Published online by Cambridge University Press: 28 June 2005
In order to investigate the importance of the host microbiota on differentiation of T cell subsets in response to infection, Swiss/NIH germ-free mice and conventional (microbiota-bearing) mice were infected with Leishmania major, and lesion development, parasite loads, and cytokine production were assessed. Germ-free mice failed to heal lesions and presented a higher number of parasites at the site of infection than their conventional counterparts. In addition, histopathological analysis indicated a higher density of parasitized macrophages in lesions from germ-free mice than in conventional mice. The initial production of interleukin (IL)-12 and interferon-gamma (IFN-γ) in germ-free mice was comparable to the conventional controls. Also, germ-free mice produced elevated levels of IFN-γ and lower levels of IL-4 throughout the course of infection, suggesting the development of a Th1 response. Macrophages from germ-free mice exposed to IFN-γ and infected with amastigotes in vitro were not as efficient at killing parasites as macrophages from conventional animals. These observations indicate that the microbiota is not essential for the development of Th1 immune responses, but seems to be important for macrophage activation.
Experimental infections with the parasite Leishmania major have established the Th1/Th2 paradigm. Thus, C57BL/6 mice infected with L. major develop a Th1 response that results in IFN-γ production, macrophage activation, and control of parasite growth and lesions (Belosevic et al. 1989; Heinzel et al. 1989; Scott, 1991). In contrast, BALB/c mice respond to this parasite by developing a Th2 response, but no macrophage activation is achieved and parasite growth is poorly controlled (Heinzel et al. 1989; Sadick et al. 1990). Although resistance and susceptibility models with clear-cut Th1 or Th2 responses were not found for other Leishmania that cause cutaneous leishmaniasis (Afonso and Scott, 1993; Barral et al. 1993; Donnelly, Lima and Titus, 1998; Lima, Dekrey and Titus, 1999; Soong et al. 1996), the Th1/Th2 correlation with resistance or susceptibility has been found to be true for all L. major isolates or clones studied.
Many factors can influence the type of immune response mounted by the host, however, the effects of the intestinal microbiota on the outcome of infections caused by Leishmania parasites are little understood. It has been argued that development of the T cell response (MacDonald and Carter, 1979) and macrophage activity (Nicaise et al. 1995, 1999) would be impaired in the absence of the normal microbiota.
We had previously shown that conventional Swiss/NIH mice are resistant to experimental L. major infection with the development of a Th1-type immune response against this infection. Moreover, we observed that germ-free animals infected with L. major which were conventionalized 3 weeks after infection presented larger lesions than conventional animals. However, similar to conventional mice, lesions in conventionalized mice peaked at 6 weeks and were progressively controlled (Oliveira et al. 1999).
In the present study, we investigated the outcome of L. major infection in germ-free Swiss/NIH mice for 13 weeks in the absence of microbiota, as compared to conventional mice. We demonstrate that germ-free Swiss mice developed non-healing lesions with high numbers of parasitized macrophages. However, in spite of the inability of germ-free mice to heal, cells from these mice produced high levels of IFN-γ. These observations indicate that the microbiota is not necessary for the development of a Th1 immune response, but is an adjuvant for macrophages to efficiently kill this intracellular parasite.
Germ-free Swiss/NIH mice were derived from a germ-free nucleus (Taconic Farms, Germantown, USA) and maintained in flexible plastic isolators (Standard Safety Equipment Co., Pallatine, USA) using classical gnotobiology techniques (Pleasants, 1974). Conventional Swiss/NIH mice are derived from germ-free matrices, and considered conventional only after 2 generations in the conventional facility. All animals were 5 to 7-week-old females. All experimental procedures were carried out according to the standards set forth in the ‘Guide for the Care and Use of Laboratory Animals’ of the National Research Council (1996).
The Leishmania major strain WHO MHOM/IL/80/Friedlin was used for these studies. Promastigotes were cultured in Grace's insect medium (Life Technologies, Grand Island, USA) supplemented with 20% fetal bovine serum (Nutricell, Campinas, SP, Brazil) 100 U/ml of penicillin and 100 g/ml of streptomycin. Metacyclic promastigotes were obtained from stationary phase of growth using Arachis hypogae agglutinin (Sigma Chemical Co., St Louis, MO) as described previously (Sacks, Hieny and Sher, 1985). Amastigotes were obtained by disruption of infected footpad tissue by homogenization in a glass tissue grinder, and tissue debris was removed by centrifugation of the suspension at 200 g. Antigen was prepared from L. major promastigote stationary phase cultures as earlier described (Hodes, 1995). In short, cells were washed 4 times in PBS, the concentration adjusted to 1×108 cells/ml, and frozen/ thawed 3 times. The antigen was homogenized, aliquoted, and maintained at −20 °C until use.
