (Fig. 8 (IIa, IIIa)). In the general case the energetic effect of the ‘symmetric’ counteraction is impaired in comparison with the effect of the executed action, as in a usual chemical system. Nevertheless, a bistate system possesses some specific qualities, which provide it with other opportunities to effectively respond to changes in the surroundings.

Fig. 8. Reactions of a usual chemical system and a prebiotic bistate system to external actions (stress). I – reaction of a usual chemical system; II – reactions of a prebiotic bistate (micro)system having negative current ratio of F/S contributions; III – reactions of a prebiotic bistate (micro)system having positive current ratio of F/S contributions.
A bistate prebiotic microsystem cannot complete the respond to stress immediately, unlike a simple chemical system. Any external influence to those systems is involved in complex circulation of substance and energy; moreover, the tendency to structural-functional complications exists due to the regular inversions of the internal physico-chemical processes and accumulation of accidental changes. It follows that penetration of an external action into the bisystem and the counteraction last for a long time. In this context the immediate symmetric reaction should be prolonged by the late reaction. Due to the changes that have occurred in the bisystem, the direction of the late respond is different from the initial one, i.e. it is ‘asymmetric’. Therefore, the following scheme of the reaction of a prebiotic bisystem to an external influence can be outlined: external action (stress, inner structural-functional deformation)→(impaired) immediate symmetric reaction or pause (gradual compensation of the stress and preparing of the efficient respond)→(efficient) late asymmetric reaction to the stress. This scheme in principle coincides with the well-known scheme formulated in the framework of the theory of stress. Because a bistate prebiotic system is open, its reaction to stress should expand into the outside world.
From the energetic point of view, an external stress contributes the additional entropy to the microsystem. As a consequence, the ratio F/S contribution considered earlier shapes the F/S + contribution (S +=S internal+S external) for the bistate microsystem under external influences. The current ratio of F/S + contribution in the prebiotic bisystem defines in many ways its counteraction to stress. In the case when the ratio is negative, the additional entropy input results in strengthening of the internal destructive processes. Both the symmetric and asymmetric reactions are inhibited due to the prevailing entropy contribution. Such microsystems lose bistate status and degrade (Fig. 8 (II b)). Extraordinary strong stress may initiate their complete destruction (Fig. 8 (II c)). The temporal positive F/S + ratio means that the input of both the symmetric and asymmetric reactions of the microsystem becomes intensified in comparison with the power of the external influence. This ability to have an intensified counteraction is the second unique biological property. Joint symmetric and asymmetric reactions of a probiont expand into the outside world and begin to transform it into the environment, i.e. the ‘physical’ medium is under the active influence of living processes (Fig. 8 (IIIb)). Unlike the immediate symmetric reaction, the late ‘asymmetric’ one inverts during the deep penetration of an external influence into the network of chemical processes (which are already inverted in probionts). This means the symmetric reaction arises after an external action, but the asymmetric reaction, on the contrary, may forestall external actions through the reflection. Continuous external influences destroy part of the energy-rich compounds and utilize the released free energy. The following intensified responses to these stresses restore the surplus of free energy plentifully. This correlative process leads to an accumulation and concentrative encapsulation of free energy in a viable probiont. In this way, optimum changes in the environment allowed the original probionts on the early Earth to develop further which gave rise to the process of biological evolution. If the strength of stress exceeded the ability of a probiont for intensified resistance (F/S + became negative), it was eliminated with the natural selection. Appearance of interactions between the probionts through interconnection in their local environments led to the emergence of primary ecological systems (Fig. 8 (IIIc)). Such cooperation facilitated survival of the probionts and promoted further evolution of the initial communities.
