Introduction
Botanical diversity plays a crucial role in the successful functioning of all natural ecosystems, as well as in part providing an essential resource for human utilization. However, evidence suggests that during the last millennium, human exploitation has led to an exponential loss in plant biodiversity and the variation existing within communities, species and genes (Hawkes et al., Reference Hawkes, Maxted and Ford-Lloyd2000). For example, approximately 34,000 plant species, from an estimated worldwide total of 270,000, are currently facing extinction (Walter and Gillett, Reference Walter and Gillett1998). It remains difficult, if not impossible, to estimate precise rates of loss of potentially exploitable genetic diversity, but it has been suggested that the combination of a growing human population combined with climatic instability is likely to accentuate the devastating loss of plant resources, with all its negative economic, political and social consequences for humanity (Hawkes et al., Reference Hawkes, Maxted and Ford-Lloyd2000). Worldwide concern for biodiversity loss and its likely impact has provided the main driving force for the implementation of global conservation initiatives. However, bearing in mind the practical financial and temporal limitations for conservation, the Convention on Biological Diversity recognized the need to improve the efficiency and effectiveness of current conservation actions (CBD, 1992). One approach to achieve this goal is to compare natural diversity with current conservation actions, and then having identified the gaps, instigate a revised conservation strategy. This approach would be widely recognized as conservation gap analysis.
There is now an extensive literature associated with gap analysis and broader conservation evaluation techniques in ecosystem conservation, which essentially identifies areas in which selected elements of biodiversity are under-represented (Margules, Reference Margules1989; Margules and Pressey, Reference Margules and Pressey2000; Balmford, Reference Balmford2003; Brooks et al., Reference Brooks, Bakarr, Boucher, Da Fonesca, Hilton-Taylor and Hoekstra2004; Dietz and Czech, Reference Dietz and Czech2005; Riemann and Ezcurra, Reference Riemann and Ezcurra2005). Burley (Reference Burley, Wilson and Peter1988) identified four steps in gap analysis: (1) identifying and classifying biodiversity; (2) locating areas managed primarily for biodiversity; (3) identifying biodiversity that is under-represented in the managed areas; (4) setting priorities for conservation action. This basic methodology can equally be applied to taxonomic and genetic diversity and its distribution in existing wild populations, as was illustrated in the recent application for cowpea [Vigna unguiculata (L.) Walp.] and its wild relatives from Africa (Maxted et al., Reference Maxted, Mabuza-Diamini, Moss, Padulosi, Jarvis and Guarino2004). For crop wild relative (CWR) conservation, the four steps involve: (1) identifying priority taxa; (2) using distributional data to identify ecogeographic breadth and complementary hotspots; (3) matching current conservation actions with the identified ecogeographic breadth and complementary hotspots to existing protected area networks (identifying the so-called ‘gaps’); (4) formulating a revised in situ and ex situ conservation strategy (Maxted et al., Reference Maxted, Dulloo, Ford-Lloyd, Iriondo and Jarvis2008).
The wheat gene pool is an ideal candidate for this application of what might be referred to as CWR gap analysis. It is the most widely consumed crop and provides the staple food for 35% of the world population (Hedge et al., Reference Hedge, Valkoun and Waines2000; Lage et al., Reference Lage, Warburton, Crossa, Skovmand and Anderson2003), with annual production levels of 632 and 627 million tons for 2004/2005 and 2005/2006, respectively (FAOSTAT, 2007). Bread wheat (Triticum aestivum, six times), durum wheat (Triticum turgidum subsp. durum, four times), emmer wheat (Triticum turgidum subsp. dicoccoides, four times) and einkorn wheat (Triticum monococcum subsp. monococcum, two times) are all cultivated species and form the primary wheat gene pool (GP-1), along with their associated wild relatives within the Triticum genus (Hedge et al., Reference Hedge, Valkoun and Waines2000). The secondary wheat gene pool (GP-2) comprises mostly species of the genus Aegilops, with an additional species (Amblyopyrum muticum) belonging to the genus Amblyopyrum (Manners and van Slageren, Reference Manners and van Slageren1998). The inter-generic hybridization between Triticum and Aegilops species, both in nature and cultivation, illustrates the close genetic links between the two genera (van Slageren, Reference van Slageren1994). For example, studies have shown that tetraploid cultivated wheats have resulted from the hybridization of wild goat grass (Aegilops speltoides) carrying the B genome and the diploid Triticum urartu species carrying the A genome (Hedge et al., Reference Hedge, Valkoun and Waines2000). In addition, studies have also provided evidence that hexaploid bread wheat (T. aestivum) has developed from a cross between tetraploid cultivated wheat and Aegilops tauschii, which has been identified as the donor of the D genome (Dvorák et al., Reference Dvorák, Luo, Yang, Damania, Valkoun, Willcox and Qualset1998).
The genus Aegilops L. is a member of the grass family (Poaceae) comprising 22 species inclusive of ten diploids, ten tetraploids and two hexaploids (Manners and van Slageren, Reference Manners and van Slageren1998). The genus has been characterized as a Mediterranean–Western Asiatic element comprising species that occur in both the Mediterranean and Irano-Turanian regions (Hedge et al., Reference Hedge, Valkoun and Waines2002). Transcaucasia is the proposed centre of origin for this genus, with suggestions that its centre of diversity follows the Fertile Crescent arc in Western Asia (van Slageren, Reference van Slageren1994). Aegilops species predominantly grow along roadsides, at the edges of cultivation and among crops (Hedge et al., Reference Hedge, Valkoun and Waines2000). For the past half-century, agricultural scientists have been engaged in the development of technologies that facilitate the utilization of Aegilops species as a gene source for the improvement of wheat production to meet the demands set by a rapidly growing population (Rajaram, Reference Rajaram2000). Monneveux et al. (Reference Monneveux, Zaharieva, Rekika, Royo, Nachit, Fonzo and Araus2000) reviewed recent progress, providing a summary of studies that had successfully transferred the tolerance of both abiotic and biotic stresses into cultivated wheat. Studies have demonstrated the successful introduction of disease and pest resistance, as well as drought, salt and frost tolerance, and the improvement of yield and quality (Lage et al., Reference Lage, Warburton, Crossa, Skovmand and Anderson2003).
However, estimations suggest that by 2020, the global demand for wheat could reach around 1000 million tons (Rajaram, Reference Rajaram2000). As a result, wheat breeders have expressed great concerns over the remaining variability that exists within the narrow wheat gene pool, and suggest that, at present, it remains insufficient to achieve future breeding objectives (Hedge et al., Reference Hedge, Valkoun and Waines2002). As such, species from the genus Aegilops are likely to play a fundamental role in providing an invaluable source of genetic diversity to expand the genetic base of wheat cultivars (Lage et al., Reference Lage, Warburton, Crossa, Skovmand and Anderson2003), and it is critical that Aegilops is effectively conserved and made available to breeders for utilization. The objective of this paper is to present an ecogeographic, GIS and gap analysis of the genus Aegilops and propose a complementary ex situ and in situ conservation status for the genus. The classification of Aegilops followed throughout the paper is that proposed by van Slageren (Reference van Slageren1994).
Material and methods
Data editing
The data presented in this paper were derived from a database compiled of 9866 unique germplasm accessions of Aegilops species compiled from four datasets. The largest proportion came from the Global Database of Wheat Wild Relatives (ICARDA, Syria), with additional data from the CGIAR system-wide information network for genetic resources (SINGER – http://singer.grinfo.net) genebank accession databases online, the European Plant Genetic Resources Internet Search Catalogue (EURISCO – http://eurisco.ecpgr.org/index.php) and the US National Plant Germplasm System (Germplasm Resources Information Network – http://www.ars-grin.gov/cgi-bin/npgs/html/index.pl). Data were standardized to a single format and duplicate observations were identified and removed to avoid biasing the final results. In addition, observations representing individuals that occur outside their documented natural range and were considered to be introductions were also removed from the dataset. In situations where the latitude and longitude data were missing, but a description of the locality was provided, online gazetteers were used to determine the geographical coordinates. In addition, the distribution of all the geo-referenced data was checked using the computer program ArcView GIS version 3.3. Each data field was indexed, and errors and invalid entries were manually corrected. Basic statistics describing the taxonomic, geographic, curatorial and ecological data were derived for the database content. The combined, corrected dataset of Aegilops germplasm accessions was then spatially analysed. The database is freely available from the senior author on request.
Ex situ conservation gap analysis
The computer program ArcGIS version 8.0 was used to produce distribution maps from the Aegilops dataset for each of the 22 species. In addition, DIVA-GIS version 5.2 (www.diva-gis.org) and the global climatic data with 2.5 min resolution (diva_worldclim_2-5m.zip) were used in the Bioclim method (Hijmans et al., Reference Hijmans, Guarino, Jarvis, O'Brien, Mathur, Bussink, Cruz, Barrantes and Rojas2005) to produce predictive distribution maps based on the climatic data. For each Aegilops species, a comparison was made between the distribution map based on the actual ex situ germplasm accession data and the predicted distribution maps generated from their climatic envelope data. Ex situ conservation gaps were identified as regions where the species was predicted to occur but had not been previously collected, areas predicted to be under-sampled. The level of the ex situ conservation priority for each of the Aegilops species was ranked (high, medium and low) as follows:
High priority. Species with < 200 germplasm accessions conserved ex situ and/or species for which ex situ collections inadequately represented their geographic range with several predicted under-sampled regions.
Medium priority. Species well represented in ex situ collections across their geographic range, with only a few predicted under-sampled regions, but with < 500 germplasm accessions conserved ex situ.