Mice were injected subcutaneously in the left hind footpad with 1×106 metacyclic promastigotes. For the infection of germ-free Swiss/NIH mice, metacyclic promastigotes in Grace's medium were aseptically transferred into sterile glass ampoules. The ampoules were sealed, chemically sterilized with 8% peracetic acid and introduced into the isolator. Another ampoule treated in the same manner was taken out of the isolator and the promastigotes were used to infect conventional Swiss/NIH mice. Measurements of the footpad thickness were taken using a caliper. The lesion size was calculated by subtracting the value of the uninfected contralateral footpad from that of the infected one.
Parasites were quantified by limiting dilution, as previously described (Vieira et al. 1996). Briefly, single-cell suspensions from individual excised lesions were plated in log-fold serial dilutions in Grace's insect tissue culture medium starting with a 1[ratio ]10 dilution. Each sample was plated in quadruplicates and read 5 days after the beginning of the culture. Results are expressed as the mean of the negative log of the titre, i.e., the dilution corresponding to the last positive well.
Four animals from each group were sacrificed at 2, 6 and 13 weeks of infection and footpad tissues were collected and fixed in 10% neutral buffered formalin. Later the material was dehydrated, cleared, embedded in paraffin, cut (3–5 μm thick), and stained with haematoxylin and eosin for microscopical examination.
Spleen cells and a pool of popliteal lymph node cell suspensions were prepared as described previously (Oliveira et al. 2000). Pools of popliteal lymph nodes were used since our Swiss/NIH mice did not show a positive mixed lymphocyte reaction in several experiments performed (our unpublished observations; Ribeiro-Sobrinho et al. 2002). This result indicates that these animals are not genetically different as to their MHC antigens. The organs were homogenized in RPMI 1640 medium (Gibco Laboratories, Grand Island, NY, USA), the concentration of cells was adjusted to 5×106 cells/ml RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 g/ml streptomycin, 25 mM HEPES, and 0·05 mM-mercaptoethanol (Gibco Laboratories), and 1 ml was plated in 24-well tissue culture plates. Some cultures were stimulated in vitro with concanavalin A (ConA, 5 g/ml, Sigma Chemical Co., St Louis, MO, USA) or 50 μl of L. major antigen. Supernatants were harvested at 72 h for IFN-gamma and IL-4 analysis and at 24 h for tumor necrosis factor (TNF) and IL-12 assays.
Peritoneal exudate cells were harvested 5 days after injection of 1 ml of thioglycollate. The concentration of cells was adjusted to 1×106 cells/ml in RPMI 1640 (supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 g/ml streptomycin, 25 mM HEPES, and 0·05 mM β-mercaptoethanol) and 1 ml plated onto glass cover-slips in 24-well tissue culture plates in the presence or absence of recombinant IFN-gamma (100 U/ml, Genzyme, Cambridge, MA, USA). Cultures were incubated for 2 h at 37 °C, 5% CO2 and humidified atmosphere, after which the macrophages were exposed to 2 amastigotes per macrophage for 2 h. After this incubation, the supernatants containing non-adherent cells were removed and adherent cells were washed 3 times with warm RPMI 1640 medium to remove non-ingested amastigotes and fresh medium with or without IFN-gamma (100 U/ml) was added again. The cells were cultured for 72 h (37 °C, 5% CO2, humidified atmosphere). Supernatants were collected for NO assays. The percentage of macrophages containing intracellular amastigotes was determined by optical microscopy examination of stained cover-slips (Panótico Rápido Laborclin, Pinhais, PR, Brazil).
IFN-gamma, IL-4 and IL-12 were measured using specific 2-site ELISA as previously described (Oliveira et al. 2000). IFN-gamma was measured using R46A2 rat monoclonal antibody as a capture antibody and a rabbit polyclonal anti-IFN-gamma antiserum (kind gift from Dr J. P. Farrell, University of Pennsylvania, Philadelphia, PA, USA) was used as a secondary antibody followed by donkey horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). IL-4 was measured using 11B11 rat monoclonal antibody as a capture antibody and biotinylated BVD6 rat monoclonal antibody as a secondary antibody followed by streptavidin-peroxidase (Sigma Chemical Co. St Louis, MO, USA). In the IL-12 p40 ELISA, the mAb C17.15 and biotinylated C15.6 were used (kind gifts from Dr Giorgio Trinchieri, The Wistar Institute, Philadelphia, PA, USA). The levels of IFN-gamma, IL-4 and IL-12 p40 were calculated by comparison with a standard curve using recombinant IFN-gamma, IL-4 and IL-12 (Genzyme, Cambridge, MA, USA). The sensitivity for the assays were as follows: IFN-gamma: 30 pg/ml, IL-4: 0·2 U/ml and IL-12: 30 pg/ml. TNF-alpha was measured according to the manufacturer's instructions using a kit (Mouse TNF- DUOSET Genzyme Corporation, USA). Nitric oxide production was assessed using the Griess reagent (Green et al. 1982).
