INTRODUCTION
Trypanosyllis Claparède, Reference Claparède1864 is an easily recognizable genus of syllid polychaetes, characterized by the flattened, ribbon-like shape of the body, frequently conspicuously coloured, with one to two transverse red to dark bars per segment and antennae, peristomial and all dorsal cirri ranging from red to purple.
The genus counts on more than 20 species currently known, living on hard substrates, such as rocks, stony corals and algae, and has been reported worldwide (San Martín, Reference San Martín and Ramos2003). The type-species, Trypanosyllis zebra (Grube, Reference Grube1860) has been recorded throughout the Atlantic, and San Martín (Reference San Martín and Ramos2003) suggested it may have a cosmopolitan distribution, as some species described from the Caribbean, Red Sea and Pacific Ocean are possible synonyms, or represent a mix of sibling species.
In Brazil, T. zebra has been recorded from the states of Bahia and Pernambuco (Rullier & Amoureux, Reference Rullier and Amoureux1979), in the north-eastern region, and Rio de Janeiro (Attolini, Reference Attolini1997) and São Paulo (Morgado & Amaral, Reference Morgado and Amaral1985; Duarte & Nalesso, Reference Duarte and Nalesso1996; Nogueira, Reference Nogueira2000), in the south-eastern region. In addition, T. zebra has been a common species in our collections of polychaetes living on algae, sponges, ascidians and sabelariid reefs at the intertidal zone of rocky shores along the coast of the state of São Paulo. Trypanosyllis vittigera Ehlers, 1887 was recently recorded from the states of Bahia and Rio de Janeiro (Paiva, Reference Paiva, Lavrado and Ignacio2006), but this species is a synonym of T. zebra (Aguado et al., Reference Aguado, San Martín and ten Hove2008).
More recently, in a collection from Praia do Guaraú, the southernmost beach of the state of São Paulo we have sampled until present, a different, new to science species of Trypanosyllis was found. This species differs from T. zebra in body pigmentation, in having sub-bidentate falcigers and fewer rows of proventricular muscle cells.
In the present paper we describe this new species and provide a redescription of T. zebra based on our specimens. This redescription is given not only for a matter of comparison with the new species described herein, but especially because T. zebra has been reported in many localities distant from the type-locality, often with no descriptions, and since its holotype, originally deposited at the Museum für Naturkunde of Berlin, was destroyed during World War II (anonymous referee, personal communication), it is difficult to evaluate whether this is really a cosmopolitan species, or another case of a complex of sibling species. Future molecular studies could be of help to elucidate this issue.
MATERIALS AND METHODS
The material for the present study came from two independent projects in which we are engaged. The first one is the project ‘Benthic Marine Biodiversity in the State of São Paulo’ and our participation is restricted to the identification of material previously collected. The second one is the project ‘Biodiversity of Intertidal Polychaetes (Annelida: Polychaeta) on Rocky Shores off the State of São Paulo’, which is being conducted by the Laboratório de Poliquetologia, IB–USP.
The material from the project ‘Benthic Marine Biodiversity in the State of São Paulo’ was received already sorted to family and preserved in 70% ethanol. Details on the collection of that material are available at the website http://www.ib.unicamp.br/projbiota/bentos_marinho/index.htm (Amaral, Reference Amaral2001).
For the project ‘Biodiversity of Intertidal Polychaetes (Annelida: Polychaeta) on Rocky Shores off the State of São Paulo’, collections were made at low tide, on rocky shores off the cities of Ubatuba (Praia do Félix, Praia do Perequê Mirim and Praia de Domingas Dias), Caraguatatuba (Praia de Martim de Sá), São Sebastião (Praia de São Francisco, Praia da Baleia, Barra do Una, Praia do Araçá, Praia Preta, Praia de Barequeçaba, Praia de Guaecá and Praia de Toque-toque Grande), Guarujá (Praia Branca and Praia de Pernambuco), Santos (Ilha das Palmas), São Vicente (Ilha Porchat and Praia das Vacas), Itanhaém (Praia do Sonho) and Peruíbe (Praia do Guaraú).