Exchange by information with the environment is one more important factor for existence of prebiotic bisystems and probionts. Information is necessary for constructive transformations in them, while entropy contributes to their disorganization. Taking the input of information into consideration, the ratio F+I/S + contribution is more precise than the ratio F/S + contribution used above. As it was based, a bistate system possesses the highest sensitivity to external changes which is one of the critical properties. Inheriting this property, a probiont should react to the smallest changes in the environment. In fact, a living unit and its environment themselves represent a pair system. Their interaction is maintained through exchange of influences, including the exchange of energy, substance and information. Any change of the external conditions exerts a certain influence on a probiont and initiates its structural-functional deformation. The deformation can be expressed as a change of configuration of macromolecules, the appearance of new pathways of chemical reactions, etc. In fact, this deformation is a trace of the change that occurred in the environment, or a reflection of this change inside the probiont. These traces themselves represent primary (bio)information about the original changeable environment. The labile protein chains are most suitable macromolecules for immediate sensitive fixation of the primary traces, while the steady nucleotide chains are appropriate structures to conserve them. During the existence of initial living units on the Earth the traces inevitably accumulated, interacted and pressed into each other which resulted in a concentration of bioinformation. This information was kept and circulated in the probionts through the initial nucleic–protein interaction. Because the accumulated bioinformation is inevitably involved in the network of biochemical reactions, it is inevitably involved in the initiating asymmetric response to external influences as well. Based on reliable information about the environment, a probiont has a real chance to direct the asymmetric reaction towards the most profitable way. The inverted character of the asymmetric reaction provides a probiont with the ability to foresee, execute forestalling actions in the environment and correct its own behaviour through the feedback loops. In this way the third unique biological property appears – the expedient character of interaction with the environment, or the ability to exhibit expedient behaviour. Sometimes an exceptionally profitable asymmetric reaction may arise due to internal accidental changes and this may bring a revolutionary constructive change in the probiont. The combined ability to undergo intensified and expedient reactions to stress is the real means by which a biological system is able to concentrate free energy and information through active extraction from the environment. In this way a probiont develops, while distress or absence of stress leads to its further degradation and destruction because of its inability to display the intensified and expedient counteraction. So, life is constrained to develop, or evolve, in order not to be eliminated with the natural selection.
Because of the positive ratio of F+I/S + contribution in a probiont, the constructive processes are not only able to compensate for destruction but to use the excess of free energy and information to sustain and modify the current structures and functions. In fact, the ratio provides incessant self-renovating processes in viable living systems. This continuous renovation combined with the ability to divide (which appeared in prebiotic bisystems) represent the fourth unique biological property – regular self-renovation of living systems at different levels (from the molecular to the ecosystemic).
The change of chemical reactions from in vitro into the biochemical reactions in vivo means that a separate chemical reaction loses its ‘spontaneous’ status. The network of biochemical reactions is aimed at maintenance of the living state of a probiont, and the process of a single reaction is constrained by the general organized circulation of matter and energy (metabolism). Regular inversions and accidental changes inside the network of reactions continuously complicated the metabolism: new and new outgrowths appeared between the extreme products of the initial chemical reactions. Multiplication of the intermediate links in the transmission of bioinformation gave birth to specific programming and further complication of the nucleic–protein interaction inside the primary forms of life on the Earth. The impulsive character of the signals' transmission is maintained by the persistent oscillations of metabolic processes. The combination of the contradictory tendencies to differentiation and integration in the background of growth of a probiont led to the progressive ‘concentrative encapsulation’ (the maximum complexity inside the minimum space). This process resulted in complication of the inner concentration gradients and membranes. In particular, the combination of left forms of amino acids and right forms of sugars in a living cell can be considered as the final result of the continuous rise of the initial concentration gradients that started off the racemic mixtures (ratio ‘left and right amino acids’, ‘left and right sugars’ close to 50/50%) at the earliest stage of biological evolution. Moreover, disymmetric organization of a probiont (inherited from the former prebiotic bisystem) facilitated advancement of biohomochirality. The anti-entropy tendency led to the rise of other energetic gradients – bioelectric potentials, osmosis, etc.