The data obtained in each experiment were analysed using the Student's t-test or the paired Mann Whitney test (non-parametric). Values of P<0·05 were considered statistically significant.
The outcome of the experimental infection by L. major in conventional and germ-free Swiss/NIH mice was compared by measuring the development of primary lesions after infection with L. major in the hind footpad (Fig. 1). As previously described, Swiss/NIH conventional mice infected with L. major developed a lesion at the site of infection, which was comparable to that of other resistant models (Oliveira et al. 1999), and eventually healed these lesions. In contrast, Swiss/NIH germ-free mice developed larger lesions than their conventional counterparts and were unable to heal over a period of 13 weeks after the infection (Fig. 1A–C). Accordingly, parasite numbers in footpads harvested from germ-free animals were higher than in footpads from conventional mice (Fig. 2). Furthermore, 13 weeks after infection, parasites were cultured from the spleens and liver in 4 out of 5 germ-free animals in 1 experiment, while parasites were not detected in the viscera of conventional mice at this time-point.

Fig. 1. Lesion sizes in conventional (black symbols and bars) and germ-free (white symbols and bars) mice infected with 1×106 metacyclic promastigotes of Leishmania major in the hind footpad. Results from 3 experiments are shown. (A) Lesion size in conventional and germ-free mice at 2, 6 and 13 weeks of infection taken from separate groups of mice, as they were sacrificed for experiments. Bars for second and sixth week represent the average of the footpad thickness of 8 animals, the results of the thirteenth week post-infection is the average of the lesions of 20 animals per group. Lines stand for the standard deviation of the means. (B) Lesion size from individual conventional (n=8) and germ-free (n=10) mice 13 weeks after infection. (C) Course of infection in conventional and germ-free mice, measurements were taken weekly inside isolators or in the conventional animal house using a caliper. Each point represents the mean of 5 mice per group; lines stand for the standard deviation of the means. *Indicates statistical difference, P<0·05.

Fig. 2. Parasite numbers in lesions from conventional (black bars) and germ-free (white bars) mice infected with 1×106 metacyclic promastigotes of Leishmania major. Parasite numbers were evaluated by limiting dilution, as described in the Materials and Methods section. Represented are the results of 1 of 2 experiments performed, with similar results. *Indicates statistical difference, P<0·05, # indicates P=0·073.
In order to determine if the presence of the microbiota would interfere with the inflammatory process induced by infection with L. major, fragments of lesions from conventional and germ-free mice were histologically examined. At the sixth week after infection, cutaneous lesions from conventional mice presented a diffuse and moderate chronic inflammatory process (Fig. 3A) with the presence of macrophages parasitized with L. major amastigotes (Fig. 3B). In germ-free mice an intense and diffuse chronic inflammatory reaction was observed, associated with necrotic areas (Fig. 3C). The cellular exudate was characterized by several macrophages loaded with amastigotes associated with the presence of necrotic cells (Fig. 3D).

Fig. 3. Skin sections of hind footpads lesions of conventional and germ-free Swiss/NIH mice infected with 1×106 metacyclic promastigotes of Leishmania major after 6 weeks post-infection. (A) Lesions from conventional mice showing a diffuse and moderate chronic inflammatory process (H.&E. 40×). (B) Higher magnification showing the presence of macrophages parasitized with L. major amastigotes (arrow) (H.&E. 400×). (C) Lesions from germ-free mice showing an intense and diffuse chronic inflammatory reaction (H.&E. 40×). (D) Higher magnification indicating several macrophages loaded with L. major amastigotes.