Low priority. Species well represented throughout their geographic range with >500 germplasm accessions conserved ex situ and only a few, if any, under-sampled areas predicted.
In situ species richness and complementarity analysis
The DIVA-GIS software was used to identify the optimal locations for the establishment of future in situ reserves required to conserve the maximum species diversity within the Aegilops genus. Firstly, the ‘number of different classes (richness)’ method (Hijmans et al., Reference Hijmans, Guarino, Jarvis, O'Brien, Mathur, Bussink, Cruz, Barrantes and Rojas2005) was used to map the distribution of species richness in order to identify hotspot regions. The circular neighbourhood point-to-grid method was selected with a default cell size of 1° resolution. It is worth noting that, in general, botanists do not sample diversity randomly, and the bias created from collecting in easily accessible areas or in countries and regions where the botanist considers a large number of species has to be considered when interpreting maps of species richness. To address this issue, the ‘number of observations’ method (Hijmans et al., Reference Hijmans, Guarino, Jarvis, O'Brien, Mathur, Bussink, Cruz, Barrantes and Rojas2005) was also used in DIVA, to map the density of germplasm collections for all Aegilops species. Secondly, DIVA-GIS was used to study species complementarity using the iterative procedure (Rebelo and Siegfried, Reference Rebelo and Sigfried1992; Rebelo, Reference Rebelo1994) in the ‘reserve selection’ manner described by Hijmans et al. (Reference Hijmans, Guarino, Jarvis, O'Brien, Mathur, Bussink, Cruz, Barrantes and Rojas2005), which identifies the minimum number of 100 × 100 km2 grid cells that are complementary to each other in maximizing conserved Aegilops diversity.
It is often assumed that when recommending a site for the establishment of a genetic reserve for CWR species, it will be within an existing protected area (Maxted et al., Reference Maxted, Ford-Lloyd and Hawkes1997; Heywood and Dulloo, Reference Heywood and Dulloo2006; Iriondo et al., Reference Iriondo, Dulloo and Maxted2008), but Aegilops like many other CWR species are located both within and outside existing protected areas. The reasons for locating reserves in existing protected areas are that (1) these sites already have an associated long-term conservation ethos and are less prone to hasty management changes to situations where conservation value and sustainability are not considered; (2) it is relatively easy to amend the existing site management structure to facilitate genetic conservation of CWR species; (3) creating novel conservation sites can be avoided, avoiding possibly prohibitive costs of acquiring previously non-conservation managed land (Iriondo et al., Reference Iriondo, Dulloo and Maxted2008). Therefore, often the simplest way forward in economic and political terms is for countries to locate genetic reserves in existing protected areas. As such, DIVA-GIS was used to compare the distribution of complementary hotspot sites with the World Database on Protected Areas (WDPA – www.unep-wcmc.org). A spatial comparison between the complementary hotspots identified and the WDPA highlights potential National protected areas in optimal locations for the establishment of the active in situ conservation of Aegilops species.
Results
Database content
Looking first at the crude numbers of accessions and the duplication of accessions between the four primary datasets containing Aegilops accessions, by far the largest dataset is held by ICARDA with 12,476 accessions included the Global Database of Wheat Wild Relatives, SINGER includes 3569 accessions, EURISCO 7684 accessions and GRIN 3626 accessions. Comparing the accessions included the Global Database of Wheat Wild Relatives with those found in SINGER, the number in the latter are fewer but almost a complete duplicate of the former with just two additional Aegilops accessions held by CIMMYT. However, there appears to be no duplication between the Global Database of Wheat Wild Relatives and EURISCO or GRIN accessions, but eight accessions are found in both the EURISCO and GRIN datasets. Care must be taken when interpreting these results as due to extensive missing data further actual duplication between the datasets may have passed unrecognized.
Following the merging and standardization of the four datasets, duplicate observations, obvious erroneous records and those with extensive missing data were removed to provide the Aegilops accession dataset for the analysis. This significantly reduces the number of combined accessions from 27,355 to 9866, only 36.06% of the original accessions were included in the analysis. Although this includes the duplicates between information systems, it also highlights the lack of sufficiently detailed passport data for many accessions, which must seriously hamper these accessions' utilization potential.
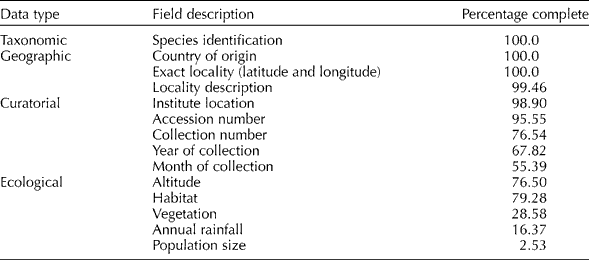
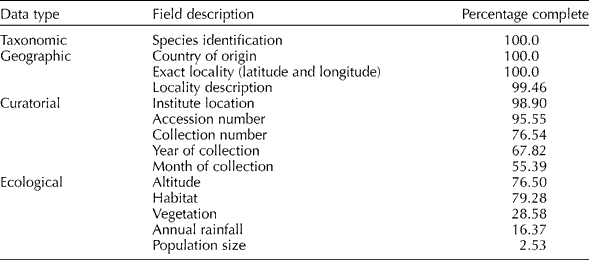
The final Aegilops accession dataset had a near-complete content for taxonomic and geographic characters for the 9866 observations (Table 1), which contrasts with the curatorial and ecological data where much data were unavailable. The database content will ultimately reflect the quality of the results produced from the ecogeographic and GIS analysis, but the relative quantities of taxonomic, geographic, curatorial and ecological data collated is similar to previous studies by Maxted et al. (Reference Maxted, van Slageren, Rihan, Guarino, Ramanatha Rao and Reid1995, Reference Maxted, Mabuza-Diamini, Moss, Padulosi, Jarvis and Guarino2004).
Table 1 Percentage completion for selected database fields
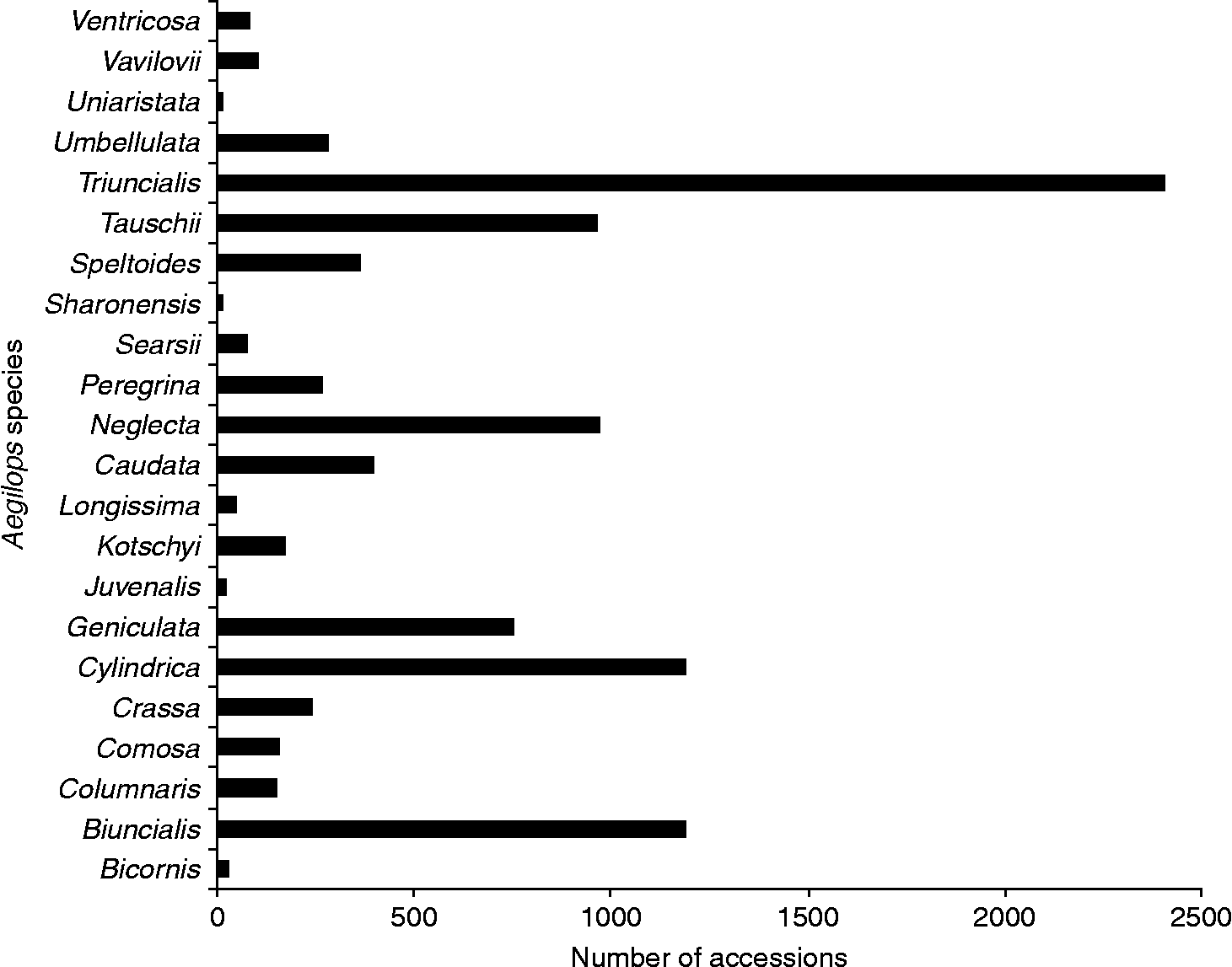
The most frequently collected species have been Aegilops triuncialis (2404), Aegilops cylindrica (1190), Aegilops biuncialis (1190), Aegilops neglecta (969) and Aegilops tauschii (962), which together account for 68.06% (6715 accessions) of the records (Fig. 1). By contrast, those species with the least number of accessions ( < 50) conserved ex situ include Aegilops sharonensis (11), Aegilops uniaristata (15), Aegilops juvenalis (20), Aegilops bicornis (29) and Aegilops longissima (48), which together only account for just over 1% (123) of the collections. It should be noted that the numbers above reflect unique accessions; some species have relatively large numbers of accessions available, but these figures mask widespread genebank duplication of larger samples or repeated sampling of a small number of populations, as is believed to be the case for Ae. longissima and Ae. sharonensis.