The method of transformation of a prebiotic bisystem into a probiont theoretically outlined includes some sequences of events that really entered into the biological organization and can be traced in the process of biological evolution. The first of them is periodic inversions of physico-chemical processes in prebiotic bistate microsystems that served as the necessary background for the further emergence of life. Such inversions back and forth from the prevalence of destructive processes to constructive ones and vice versa are reflected in two balanced tendencies in metabolism – catabolism and anabolism. The next event was the greatest anastrophe in the history of life – the change of the average ratio of F/S contribution onto the positive branch. This starting point of biological evolution was continued by the great anastrophes that followed. Some of these anastrophes concerning the early evolution of biological energy conversation were listed and considered by Baltscheffsky (Reference Baltscheffsky1997). One more theoretically based event is the necessity of external influences from the outside world (initiating an optimum level of stress) to sustain the existence of probionts. This correlates with the fundamental notions of the theory of stress. The fourth event regards lengthening of the probiont's reaction to external actions, beginning with the initial ‘symmetric’ reaction and completing with the late ‘asymmetric’ one. This difference becomes apparent after a long period of biological evolution. Thus, the ‘arsenal’ of behaviour of advanced living beings includes as the immediate adequate (‘symmetric’) reaction to a stress as expedient actions to forestall such stress in the future.
Brief scenario of the origin of life on the early Earth
Most of the scientific background knowledge from which the elaborated systemic conception of the origin of life emerged covers not only Earth, but processes in the vast explored Universe too. The key notions of ‘free energy’ and ‘entropy’ used are universal and characterize the ability or disability of a natural system to carry out work elsewhere. For instance, the eruption of ash by active volcanoes on Earth and Jupiter's satellite Io is the same kind of work that can be approximately evaluated in terms of the spent free energy. The opposite spontaneous and non-spontaneous processes are universal as well. They are described by the transition of a natural system to a more or less probable state. Cooling of a lava flow on Earth and cooling of the entire Mars planet are similar processes related with a spontaneous process – heat conductivity. All stars, planets and other space bodies in the Universe are composed of about 100 elements systematized in the Periodic Table. In this context, the behaviour of chemical systems elsewhere in the Universe should comply with the fundamental laws determined on Earth, in particular with the Le Chatelier principle, the Onsager theorem and the theory of dissipative structures used in this work. For example, outflow of matter from a star occurs simultaneously with outflow of heat energy as follows from the Onsager theorem. The non-unique properties of biological systems distinguished on Earth (Table 2) correlate with similar properties of variable non-biological natural systems, which are widespread in the Universe. Among these non-biological systems are: crystals and minerals, many of which have been explored on Earth, Moon, Mars and meteorites; magmatic (volcanic) systems on Earth, Io, early Mars and Venus, etc.; stars in our Galaxy and beyond. The organic microsystems, which are considered as prebiotic for the early Earth, were composed of diverse organic compounds that have been detected in space and meteorites (from simple hydrocarbons up to amino acids). Taking these reasons into account, the three stages distinguished in principle can be considered in the context of a general scenario of the origin of life in the Universe.
Much data relevant to the origin of life on the early Earth has now been obtained. The argued principal way of transformation of prebiotic microsystems into simplest living units should be integrated with this data in the future. In what follows, a sketch of the scenario of the origin of life on Earth is outlined. Although the scenario is still not elaborated in detail, it illustrates the author's approach to clarification of this problem.
The key consequence of the systemic conception is that the transformation of prebiotic microsystems into probionts might happen only at the non-equilibrium state of the bifurcate transition and in a liquid geological medium with fluctuating (oscillating) parameters. This thesis suggests an explanation for why self-maintaining dynamic processes and the ability to self-evolve did not appear in various prebiotic models during the experiments conducted under stable conditions in chambers. So, according to the conception, significant fluctuations of thermodynamic and physico-chemical parameters (pressure, temperature, concentrations of compounds, pH, Eh) stimulated the origin-of-life process. Comparing in this context two alternate liquid media for the origin of life – an ocean and a hydrothermal system – the following conclusion can be inferred. The scale of the fluctuations in the oceans is very limited due to the close minimum and maximum values (pH 8.0–8.4, salinity 30–38 g/l, etc.), while in hydrothermal systems it can be very large because of the highly different extremes (pH 1–12, salinity 1–500 g/l, temperature 30–400°C). Macrofluctuations in present hydrothermal systems are initiated by the nearest earthquakes, tectonic dislocation, volcanic eruptions or internal explosive events, and they create highly non-equilibrium conditions. Irregular microfluctuations and regular micro-oscillations often occur in hydrothermal media. For instance, thermodynamic oscillations in hydrothermal systems of the Kamchatka peninsula (an active volcanic region) and the Mura field in Slovenia (a non-active volcanic region) have the following characteristics: the average amplitude of pressure is 0.5–1 bar, temperature is about 2°C, the period of oscillations is from 20 to 70 minutes (Kompanichenko et al. Reference Kompanichenko, Frisman, Fishman, Savenkova and Shlufman2007; Kralj & Kralj Reference Kralj and Kralj2000). In this connection the author considers ancient hydrothermal systems and their submarine or/and terrestrial discharges as the most probable medium for the origin of life, like many other scientists (Corliss et al. Reference Corliss, Baross and Hoffman1981; Kompanichenko Reference Kompanichenko1996, Reference Kompanichenko2004; Schwartzman & Lineweaver Reference Schwartzman and Lineweaver2004; Holm & Andersson Reference Holm and Andersson2005; Russell et al. Reference Russell, Hall, Boyce and Fallick2005; Holm et al. Reference Holm2006).