At the thirteenth week of infection sections of cutaneous lesions in footpads from conventional animals showed a moderate and diffuse chronic inflammatory reaction (Fig. 4A). The cellular exudate was comprised of macrophages, lymphocytes and rare neutrophils. Higher magnification showed no signs of parasites (Fig. 4B). In contrast, germ-free mice showed an intense and diffuse chronic inflammatory reaction (Fig. 4C), and the cellular exudate contained macrophages, lymphocytes and some plasma cells. Moreover, a severe chronic granulomatous inflammatory reaction was observed. The granuloma contained mainly vacuolated macrophages heavily parasitized with amastigotes (Fig. 4D).

Fig. 4. Skin sections of hind footpads lesions of conventional and germ-free Swiss/NIH mice infected with 1×106 metacyclic promastigotes of Leishmania major after 13 weeks post-infection. (A) Healed lesion from a conventional mouse (H.&E. 40×). (B) Higher magnification showing no signs of parasites, but showing a discreet inflammatory process (H.&E. 400×). (C) Lesion from germ-free mouse showing an intense and diffuse granulomatous inflammatory reaction (H.&E. 40×). (D) Higher magnification showing nodules of macrophages loaded with L. major amastigotes (arrow).
Early production of IL-12 and IFN-gamma is essential for resistance to infection with L. major (Heinzel et al. 1993; Sypek et al. 1993). Hence, we investigated the production of these cytokines by lymph node cells from Swiss/NIH conventional and germ-free mice 2 days after infection. As seen in Fig. 5, lymph node cells from both groups of animals secreted similar levels of IL-12 and IFN-gamma. Moreover, no differences in in vitro IFN-gamma production was found between the two groups at 2, 6 and 13 weeks after infection, both in lymph node and spleen cell cultures stimulated with L. major antigens (Fig. 6A and B). Importantly, IL-4 production was detected only in lymph node cell cultures, at the second week of infection and in similarly low levels in both groups of animal (Fig. 6C). At 2 weeks post-infection, similar levels of TNF-alpha were also found in cultures of lymph node cells from conventional and germ-free mice, and the addition of L. major antigen in vitro increased the production of TNF-alpha by lymph node cells (Fig. 6D).

Fig. 5. IL-12 (A) and IFN-gamma (B) production by lymph nodes cells from conventional and germ-free Swiss/NIH mice in the beginning of infection. Cells from non-infected animals (white bars) and from animals infected with 1×106 metacyclic promastigotes of Leishmania major for 2 days (hatched bars) were cultured in vitro for 24 h in the absence of antigen, as described in the Materials and Methods section. The bars represent the average of 4 animals per group, vertical lines stand for the standard deviations of the means. Data are from 1 of 2 experiments performed with similar results. ND, not detected.

Fig. 6. Cytokine production by conventional and germ-free Swiss/NIH mice infected with Leishmania major. (A) IFN-gamma production by antigen-stimulated lymph node cells from conventional (black bars) or germ-free (white bars) 2, 6 and 13 weeks after infection with L. major. (B) IFN-gamma production by antigen-stimulated spleen cells from conventional (black bars) or germ-free (white bars) 2, 6 and 13 weeks after infection with L. major. (C) IL-4 production by antigen-stimulated lymph node or spleen cells from conventional (black bars) or germ-free (white bars) mice 2 weeks after infection with L. major. (D) TNF-alpha production by lymph node cells from conventional and germ-free mice 2 weeks after infection in the presence (white bars) or absence (hatched bars) of Leishmania major antigens. Lymph node cells were pooled from 5 mice in each group. Spleen cell results are the mean production of individual cultures from 5 mice in each group. All experiments were repeated once and similar results were obtained.
Peritoneal macrophages from conventional and germ-free mice were infected in vitro with L. major amastigotes in the presence or absence of IFN-gamma and incubated for 72 h. It was found consistently that macrophages from germ-free mice were less effective in killing parasites than macrophages from conventional mice. Both macrophages were equally permissive to infection with parasites (percentage infection of 39·7±19·9 for cells from germfree mice and 30·2±24·3 for conventional mice). Similar results were found when promastigotes were used to infect macrophages (data not shown). However, a higher percentage of macrophages were infected when amastigotes were used, therefore results were more clear-cut in these conditions. Variability among experiments was very large. Still, when the data from 4 experiments were pooled and the results were expressed as the percentage reduction of infected macrophages upon activation with IFN-gamma (Fig. 7), a statistical difference was found, which indicated that, indeed, macrophages from germ-free mice were less effective in killing L. major when activated with IFN-gamma. Slightly less nitric oxide was produced by macrophages from germ-free mice in these cultures (88·3 mM±34·0 for germ-free and 121·5 mM±50·9 for conventional mice, P=0·062 by the Mann Whitney paired test).