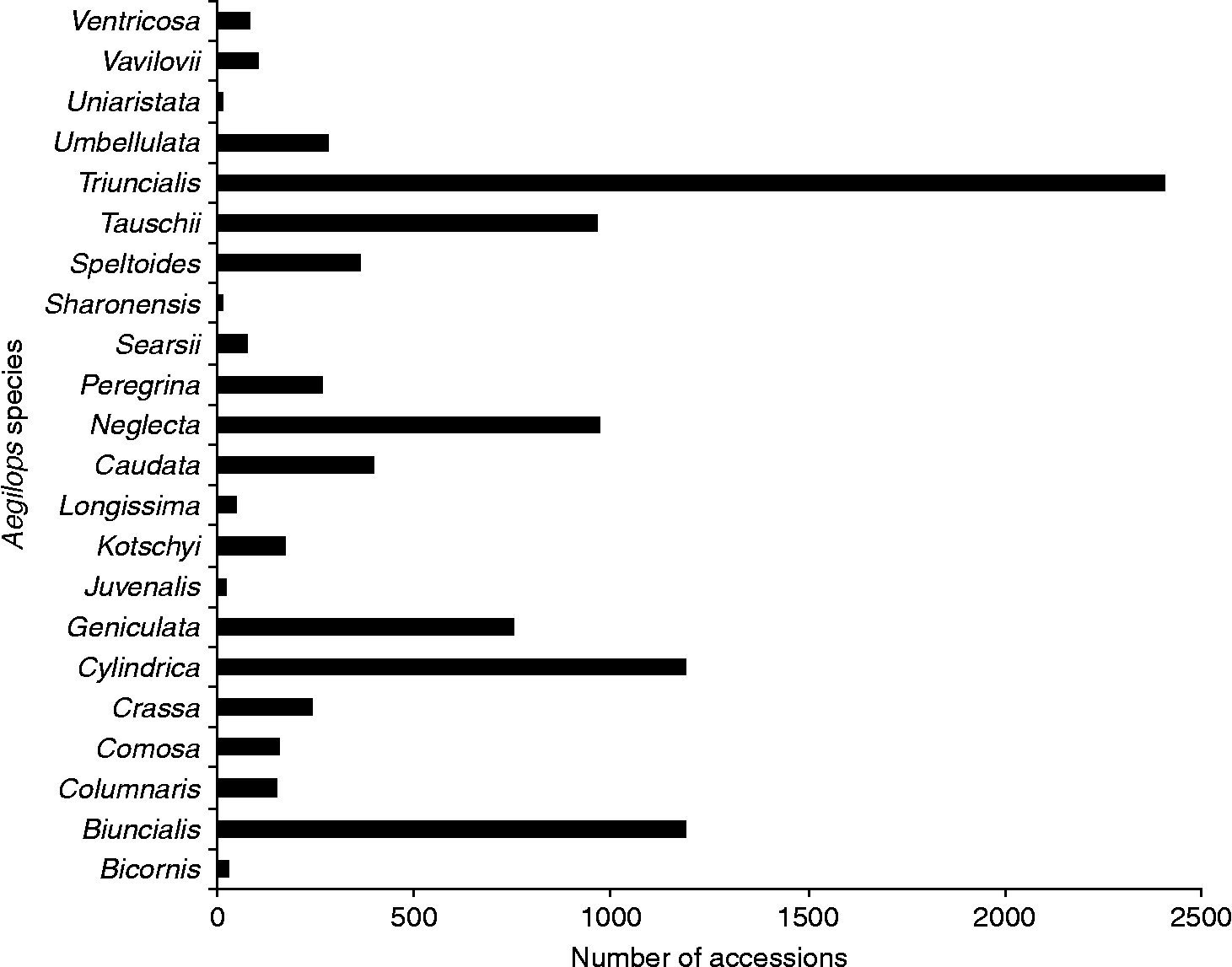
Fig. 1 Number of germplasm accessions for each Aegilops species.
Geographic distribution
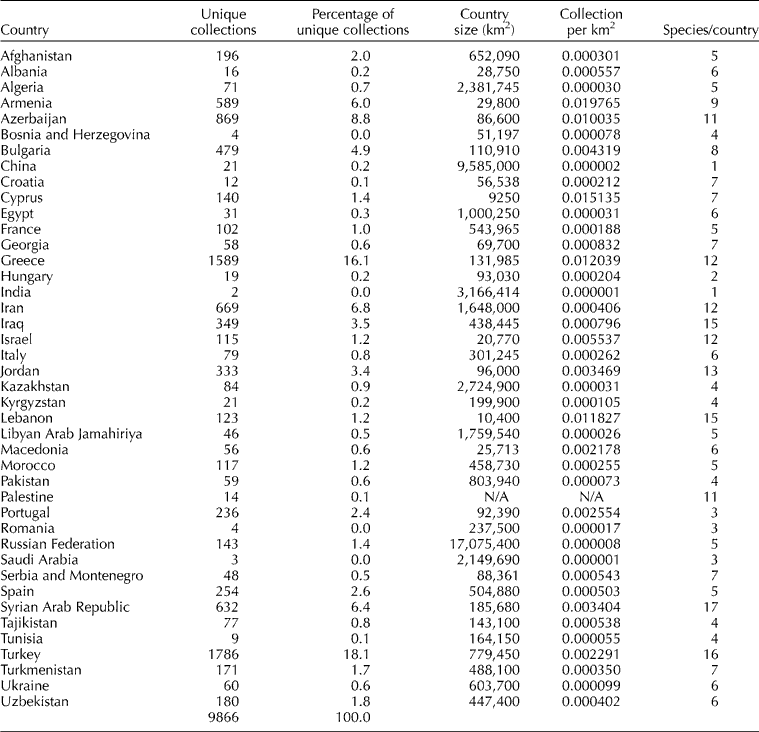
The genus Aegilops has been characterized as a Mediterranean–Western Asiatic element by van Slageren (Reference van Slageren1994) and analysis of the collections showed that 68% (6768 unique accessions) were collected in Central and West Asia and North Africa (CWANA), with the remaining 32% (3098 accessions) from Europe. Just over half the records in the database (56.2%) were collected from five countries: Turkey, which alone accounts for 18.1% of the records, Greece, Azerbaijan, Iran and Syria (Table 2). However, absolute country counts do not take into account of the relative size of the country itself, therefore the countries with the highest concentration of accessions collected per unit area are: Armenia, Cyprus, Greece, Lebanon and Azerbaijan.