The most probable range of temperature in the geological Cradle of Life can be evaluated as 70–150°C. The low extreme magnitude characterizes the seawater temperature of the Archaean ocean. The oxygen isotopic record in marine charts shows the temperature of the Archaean ocean to be +80°C at age 3.8 billion years ago (Ga) and +70°C (±15°C) at age 3.5 Ga (Knauth Reference Knauth, Clanet and Chaudhuri1992; Knauth & Lowe Reference Knauth and Lowe2003). A similar result was obtained on the basis of the exploration of the silicon isotopic compositions (Chaussidon & Franquis Reference Chaussidon and Franquis2006). The oldest records of life are about 3.5 Ga in accordance with exploration of microfossils and 3.85 Ga on the basis of biogeochemical data (Schidlowski Reference Schidlowski1997; Schopf et al. Reference Schopf, Kudryavtsev, Argesti, Wdowiak and Czaja2002; Westall et al. Reference Westall, Reymond and Gibson2006). So, the minimum temperature of liquid surroundings around submarine hot springs can be evaluated to be 70°C at the time of the origin of life on Earth (3.8–4.4 Ga). The high extreme value of temperature in the geological Cradle of Life (150°C) is conditional. The hydrothermal process develops on the background of a temperature decrease from 400–450°C down to 40–50°C. Now it has been proved that microorganisms may exist at the maximum temperature of 121°C.
Organic compounds of main prebiotic models (RNA World macromolecules, liposomes, proteinoide microspheres, aromatic hydrocarbons) in principle may exist inside this range of thermodynamic conditions. The major problem is to integrate the low thermal stability of nucleotides (50–60°C, as an exception up to 90°C) into a hydrothermal origin of life scenario. Although a temperature of 100–200°C is suitable for the synthesis of amino acids, their further behaviour in hot solutions follows two different trends – either to polymerize or to destroy. The important consequence of the suggested systemic conception is that the stability of various organic molecules in usual chemical systems, on the one hand, and inside the bistate microsystems and especially probionts, on the other, can be different. Unlike usual chemical reactions in vitro, organic molecules in probionts (and particularly those in prebiotic bisystems) are involved in an organized network of (bio)chemical reactions in vivo that may significantly increase their stability. The ostensive example is comparison of the highest thermal stability of RNA in experimental chemical systems (50°C) and in hyperthermophilic Archaea (120°C). The simplest living probionts and even prebiotic bistate microsystems possess the specific organization that stimulates inner constructive transformations. In this way RNA macromolecules might be gradually synthesized by precursors inside the bistate microsystems and probionts under much higher temperatures than 50–60°C.
The behaviour of the biologically important organic molecules and the suggested prebiotic models has still not been experimentally explored under non-equilibrium oscillating conditions. For this reason, it is unclear which of the models or their combination is the most suitable to be transformed into the initial forms of life on the early Earth. The author uses for further consideration the conditional prebiotic microsystem composed of a mixture of the biologically important organic molecules (lipids, amino acids, nucleic acids) and/or their precursors. The optimum ratio between them can be determined later on.