Fig. 7. Killing of Leishmania major by elicited peritoneal macrophages from conventional or germ-free mice. The percentage reduction of the number of macrophages infected with L. major in response to treatment with 100 U/ml of IFN-gamma is presented. The percentages of infected macrophages in control (no IFN-gamma added) wells were 39·7±19·9 for cells from germ-free mice and 30·2±24·3 for conventional mice. The percentage of infected macrophages was determined after 72 h of culture, as described in the Materials and Methods section. A total of 400 macrophages were counted per group. Bars stand for the average of the 4 experiments performed, lines represent the standard deviations of the means. *Indicates statistically significant difference by the paired Mann Whitney test (P=0·0286).
Animals are associated with a variety of microorganisms, which are called the normal or indigenous microbiota (Berg, 1996). These associated microbiota influence the host homeostasis, and several studies have indicated that exposure to microorganisms may influence the host immune response and susceptibility to disease (Rodrigues et al. 1996; Vieira et al. 1998; Maia et al. 2001). Germ-free animals have been major tools in investigating host-microbiota relationships (Rodrigues et al. 1996; Vieira et al. 1998; Filho-Lima, Vieira and Nicoli, 2000; Maia et al. 2001). When infected with pathogens in the absence of the normal microbiota, animals may be either more resistant or more susceptible to parasites. Hence, intestinal microbiota is essential for the establishment of some gut infections (Phillips and Wolfe, 1959; Torres et al. 1992). On the other hand, infection with the intracellular parasite Trypanosoma cruzi is more severe in germ-free mice, as shown by a higher mortality, more intense parasitism, and more aggressive inflammatory response when compared to the infection in conventional mice (Silva et al. 1987; Furarah et al. 1991). Infection with Leishmania amazonensis was less severe in germ-free mice if compared to conventional controls when we used Swiss/CFW mice (Vieira et al. 1987). However, Swiss/NIH germ-free and conventional mice behaved similarly when infected with L. amazonensis, regardless of the association with the normal microbiota (our unpublished observation). We had previously shown that conventional Swiss/NIH mice develop an ultimately self-healing lesion around the inoculation site. This pattern of resistance to experimental L. major infection is similar to that observed in C57BL/6 mice, characterized as resistant to this infection (Oliveira et al. 1999). In addition, resistance to L. major in Swiss/NIH mice is associated with the development of a Th1-type immune response against L. major (Oliveira et al. 1999; and data presented herein), since similar levels of IFN-gamma are produced by both strains at 2 weeks of infection. We also found that germ-free mice infected with L. major while in the germ-free state and conventionalized 3 weeks after infection (i.e. associated with the normal mouse microbiota) had significantly larger lesions than the conventional controls. However, similar to conventional mice, these conventionalized animals resolved their lesions (Oliveira et al. 1999). Here, we show that germ-free mice infected with L. major and kept in the gnotobiotic state (associated only with L. major) for 13 weeks after infection failed to resolve lesions. Accordingly, these mice did not control parasite growth for the duration of the experiments, as determined both by limiting dilution and histopathological analysis. At the thirteenth week of infection, while conventional mice showed a discrete inflammatory reaction indicating regression of the inflammatory process, germ-free mice showed an intense chronic inflammatory reaction with many vacuolated macrophages, loaded with Leishmania amastigotes. Taken together, these results show that the presence of the indigenous microbiota is important for effective resistance to infection with L. major.