Table 2 Geographic distribution of Aegilops collections

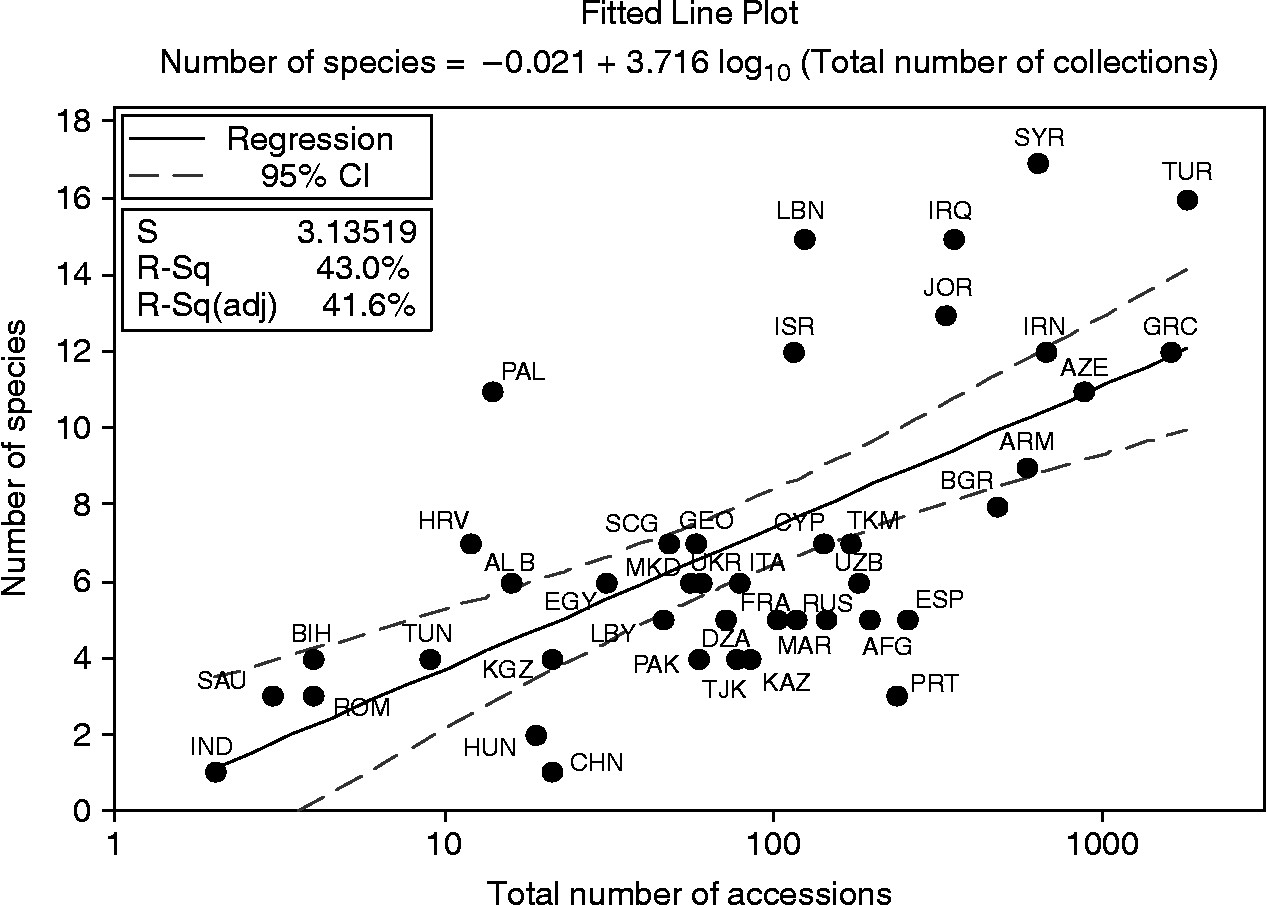
The countries with the highest Aegilops species richness were Syria (17 species), Turkey (16 species) and Iraq (15 species). However, it has been suggested that unequal sampling across a species native range may lead to underestimation of species richness in the under-sampled areas (Maxted et al., Reference Maxted, Mabuza-Diamini, Moss, Padulosi, Jarvis and Guarino2004). This was tested using the regression analysis of the number of Aegilops species recorded in each country and the number of accessions collected from that country. The regression line (y = −0.021 + 3.716 log10x) with 95% confidence intervals is presented in Fig. 2. The use of regression analysis enables over-sampled countries, in relation to the total sampling effort, to be identified below the lower confidence interval, and under-sampled countries, in comparison with the total sampling effort, to be identified above the upper confidence interval (Maxted et al., Reference Maxted, Mabuza-Diamini, Moss, Padulosi, Jarvis and Guarino2004). The regression analysis illustrated that none of the countries rich in Aegilops species can be considered over-sampled. In fact, Fig. 2 suggests that these countries can each be considered under-sampled, highlighting the potential for finding additional Aegilops diversity.
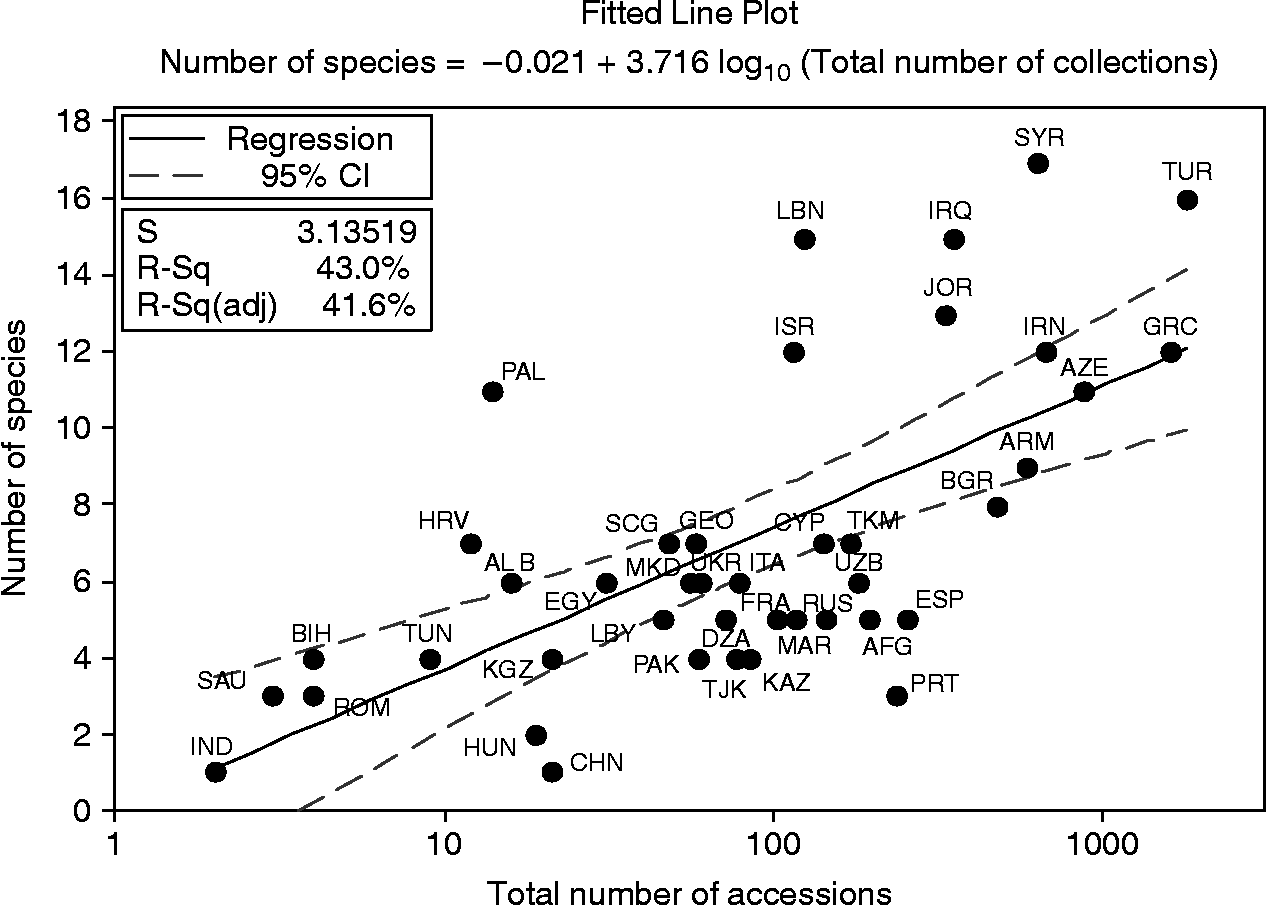
Fig. 2 Regression of Aegilops species against accessions for each country.
Ex situ conservation gap analysis
A comparison between the distribution and the predicted distribution maps produced using GIS provides an indication of the ex situ conservation status of each Aegilops species. The predicted size and range of these potential gaps varied considerably between species, highlighting differences in their ex situ representation and their conservation status. A summary of this analysis for all 22 Aegilops species and the ex situ collection priorities is presented below.
Section: Aegilops
Ae. biuncialis Vis. is relatively well represented in ex situ collections throughout its native geographic range. The majority of germplasm accessions have been collected in western Asia, Caucasus and south-eastern Europe, with the exception of Italy which is identified as a potential conservation gap. However, relatively few ex situ accessions have been collected in south-western Europe and northern Africa, even considering the distribution of Ae. biuncialis is likely to be less frequent in these areas. However, possible conservation gaps are identified in western Spain and the northern coastal regions of both Algeria and Morocco.
Aegilops columnaris Zhuk. appears relatively well represented in ex situ collections across its native geographic range, but Jordan, Lebanon, Armenia and Azerbaijan appear more intensely collected than other countries. The conservation gap analysis predicts gaps in north-western Iran, where Ae. columnaris appears inadequately sampled, and also western and central regions of Turkey. A few germplasm accessions suggest that the distributional range of Ae. columnaris extends as far as Afghanistan and Turkmenistan in Central Asia. Climatic predictions show that this species may also extend into southern Uzbekistan and western Tajikistan, suggesting that future exploration in these regions may prove fruitful.
Aegilops geniculata Roth appears relatively well represented in ex situ collections across the majority of its native geographic range. Ex situ collection has been particularly abundant in Lebanon, Israel, western Turkey, Greece, northern Morocco and southern Portugal. A comparison between the distribution of ex situ collections and the predicted distribution of Ae. geniculata suggests that further collection in Spain, southern Greece and south-western Turkey is desirable.
Aegilops kotschyi Boiss. appears inadequately represented in ex situ collections. The majority of germplasm accessions have been collected in eastern Jordan, southern Syria and northern Iraq. The conservation gap analysis for this species identifies Cyprus, north-western Libya, Tunisia and north Syria as potential under-sampled areas. In addition, Ae. kotschyi is also predicted to possibly occur in northern Algeria, Morocco and southern Spain, but Tutin and Humphries (Reference Tutin, Humphries and Tutin1980) suggested that this species is not a European native and its geographic range only extends as far as Tunisia in northern Africa. Thus, further exploration of the distribution range of Ae. kotschyi is required.
Ae. neglecta Req. ex Bertol. is relatively well represented in ex situ collections. However, a comparison between the actual and predictive distributions of Ae. neglecta suggests that gaps in the ex situ conservation of this species occur in the northern coastal regions of north Africa.
Aegilops peregrina (Hack. in J. Fraser) Maire & Weiller appears inadequately represented in ex situ collections. This species has been intensely collected in areas of western Asia, particularly in Israel, Lebanon, Jordan and Cyprus. The analysis predicts that Ae. peregrina is likely to be also found on the south-west coast of Turkey, which is confirmed from the flora (Davis, Reference Davis and Davis1985) but no germplasm collections are available. van Slageren (Reference van Slageren1994) indicated that Ae. peregrina is limited in its African distribution to Egypt; however, germplasm accessions and spatial analysis highlight Libya as a potential area for further exploration.