The general scenario
About four billion years ago intensive tectonic-volcanic processes maintained non-equilibrium conditions and an inhomogeneous distribution of substance in hydrothermal systems on the early Earth. In this way some local parts in hydrothermal media were enriched with organic matter. Organic compounds could penetrate into hydrothermal systems from the surface (exogenous or cosmic source), or to be synthesized in hot solutions, for instance in the course of Strecker and Fischer–Tropsch synthesis reactions (Holm & Andersson Reference Holm and Andersson2005). With the background of the gradual temperature decrease, organic molecules segregated into numerous microsystems disseminated in the local high-temperature aquatic zones. Significant changes of conditions in the hydrothermal medium (macrofluctuation) turned the microsystems into the bifurcate state, i.e. the intermediate unstable state between stability and destruction. The following smaller micro-oscillations of thermodynamic and physico-chemical parameters in the optimum regime stabilized the bifurcate state of some of these microsystems. They were transformed into prebiotic bistate microsystems, while the remainder of this huge number of microsystems left the bifurcate area and either transited to a passive existence in the medium or were destroyed. Under continuous optimum oscillations in the medium some of the prebiotic bistate microsystems transited into living probionts, others lost their own bistate status and were eliminated from further evolution into life by the natural selection. The ability of the initial probionts to support existence much rose through the established interaction between them in these local zones. This process resulted in the formation of a minimum self-sufficient living system – a community of probionts interacting with the environment. Then the habitats of the communities expanded from the submarine hydrothermal media or/and (hot) terrestrial groundwater systems into the hot ocean. The evolution of probionts into advanced forms of the universal ancestor and then into (hyper)thermophilic Archaea and Bacteria proceeded through substantial transformations, some of which have been investigated already: periodic radical constructive reorganizations (anastrophes) in biological energy conversation (Baltscheffsky Reference Baltscheffsky1997), reorganizations of the initial genetic structures in accordance with ‘self-perfecting logic’ (Wong & Xue Reference Wong, Xue, Palyi, Zucci and Caglioti2002), persistent rise in the level of accuracy in translation (Woese Reference Woese1987), etc. The scenario outlined corresponds with the position of hyperthermophiles at the root of the Philogenetic Tree (Stetter Reference Stetter1995; Xue et al. Reference Xue, Tong, Marck, Grosjean and Wong2003) and explains the incredible ability of Archaea to survive under diverse extreme conditions.
Conclusion
The presented succession of events leading to the origin of life is a theoretical construction and needs further confirmation. Some scientific methods that may check and develop various aspects of the systemic conception are suggested below.
1. Laboratory experimental research of diverse prebiotic models at the state of bifurcate transition and under oscillating conditions in the experimental chamber. The goal of the experiments is to corroborate or demolish the thesis concerning the real existence of the bistate type of natural system, including its ‘prebiotic version’ useful in the evolution to life.
2. Natural experimental exploration of changeability of the thermodynamic and physico-chemical parameters (pressure, temperature, concentrations of compounds, pH, Eh) in submarine and terrestrial hydrothermal systems, including amplitudes, frequencies and periods of the oscillations. Also it is important to determine how the hydrothermal oscillations depend on the close volcanic and tectonic (seismic) events. The events were much more intensive on the early Earth, and might significantly influence the scale of the fluctuations. These investigations are aimed at characterizing the probable geological Cradle of Life. Then the obtained characteristics can be used to conduct laboratory experiments.
3. Natural and laboratory experimental research directed towards further clarification of the set of organic compounds which could serve as the substantial basis for original prebiotic microsystems formed in the ancient hydrothermal systems.
4. Theoretical substantiation of thermodynamic aspects of the conception in detail. In this paper the most general terms ‘free energy contribution’ and ‘entropy contribution’ were used. Furthermore, the ratio between them should be considered in the framework of more special knowledge and terminology of non-equilibrium thermodynamics.
5. Theoretical modelling of a dynamic system with changeable parameters that oscillate around the point of bifurcation.
Acknowledgements
The author is profoundly grateful to Professor David W. Deamer (USA), Efim J. Frisman (Russia), Gyula Palyi (Italy), Petr S. Posohov (Russia), Jeffrey T.-F. Wong (Hong Kong) and Alla L. Voronina (Russia) for fruitful discussions and assistance during elaboration of the conception.