One mechanism that could explain the differences in behaviour between germ-free and conventional animals is that the microbiota would influence the kind of response that is made when animals are faced with infection. The ideal model to address this issue would the infection with L. major. IFN-gamma secreted by CD4+ T (Th1) lymphocytes is known to be essential for effective killing of parasites by macrophages (Titus, Kelso and Louis, 1984; Belosevic et al. 1989;). It has also been demonstrated that IFN-gamma secreted early during infection with L. major plays an important role in the development of Th1 phenotype (Titus et al 1984; Scharton and Scott, 1993); development of this phenotype was shown to be dependent on IL-12 (Scharton-Kersten et al. 1995). On the other hand, susceptibility to L. major infection in mice is characterized by the differentiation of CD4+ T lymphocytes into the Th2 subtype, which secretes high amounts of IL-4 (Locksley et al. 1991). IL-4 inhibits macrophage activation leading to high intracellular parasitism and a non-healing lesion at the inoculation site with L. major (Liew et al. 1989; Locksley et al. 1991). In addition, the development of the Th2 subtype of CD4+ T cells inhibits the development of a Th1 response and the activation of macrophages by IFN-gamma. This archetypal response correlated well with the initial findings by Parish (1972), Parish and Liew (1972) and Lagrange, Mackaness and Miller (1974) who had suggested that there was an inhibition of the production of antibodies to a given antigen when the cell-mediated immune response prevailed, and vice-versa. Additionally, McDonald and Carter (1979) established that the development of a delayed-type hypersensitivity to sheep red blood cells (a putative Th1 response) was dependent on the presence of the normal microbiota, while germ-free mice could produce antibodies to this antigen (a putative Th2 response) similar to conventional animals. Subsequently, it was shown that the normal microbiota enhanced IL-12 production in spleen-derived macrophages (Nicaise et al. 1999). Hence, it was tempting to predict that germ-free mice, which are more susceptible than conventional mice to infection with L. major, would tend to respond to this parasite in a Th2 fashion. Indeed, preliminary data using a different source of Swiss mice than the one used herein suggested that infection with L. major would trigger a higher level of IL-4 production by germ-free mice (Vieira and Scott, 1996). However, in this paper we show that the enhanced susceptibility of germ-free animals to L. major was not due to a higher production of IL-4. IFN-γ was produced at similar levels by germ-free and conventional animals in response to L. major at the time points investigated. Also, early production of IL-12 and IFN-γ in the lymph nodes, which are clearly shown to be essential for the development of a Th1 response during infection with L. major (Scharton and Scott, 1993; Scharton-Kersten et al. 1995) are similar in both groups of mice. Peritoneal cells from germ-free and conventional mice injected intraperitoneally with L. major produce equivalent levels of IL-12 (data not shown). Thus, the higher susceptibility of germ-free mice to L. major could not be explained by their bias toward a Th2 response.
Although the increased susceptibility of BALB/c mice to L. major seems to be related to the presence of the cytokine IL-4 (Scharton-Kersten et al. 1995; Launois et al. 1995, 1997), the reason for this bias towards IL-4 production is unknown. An early IL-4 burst in both susceptible and resistant strains occurs (Scharton-Kersten et al. 1995). It has been demonstrated that there is expansion of a memory IL-4 producing CD4+ T cell population a few hours after infection of BALB/c mice with L. major, but the same population is present and expands in B10D2 (resistant) mice (Julia et al. 2000; Malherbe et al. 2000). Interestingly, these memory cells bind to a peptide from the LACK antigen of Leishmania which sequence is homologous to peptides found in some subdominant components of the normal microbiota (Escherichia coli and Enterococcus faecalis) but not in other residual (Proteus mirabilis) or residual and potentially pathogenic (Clostridium perfringens) components of the microbiota. Hence, when conventional BALB/c mice were decontaminated with antibiotics the early IL-4 burst was abrogated. In addition, germ-free BALB/c mice did not present the early IL-4 production found in conventional mice. Germ-free BALB/c mice were, however, as susceptible as conventional mice to L. major, ruling out the importance of the microbiota-primed memory cell-mediated early IL-4 production in susceptibility. Moreover, contrary to conventional BALB/c mice, germ-free BALB/c mice were still somewhat susceptible to L. major after anti-IL-4 treatment (Julia et al. 2000). Here we show that in Swiss/NIH mice (a resistant mouse strain) the germ-free state confers greater susceptibility to infection with L. major and that this susceptibility does not correlate with higher IL-4 production. We had previously shown that conventional Swiss mice produce lower levels of IL-4 than BALB/c mice, and that the IL-4 production by Swiss mice at 4 weeks of infection was similar to that of C57BL/6 mice (Oliveira et al. 1999). Hence, the levels of IL-4 found at 2 weeks of infection are not typical of a Th2 response. More importantly, mice remain susceptible even whilst producing similar levels of IFN-gamma to conventional mice. It is important to note that lesions found in germ-free Swiss mice are in no way similar to the ones found in BALB/c mice: BALB/c mice present large uncontrolled lesions that can reach 7 mm (Oliveira et al. 1999) while germ-free Swiss mice displayed non-healing lesions that were always smaller than 3 mm. Furthermore, the histological aspect of the lesions was also very diverse: Swiss/NHI germ-free mice had a large lymphocytic infiltrate that is mostly absent in BALB/c mice (Santiago et al. 1999). One possible explanation for the higher susceptibility would be that germ-free mice produce higher levels of IL-10, since this cytokine has also been shown to be important for susceptibility to L. major (Noben-Trauth et al. 2003). Indeed, during acute inflammation, germ-free mice produce higher levels of IL-10 (Souza et al. 2004). Moreover, spleen cells from germ-free mice, when cultured with Trypanosoma cruzi homogenate, produce higher levels of IL-10 than cells from conventional mice, albeit there is no difference in the IL-10 levels during infection (Duarte et al. 2004). This possibility deserves further investigation and would explain the deficient activation of macrophages to kill parasites.