Aegilops triuncialis L. has by far the largest number of germplasm accessions conserved ex situ compared with other Aegilops species. These collections are representative of the geographic range of the species and, as a result, the conservation of Ae. triuncialis is not considered a priority. However, the conservation gap analysis highlights possible under-collected regions in the northern coastal regions of Algeria and Pakistan.
Ae. umbellulata Zhuk. has >250 germplasm accessions conserved ex situ and these appear evenly distributed across its geographic range. Comparing the predicted distribution of Ae. umbellulata with the distribution of ex situ collections suggests areas of western and south-eastern Turkey, and north-western Syria as under-sampled and further collection is required.
Section: Comopyrum (Jaub & Spach) Zhuk
Aegilops comosa Sm. in Sibth. & Sm. is insufficiently represented in ex situ collections from throughout its native geographic range. The comparison of ex situ collections of this species and its predictive distribution highlights areas in north-western Turkey which have not been previously collected or are under-sampled.
Aegilops uniaristata Vis. is rare throughout its range (van Slageren, Reference van Slageren1994) and is represented by only 15 unique germplasm accessions conserved, suggesting that this species is also inadequately represented in ex situ collections and should be given conservation priority. The conservation gap analysis predicts under-sampled regions in north-western Turkey and southern Greece, although van Slageren (Reference van Slageren1994) suggested that the species is extinct or extremely rare in this area of Turkey today.
Section: Cylindropyrum (Jaub & Spach) Zhuk
Ae. cylindrica Host is well represented in ex situ collections. Areas within the Caucasus and Central Asia appear particularly intensely collected compared with other regions. A comparison between the distribution of ex situ collections and the predicted distribution of this species indicates a good match and therefore this species is not considered a priority for future collection.
Aegilops caudata L. has nearly 400 germplasm accessions conserved ex situ and these are evenly distributed across its range, with the exception of more abundant collections on the Aegean Islands. Comparing the predicted distribution of Ae. caudata with the distribution of ex situ collections identifies north-western Turkey as a priority for further ex situ conservation.
Section: Sitopsis (Jaub & Spach) Zhuk
Ae. bicornis (Forssk.) Jaub & Spach is inadequately represented in ex situ collections with only 29 unique germplasm accessions currently conserved. A comparison of the predicted distribution of Ae. bicornis with the distribution of ex situ collections indicates that conservation efforts should be focussed on the coastal regions of north-western Egypt and north-eastern Libya.
Ae. longissima Schweinf. & Muschl. has only 48 unique germplasm accessions conserved ex situ; however, these collections are evenly distributed across the narrow geographic range of the species. The conservation gap analysis highlights south-western Israel as an area under-sampled and southern Syria as an area also likely to contain this species.
Aegilops searsii Feldman & Kislev ex Hammer has only 74 accessions conserved ex situ, but these are distributed throughout its narrow geographical range, suggesting that this species is in fact relatively well represented in ex situ collections especially in the western mountain ranges of Jordan. The conservation gap analysis highlights south-east Syria as a relatively under-sampled area.
Ae. sharonensis Eig has only 11 unique germplasm accessions represented in ex situ collections; however, this species is the most geographically restricted species within the genus. It already has a high number of duplicated accessions within germplasm collections, indicating that conservation efforts should focus on the collection of novel populations. A comparison of the actual distribution of this species with its predicted distribution highlights a comparatively under-collected area in northern Israel.
Ae. speltoides Tausch is relatively well represented in ex situ collections with 359 germplasm accessions conserved across its geographic range. A comparison of ex situ collections with the predicted distribution indicates that this species may be over-collected in southern Turkey and northern Iraq, but comparatively under-collected in central and western parts of Turkey. However, given the potential of this particular species for wheat improvement, the fact that it contributed the maternal genome and cytoplasm to two tetraploid wheat species, T. turgidum and T. timopheevii (Dvorák et al., Reference Dvorák, McGuire and Cassidy1988) and its intrinsically high genetic diversity (Sasanuma et al., Reference Sasanuma, Chabane, Endo and Valkoun2002), it is recommended that further collection is made throughout its range.
Section: Vertebrata Zhuk. emend Kihara
Aegilops crassa Boiss. is represented by 238 germplasm accessions conserved ex situ and these are well distributed throughout its range. Relatively over-collected regions include northern Iraq and north-western Iran, but the comparison of distribution of ex situ collections with the predicted distribution identifies potential conservation gaps in south-eastern Turkmenistan and eastern Iran.
Ae. juvenalis (Thell.) Eig is represented by only 20 germplasm accessions and therefore appears inadequately conserved ex situ. This species occurs in dispersed locations across central Asia and northern regions of the Fertile Crescent. Evidence suggests that this species is uncommon throughout its range, which may account for its insufficient representation in ex situ collections. The conservation gap analysis identifies under-collected regions in northern Syria, southern Uzbekistan and southern Turkmenistan.
Ae. tauschii Coss. appears well represented in ex situ collections across the majority of its native range, possibly as a result of its wide usage in bread wheat breeding. Ex situ collection has been particularly abundant in Caucasus, at the centre of its distribution, and also in central Asia. A comparison between ex situ collections and its predictive distribution identifies north-western and north-eastern Iran and southern Turkmenistan as possible conservation gaps. It shows a high tolerance to abiotic stresses (drought, heat and cold), and so there may also remain significant uncollected diversity located in desert marginal areas of central and north-eastern Syria, regions that remain insufficiently explored.
Aegilops vavilovii (Zhuk.) Chennav. has 104 ex situ conserved germplasm accessions, but these appear evenly distributed across the geographic range of the species. van Slageren (Reference van Slageren1994) suggested that Ae. vavilovii distribution is restricted to western Asia; however, by contrast, the spatial analysis predicts the species presence in areas of central Asia including Afghanistan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan, which may indicate that the geographical range of Ae. vavilovii extends further into Asia than previously recorded. The passport data associated with these accessions collected in this region indicate that collections were made more recently in 1999 and 2002, which may provide an explanation of why Ae. vavilovii had not previously being reported in this region. However, this is an area that requires further investigation to establish whether the accessions collected in this region have been correctly identified and to determine whether they are a result of introduction or are native to this region.
Aegilops ventricosa Tausch is represented in ex situ collections by only 83 germplasm accessions, but these appear to represent the geographic range, with the exception of northern Tunisia, which can be considered a potential conservation gap. In addition, a comparison between the distribution of ex situ collections and the predicted distribution of this species also highlights areas of southern Italy, northern Morocco and southern Spain as under-sampled regions. A summary of the ex situ conservation priority ranking for each Aegilops species is presented in Table 3 for species and Table 4 for countries.
Table 3 Aegilops species ex situ conservation priorities

Table 4 Country priorities for Aegilops species ex situ conservation

a High priority.
b Low priority.
c Medium priority.
In situ species richness and complementarity analysis
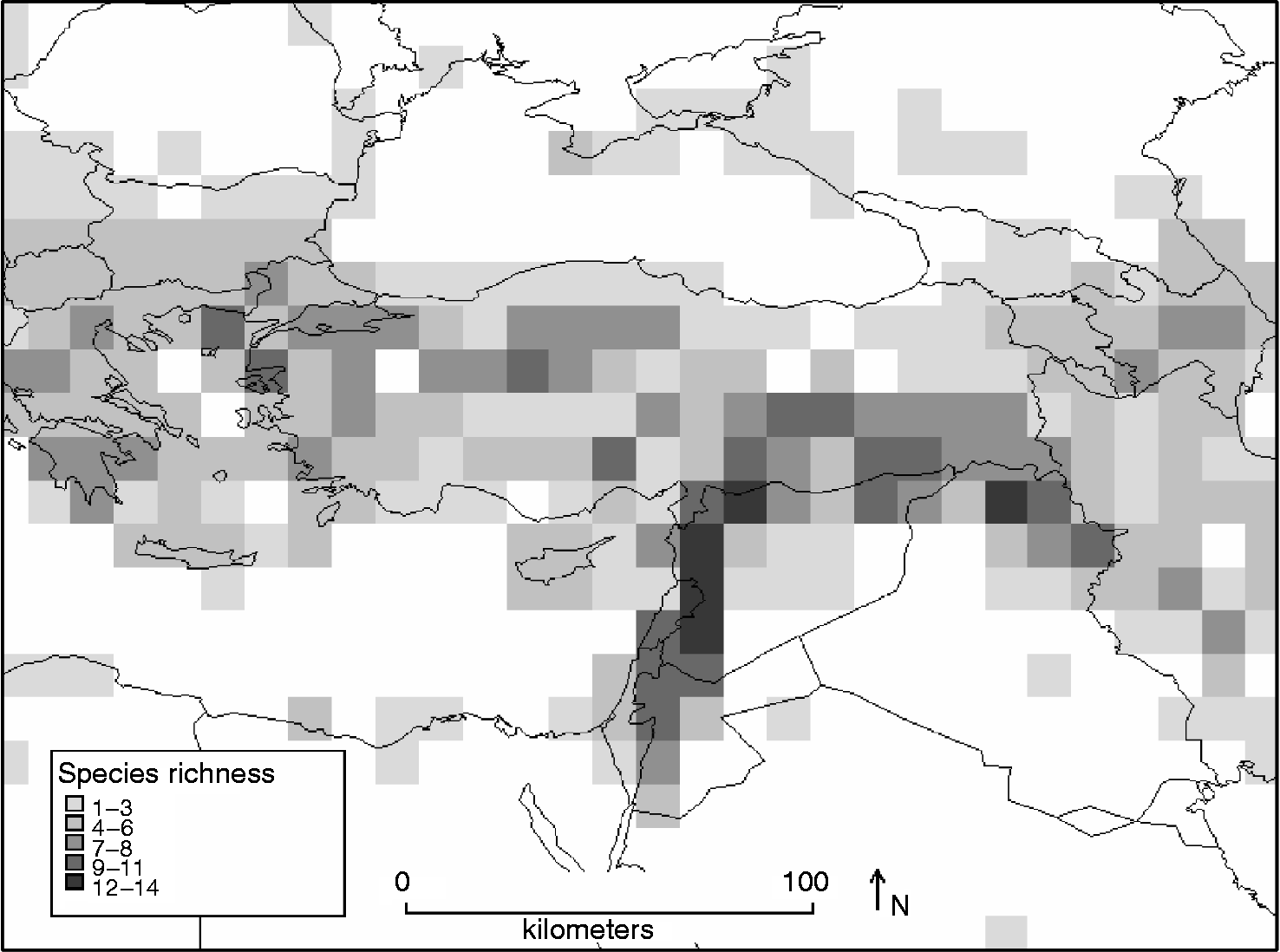
Mapping species richness identifies north-west Jordan, Israel, Lebanon, western Syria, Iraq and Turkey as areas containing more than nine Aegilops species. Figure 3 highlights two main hotspots containing 12–14 Aegilops species: western Syria (covering Damascus, Homs, Hama, Idlib and Halab provinces), north-east Lebanon (north, central and east Bekaa valley) and northern Iraq (Ninawa and Arbil provinces). The mapping of the density of germplasm collections for all Aegilops species (Fig. 4) shows the most highly-sampled areas, each with >150 unique accessions collected, which include southern Greece, Armenia and Azerbaijan, as well as western Syria and northern Lebanon. The area of northern Iraq identified in Fig. 3 as hotspot does not correspond to an area of high collection and further collection in this area is required. Southern Lebanon, northern Israel and eastern Jordan also form the region occupied by the most geographically restricted species within the genus, including the endemics Ae. searsii, Ae. longissima and Ae. sharonensis which also suggest further conservation action in this area.