Peritoneal and bone-marrow macrophages from germ-free mice that were exposed to the normal microbiota as adults produce higher levels of IL-1, IL-6 and TNF-alpha than macrophages from germ-free mice (Nicaise et al. 1993). Subsequently, it was shown that the normal microbiota enhanced IL-12 production in spleen-derived macrophages (Nicaise et al. 1999). Pro-inflammatory cytokine basal levels were also higher in sera from microbiota-associated mice (Dahlgren, Midtvedt and Tarkowski, 1995; Nicaise et al. 1999). Germ-free mice show impaired clearance of bacteria injected intravenously (Neumann et al. 1998; Rodrigues et al. 2000) and respond to this stimulus with secretion of TNF-α, IL-12 and IFN-γ more slowly than mice associated with one gut microorganism (Rodrigues et al. 2000). Hence, macrophages from mice that are exposed to the normal microbiota would seem to be in a more activated state than macrophages from an animal that has not been exposed to living bacteria in their organism. Although germ-free animals are not antigen-free and are not free of exposure to dead bacteria present in commercial diets, the presence of the conventional microbiota seems to keep macrophages secreting cytokines at higher basal levels. Thus, it is not surprising that macrophages from germ-free mice could not be activated to kill L. major as efficiently as macrophages from conventional mice. In a different experimental model we showed that germ-free mice produce more IL-10 than conventionals (data not shown). Since IL-10 is a potent inhibitor of macrophage activation (Gazzinelli et al. 1992; Oswald et al. 1992), we could hypothesize that, in spite of the addition of IFN-gamma in vitro and of the presence of IFN-gamma and TNF-alpha in vivo, macrophages from germ-free mice were not as activated as macrophages from conventional mice, which allowed growth of parasites.
The hygiene hypothesis states that in the absence of a certain level of exposure to microorganisms, an exacerbated immune response may occur, either of the Th1 or of the Th2 type (Wills-Karp, Santeliz and Karp, 2001; Yazdanbakhsh, Kremsner andVan Ree, 2002). On the other hand, recent data have pointed to IL-6 as a putative regulator of the inflammatory response, even before it is triggered (Hori, Nomura and Sakaguchi, 2003; Pasare and Medzhitov, 2003; Powrie and Maloy, 2003). Our data show that the lack of antigenic stimulus in the adult mouse does not induce an exacerbated immune response against a single invasive microorganism. On the contrary, it seems that the effector mechanisms in germ-free mice are kept on hold due to the lack of constant stimulation by the normal microbiota.
In conclusion, we have shown that the inability of germ-free mice to heal lesions during L. major infection could not be explained by low levels of IFN-gamma or higher production of IL-4. Instead, higher susceptibility to this parasite correlated with a less efficient killing of parasites in vitro by macrophages exposed to IFN-gamma. Hence, our results indicate that the normal microbiota does not bias towards a type 1 or type 2 immune response, but seems to be essential for the maintenance of macrophages in a more activated state that allows them to be responsive to activating stimuli.
This work was supported by FAPEMIG grant number CBS 117895 and NIH grant number AI-35914. J.R.N., E.C.V., M.N.M. and L.Q.V. are recipients of CNPq fellowships, M.R.O. was a fellow of CAPES and was on leave from the Universidade Federal da Paraíba, PB, Brazil. The authors are indebted to Ronilda Maria de Paula (in memorium), Maria Helena Alves de Oliveira and Antonio Mesquita Vaz for technical assistance and animal care. VARIG Airlines transported matrices and experimental germ-free animals to Brazil with great efficiency, care and expertise.
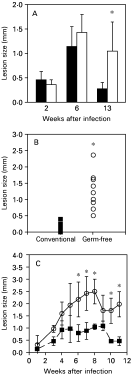
Fig. 1. Lesion sizes in conventional (black symbols and bars) and germ-free (white symbols and bars) mice infected with 1×106 metacyclic promastigotes of Leishmania major in the hind footpad. Results from 3 experiments are shown. (A) Lesion size in conventional and germ-free mice at 2, 6 and 13 weeks of infection taken from separate groups of mice, as they were sacrificed for experiments. Bars for second and sixth week represent the average of the footpad thickness of 8 animals, the results of the thirteenth week post-infection is the average of the lesions of 20 animals per group. Lines stand for the standard deviation of the means. (B) Lesion size from individual conventional (n=8) and germ-free (n=10) mice 13 weeks after infection. (C) Course of infection in conventional and germ-free mice, measurements were taken weekly inside isolators or in the conventional animal house using a caliper. Each point represents the mean of 5 mice per group; lines stand for the standard deviation of the means. *Indicates statistical difference, P<0·05.