Fig. 3 Species richness of Aegilops in 100 × 100 km2 grid cells.
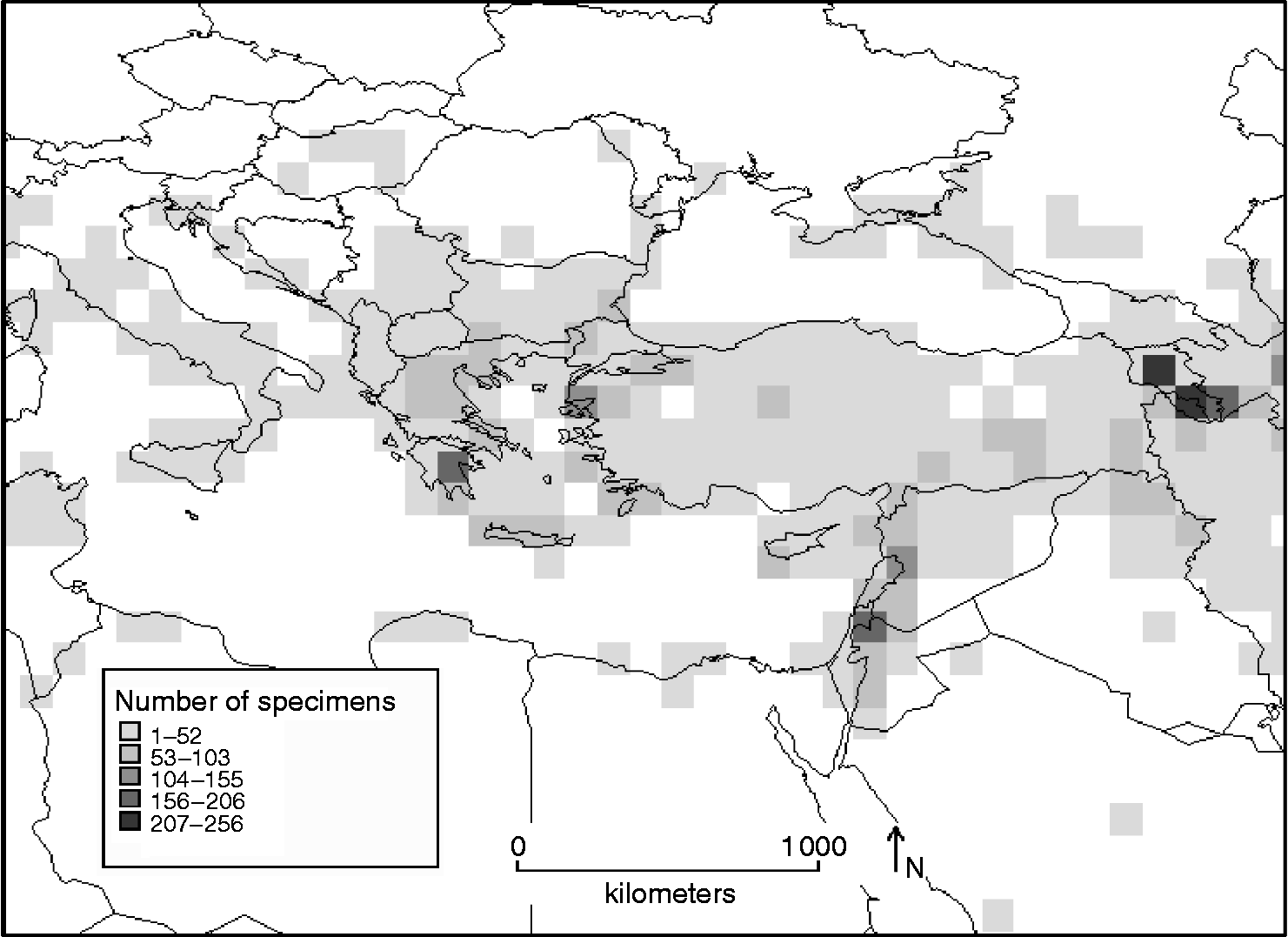
Fig. 4 Density of collections in 100 × 100 km2 grid cells for Aegilops species.
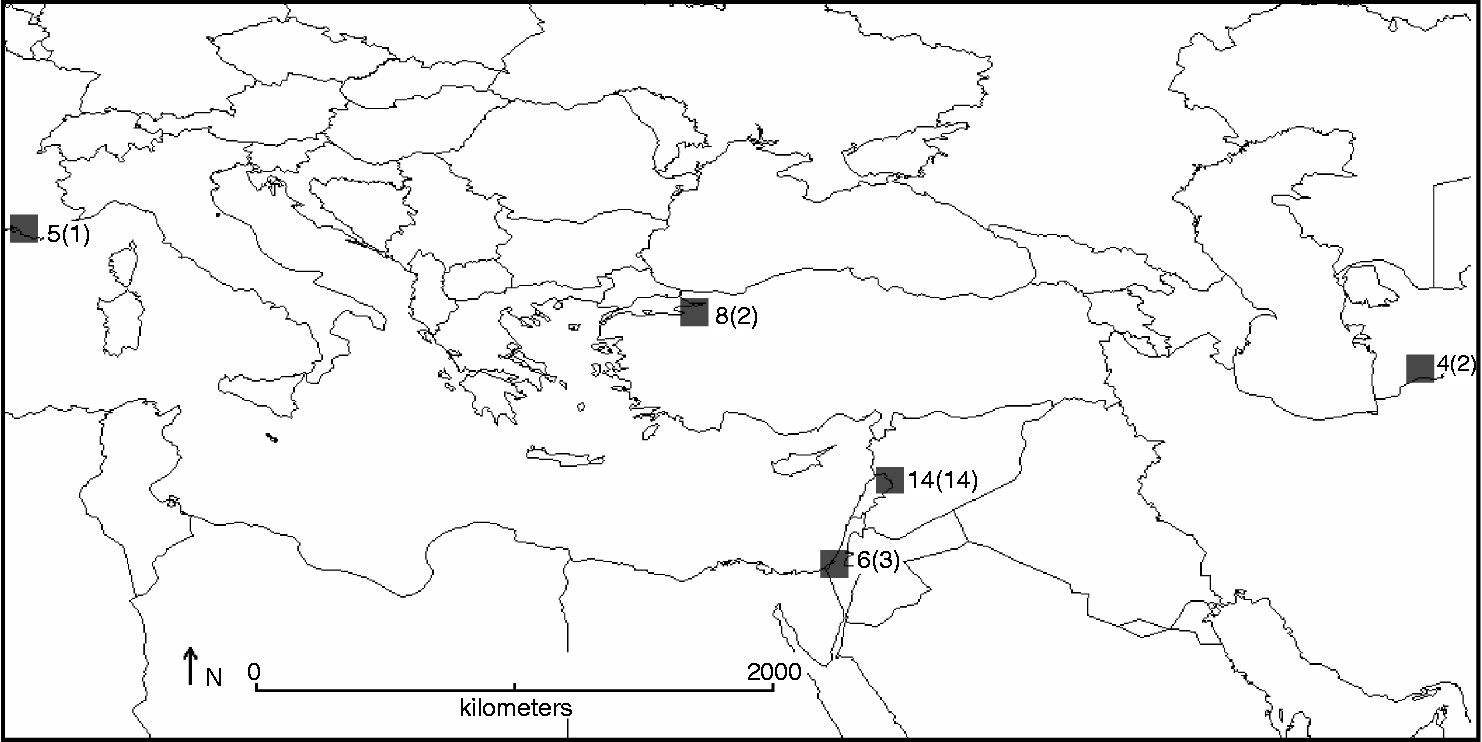
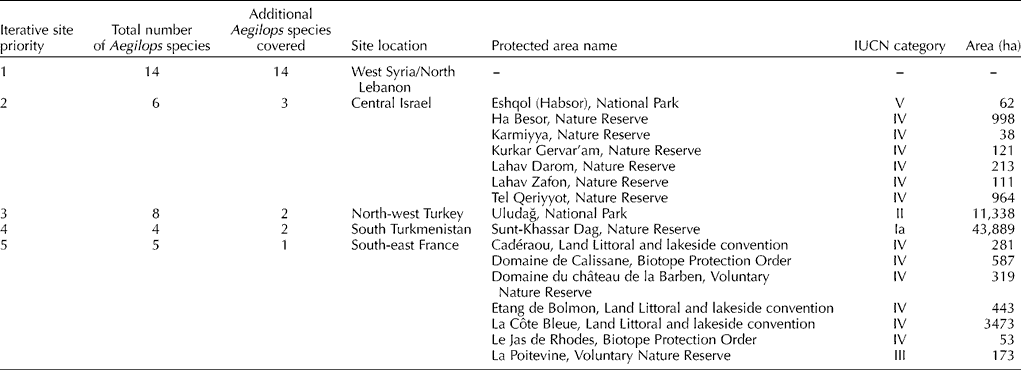
Complementarity analysis identified the five 100 × 100 km2 grid cells required to capture all 22 species in the Aegilops genus (Fig. 5), giving the most suitable sites to implement complementary genetic reserve conservation for the Aegilops gene pool. Iteration 1 corresponded to the most species rich hotspot in western Syria and North Lebanon, containing 14 species, iteration 2 to central Israel containing a further three species, iteration 3 to north-west Turkey and iteration 4 to south-west Turkmenistan both containing a further two species each and, finally, iteration 5 to south-east France containing the one additional species.
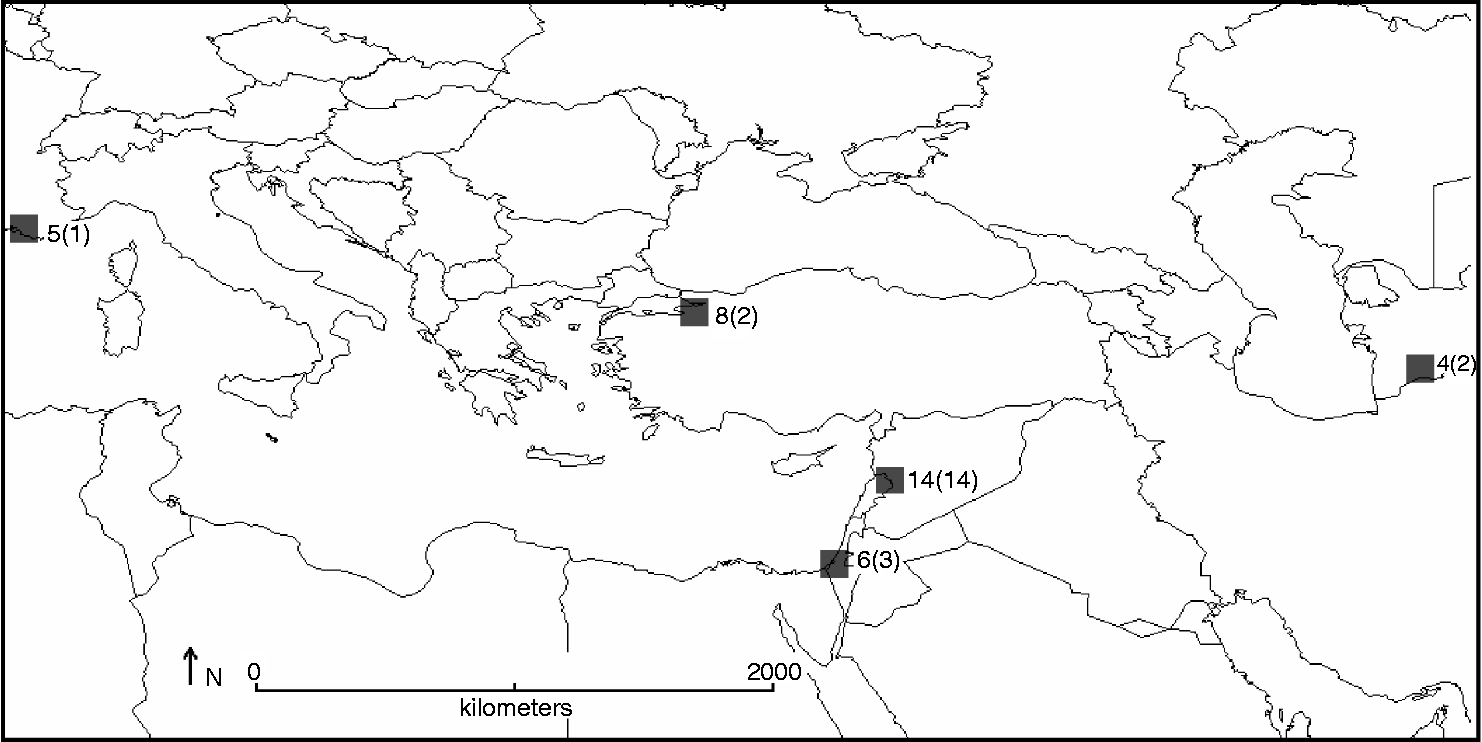
Fig. 5 Location of five complementary Aegilops species diversity hotspots (total number of Aegilops species present in each shown, as well as additional Aegilops species not found at other sites in brackets).
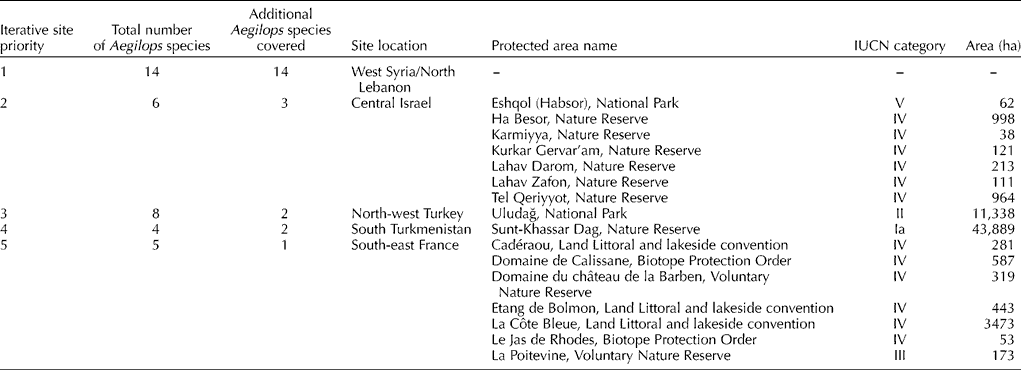
Assessment of the current in situ conservation activities in these optimal complementary locations identified 16 National protected areas with IUCN categories for the two to five locations (Table 5). However, the first area selected, West Syria/North Lebanon, with the highest concentration of Aegilops species diversity contains no internationally recognized protected areas. The recent Global Environment Facility-funded project ‘Conservation and Sustainable Use of Dryland Agrobiodiversity in West Asia’ established two genetic reserves in north-east Lebanon at Arsal and Balbak. These reserves were established to conserve genetic diversity of wild forage legumes, fruit trees, vegetables and cereals and both sites contain significant Aegilops species diversity. Therefore, it is recommended that the in situ genetic conservation of Aegilops species diversity is made a priority at these two sites. Although the same Dryland Agrobiodiversity project established two genetic reserves in Syria, these were not in the priority location identified. However, Maxted (Reference Maxted1990) identified a priority site for the in situ conservation of faba bean relatives in the legume genus Vicia in the valley below Qal'at Al Hosn in Homs province, Syria. This valley also contains Triticum baeoticum Boiss., T. urartu Tumanian ex Gandilyan, T. turgidum L. subsp. dicoccoides (Körn. Ex Asch. & Graebn.) Thell. and Hordeum vulgare L. subsp. spontaneum (C. Koch.) Thell. (Valkoun et al., Reference Valkoun, Giles Waines, Kanopka, Damania, Valkoun, Willcox and Qualset1998). A recent study by Keiša et al. (Reference Keiša, Maxted and Ford-Lloyd2007) has shown that this area is being developed rapidly for tourism and is highly threatened with genetic erosion, so designation and site protection was a priority for Vicia but is also now an equal priority in terms of Aegilops species diversity in situ conservation.
Table 5 Potential sites to establish priority genetic reserves to conserve Aegilops species diversity
It should be stressed that any form of gap analysis is constrained in its precision by the data resolution available. In the analysis, the geographic Aegilops occurrence data were analysed in the form of 100 × 100 km2 grid presence/absence derived from point location data for each species, so the presence of an Aegilops species in a grid cannot be taken to imply the presence of that species in every protected area within that grid. Therefore, in each case, confirmation of the prediction will be required, possibly involving field visits, before the final sites are selected for Aegilops genetic reserve establishment.
Discussion
The ecogeographic analysis of Aegilops species found that all 22 species are represented in ex situ collections, but the number of accessions (and their genetic diversity) varies considerably for species from 11 to 2404. Just fewer than 25% of all unique Aegilops accessions conserved ex situ were Ae. triuncialis, while Ae. biuncialis, Ae. cylindrica, Ae. geniculata, Ae. neglecta and Ae. tauschii also had >500 unique accessions conserved per species. The remaining 16 Aegilops species were all represented by < 500 accessions, and Ae. bicornis, Ae. juvenalis, Ae. longissima, Ae. sharonensis and Ae. uniaristata may be considered under-collected.