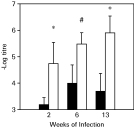
Fig. 2. Parasite numbers in lesions from conventional (black bars) and germ-free (white bars) mice infected with 1×106 metacyclic promastigotes of Leishmania major. Parasite numbers were evaluated by limiting dilution, as described in the Materials and Methods section. Represented are the results of 1 of 2 experiments performed, with similar results. *Indicates statistical difference, P<0·05, # indicates P=0·073.

Fig. 3. Skin sections of hind footpads lesions of conventional and germ-free Swiss/NIH mice infected with 1×106 metacyclic promastigotes of Leishmania major after 6 weeks post-infection. (A) Lesions from conventional mice showing a diffuse and moderate chronic inflammatory process (H.&E. 40×). (B) Higher magnification showing the presence of macrophages parasitized with L. major amastigotes (arrow) (H.&E. 400×). (C) Lesions from germ-free mice showing an intense and diffuse chronic inflammatory reaction (H.&E. 40×). (D) Higher magnification indicating several macrophages loaded with L. major amastigotes.

Fig. 4. Skin sections of hind footpads lesions of conventional and germ-free Swiss/NIH mice infected with 1×106 metacyclic promastigotes of Leishmania major after 13 weeks post-infection. (A) Healed lesion from a conventional mouse (H.&E. 40×). (B) Higher magnification showing no signs of parasites, but showing a discreet inflammatory process (H.&E. 400×). (C) Lesion from germ-free mouse showing an intense and diffuse granulomatous inflammatory reaction (H.&E. 40×). (D) Higher magnification showing nodules of macrophages loaded with L. major amastigotes (arrow).
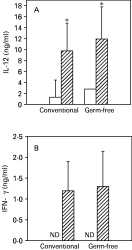
Fig. 5. IL-12 (A) and IFN-gamma (B) production by lymph nodes cells from conventional and germ-free Swiss/NIH mice in the beginning of infection. Cells from non-infected animals (white bars) and from animals infected with 1×106 metacyclic promastigotes of Leishmania major for 2 days (hatched bars) were cultured in vitro for 24 h in the absence of antigen, as described in the Materials and Methods section. The bars represent the average of 4 animals per group, vertical lines stand for the standard deviations of the means. Data are from 1 of 2 experiments performed with similar results. ND, not detected.

Fig. 6. Cytokine production by conventional and germ-free Swiss/NIH mice infected with Leishmania major. (A) IFN-gamma production by antigen-stimulated lymph node cells from conventional (black bars) or germ-free (white bars) 2, 6 and 13 weeks after infection with L. major. (B) IFN-gamma production by antigen-stimulated spleen cells from conventional (black bars) or germ-free (white bars) 2, 6 and 13 weeks after infection with L. major. (C) IL-4 production by antigen-stimulated lymph node or spleen cells from conventional (black bars) or germ-free (white bars) mice 2 weeks after infection with L. major. (D) TNF-alpha production by lymph node cells from conventional and germ-free mice 2 weeks after infection in the presence (white bars) or absence (hatched bars) of Leishmania major antigens. Lymph node cells were pooled from 5 mice in each group. Spleen cell results are the mean production of individual cultures from 5 mice in each group. All experiments were repeated once and similar results were obtained.
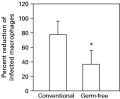
Fig. 7. Killing of Leishmania major by elicited peritoneal macrophages from conventional or germ-free mice. The percentage reduction of the number of macrophages infected with L. major in response to treatment with 100 U/ml of IFN-gamma is presented. The percentages of infected macrophages in control (no IFN-gamma added) wells were 39·7±19·9 for cells from germ-free mice and 30·2±24·3 for conventional mice. The percentage of infected macrophages was determined after 72 h of culture, as described in the Materials and Methods section. A total of 400 macrophages were counted per group. Bars stand for the average of the 4 experiments performed, lines represent the standard deviations of the means. *Indicates statistically significant difference by the paired Mann Whitney test (P=0·0286).