The spatial analysis showed that Ae. triuncialis was the most widely distributed within the genus, with collections from southern Europe, western Asia, central Asia, the Indian continent and north Africa. Therefore, it can be argued that the extensive number of germplasm accessions conserved is not a result of over-collection but reflects an attempt to sample Ae. triuncialis from throughout its extensive geographic range. Similar results from spatial analysis are evident for Ae. biuncialis, Ae. geniculata, Ae. neglecta and Ae. cylindrica. However, Ae. tauschii has a comparatively restricted geographic range, but the relatively large number of 962 unique germplasm accessions conserved ex situ is likely to reflect the fact that Ae. tauschii has been identified as the donor of the D genome in hexaploid bread wheat and is widely used as a gene source for wheat improvement (Mujeeb-Kazi et al., Reference Mujeab-Kazi, Rosas and Roldan1996), particularly for abiotic and biotic stress tolerance (Fritz et al., Reference Fritz, Cox, Gill and Sears1995). For example, the transfer of resistance to leaf rust (Kerber and Dyck, Reference Kerber and Dyck1969), wheat curl mite (Eriophyes tulipae; Whelen and Thomas, Reference Whelen and Thomas1989), Karnal bunt (Tilletia indica Mitra; Villareal et al., Reference Villareal, Mujeeb-Kazi, Fuentes-Davila, Rajaram and Del Toro1994), stripe rust (Ma et al., Reference Ma, Singh and Mujeeb-Kazi1994) and Hessian fly (Cox and Hatchett, Reference Cox and Hatchett1994), as well as drought, frost and salt tolerance (Monneveux et al., Reference Monneveux, Zaharieva, Rekika, Royo, Nachit, Fonzo and Araus2000). It is now evident that there is a broader use of Aegilops species as a gene source in wheat breeding programmes, but species differ substantially in their ease of use; Ae. tauschii being the most straightforward to utilize.
The spatial analysis of the Aegilops species represented by < 50 accessions conserved ex situ found that they were each geographically restricted; Ae. bicornis was mainly found in the coastal regions of northern Africa, Cyprus with a few inland sites in west Asia, Ae. juvenalis was found in Turkmenistan, Uzbekistan and northern parts of the Fertile Crescent, including Syria and Jordan, Ae. longissima was restricted to Israel, Palestine, Jordan and Egypt, Ae. sharonensis was an Israeli endemic and Ae. uniaristata being found in south-eastern Europe, particularly Greece and occasionally Turkey. The geographic range of each of these species is comparatively limited and therefore the lower numbers of ex situ collections may simply reflect their rare or uncommon status (van Slageren, Reference van Slageren1994).
To assist the identification of under-collected areas for Aegilops species, the pattern of distribution based on the germplasm accession data alone was contrasted with their predicted distribution based on the climatic envelope generated from the germplasm accession using DIVA-GIS. Potential under-sampled areas were identified and the size and range of these predicted conservation gaps varied considerably between species. This process highlighted ex situ conservation priorities in Cyprus, Egypt, Greece, Iran, Israel, Libya, Spain, Syria, Tajikistan, Tunisia, Turkey, Turkmenistan and Uzbekistan, as well as species priority for Ae. bicornis, Ae. comosa, Ae. juvenalis, Ae. kotschyi, Ae. peregrina, Ae. sharonensis, Ae. uniaristata and Ae. vavilovii species conservation. It is also noted that Ae. neglecta and Ae. crassa contain both tetraploid and hexaploid cytotypes, and for Ae. neglecta, the two forms can be distinguished morphologically on the basis of leaf stomata size and frequency (Aryavand et al., Reference Aryavand, Ehdaie, Tran and Waines2003), so where possible when collecting these two forms they should be identified and kept distinct.
There is an extensive literature relating to the in situ conservation of cereal genetic diversity, particularly of Triticum species in Israel (Anikster and Noy-Meir, Reference Anikster and Noy-Meir1991; Horovitz and Feldman, Reference Horovitz and Feldman1991; Noy-Meir et al., Reference Noy-Meir, Agami and Anikster1991; Anikster et al., Reference Anikster, Feldman, Horovitz, Maxted, Ford-Lloyd and Hawkes1997) and perhaps the most internationally well-known site for Triticum in situ conservation, Ammiad in Galilee, has recently been formally established as a protected area (Kaplan, Reference Kaplan, Maxted, Ford-Lloyd, Kell, Iriondo, Dulloo and Turok2007). However, a review of the literature suggests that there is only a single genetic reserve designated specifically to conserving Aegilops species, that being within the Ceylanpinar State Farm, south-eastern Turkey (Karagöz, Reference Karagöz, Zencirci, Kaya, Anikster and Adams1998), but this is inadequate to conserve Aegilops species diversity alone. The spatial analysis of the Aegilops species diversity identified five complementary areas for the optimal locations of genetic reserves for the active in situ conservation for Aegilops species. The regions identified were located in Syria and north Lebanon, central Israel, north-west Turkey, Turkmenistan and south France. Therefore, based on the results, it is recommended that at least one genetic reserve is initiated in each of the regions identified to conserve the maximum proportion of Aegilops diversity. A comparison between the five optimal locations for genetic reserves and the WDPA database highlighted 16 national protected sites predicted to contain Aegilops species diversity in four of the five locations. The location identified with the highest Aegilops species diversity, west Syria/north Lebanon, has no currently internationally recognized protected areas. However, it is recommended that Aegilops genetic diversity conservation is given priority in two existing genetic reserves in north-east Lebanon and a novel protected area is established in the valley of Qal'at Al Hosn, Syria. It is unlikely that the management and monitoring of any of these sites currently include Aegilops species as a priority but reserve managers should be encouraged to amend the reserve management plan to better facilitate Aegilops conservation. It is beyond the scope of this study to provide specific management details for these reserves because these are likely to vary from site to site and from species to species, but as the majority of Aegilops species show a preference for disturbed habitats such as roadsides, grasslands and forests, it is likely that the species will require active management to maintain diversity.
The question might be asked why we need to establish genetic reserves when the Aegilops species are already present in protected areas. Within the centre of diversity for Aegilops species, as elsewhere, the majority of protected areas are established to conserve specific habitats or faunal elements, fewer are for flora and, as noted above, only one for Aegilops species. So within existing protected area networks, Aegilops species are unlikely to be targeted for routine demographic monitoring. Maxted et al. (Reference Maxted, Ford-Lloyd and Hawkes1997) distinguished between active and passive protected area conservation, where active management implies some form of dynamic intervention at the site, even if that intervention were simply limited to demographic monitoring of target populations. Passive conservation involves little or no intervention, and, by definition, there is no management or monitoring of target population. There may be general ecosystem management and Aegilops species present will be passively conserved if the entire ecosystem or habitat is stable, but genetic diversity within individual species could be eroded or changed. Also, if the goal is Aegilops genetic conservation, then it may be important for the patterns of genetic diversity and the natural dynamics of that diversity to be better understood. Therefore, completely passive conservation of Aegilops in protected areas is unlikely to prove effective in long-term Aegilops genetic conservation. A more active demographic and genetic monitoring and management of target Aegilops populations offered by genetic reserve conservation is required. Also as noted above, the management of CWR may differ significantly from that required for more traditional protected areas where the objective will be to sustain climax communities. For example, Aegilops populations, like other major CWR complexes, are often located in pre-climax communities (Jain, Reference Jain, Frankel and Hawkes1975; Maxted et al., Reference Maxted, Ford-Lloyd and Hawkes1997; Stolton et al., Reference Stolton, Maxted, Ford-Lloyd, Kell and Dudley2006) and therefore the site management may need to be intense; where some Aegilops populations are closely associated with traditional farming practices, the genetic reserve management would require the maintenance of that farming system.
Having presented the future ex situ and in situ conservation priorities for Aegilops species, it is necessary to review the limitations associated with the methodologies applied. The accuracy of the conservation priorities ultimately reflects the quality of the Aegilops ecogeographic data collated and synthesized. The synthesis involved extensive manual checking for errors, filtering and standardizing of the passport data collated. However, with such a relatively large dataset, it is not possible to be sure that all duplicate accessions were removed prior to the analysis. In addition, the accuracy of the analysis results relies on the information obtained from the original collectors and those who have manipulated the data in the Global Database of Wheat Wild Relatives, EURISCO, GRIN and SINGER datasets subsequently. For example, this study assumed that the identification of each accession within the database was correct, as it was not within the scope of the study to confirm each accession's identification individually. Given these points, every practical step was taken to reduce errors and the dataset analysed was composed from four internationally recognized datasets. The Aegilops species dataset analysed was the most comprehensive compiled with 9866 unique accessions, which is thought to provide a sound basis for the conservation strategy outlined.
It is evident that Aegilops species provide an invaluable gene source for the improvement of wheat cultivars (Monneveux et al., Reference Monneveux, Zaharieva, Rekika, Royo, Nachit, Fonzo and Araus2000). The efficient conservation of Aegilops species is essential in order to assist plant breeders in attaining high wheat production demands estimated for the future (Hedge et al., Reference Hedge, Valkoun and Waines2002). The conservation strategy outlined reviews current conservation status of Aegilops species, identifies conservation gaps, provides detailed species and country priorities for further seed collection activities and identifies the five priority regions and existing protected areas where genetic reserves should be established as part of a coherent conservation strategy for Aegilops species diversity.
Acknowledgements
We thank M. van Slageren for his advice on Aegilops species diversity and A. Jarvis, M. Mitchell and J. Magos-Brehm for their assistance with the GIS analysis.