The rocky shores were sampled by scraping the rocks to extract small amounts of tufts of algae, colonies of sponges, small pieces of sabelariid reefs and ascidians. The material was studied alive under a stereomicroscope, polychaetes were sorted, relaxed in menthol solution, fixed in 4% formaldehyde, then washed and stored in 70% ethanol.
Further analysis under stereo- and light microscopes were made from specimens and detached parapodia permanently mounted on slides in glycerine jelly, in the case of Trypanosyllis zebra, or polyvinyl-lactophenol (PVLP), in the case of T. aurantiacus sp. nov. For the examination under SEM, one specimen of T. zebra and two specimens of T. aurantiacus sp. nov., were critical point dried and covered with 25 nm of gold. Trypanosyllis zebra was examined at the Laboratório de Microscopia Eletrônica, Instituto de Biologia, Universidade Estadual de Campinas (UNICAMP), and T. aurantiacus sp. nov. was examined at Laboratório de Microscopia Eletrônica, Museu de Zoologia, Universidade de São Paulo (MZUSP).
Photographs under light microscope were taken with an Olympus C-7070 digital camera attached to an Olympus BX51 microscope and edited with Adobe Photoshop CS software.
Type material is deposited at the MZUSP, Brazil, and the Zoological Museum of the University of Copenhagen (ZMUC), Denmark. Specimens observed under SEM are deposited in the collection of the Laboratório de Poliquetologia, IB–USP, but were not allocated collection numbers.
TYPE-SPECIES
Syllis zebra Grube, Reference Grube1860, designated by Claparède (Reference Claparède1864).
DIAGNOSIS
Medium to large sized syllines, up to 13 cm long, with nearly 500 segments, with flattened, ribbon-like body. Prostomium with three antennae, two pairs of eyes, sometimes with one pair of anterior eyespots, and one pair of short, oval, completely separated palps. Antennae, peristomial cirri, dorsal cirri throughout and anal cirri moniliform. Peristomium usually dorsally reduced, with two pairs of peristomial cirri. Parapodia with compound chaetae, sometimes secondarily simple due to fusion between shaft and blade; dorsal and/or ventral simple chaetae present sometimes, on posteriormost parapodia. Pharynx with an anterior trepan, a central, larger tooth may also be present. Reproduction by budding Tetraglene-type stolons.
REMARKS
Trypanosyllis was split into four subgenera based on the presence of a central pharyngeal tooth in addition to the trepan, on the presence of long cirrophores on dorsal cirri, and on the morphology of chaetae, if compound or secondarily simple (Imajima & Hartman, Reference Imajima and Hartman1964).
According to this classification, Trypanosyllis (Trypanosyllis) Claparède, Reference Claparède1864 has a pharynx armed with a terminal trepan, usually with 10 small teeth, and a subdistal mid-dorsal tooth, and chaetae as compound falcigers only. Trypanosyllis (Trypanedenta) Imajima & Hartman, Reference Imajima and Hartman1964 is similar to Trypanosyllis (Trypanosyllis), but lacks a mid-dorsal pharyngeal tooth. Trypanosyllis (Trypanobia) Imajima & Hartman, Reference Imajima and Hartman1964 also lacks a mid-dorsal pharyngeal tooth and, in addition, it has dorsal cirri throughout with long cirrophores and all chaetae secondarily simple. Finally, Trypanosyllis (Trypanoseta) Imajima & Hartman, Reference Imajima and Hartman1964 was characterized by having a cylindrical body, not dorsoventrally depressed as found in the other three subgenera, possessing a trepan with 10 teeth and a mid-dorsal tooth in the pharynx, and only simple chaetae throughout.
This classification was questioned by San Martín (Reference San Martín1984), who stated that the presence of a mid-dorsal pharyngeal tooth is frequently dependent on the ontogenetic status of the animal, with such tooth present in juveniles, but not in adults. In regards to Trypanobia, San Martín (Reference San Martín1984) suggested it should be raised to genus level. Except for Kudenov & Harris (Reference Kudenov, Harris, Blake, Hilbig and Scott1995), subsequent authors (Uebelacker, Reference Uebelacker, Uebelacker and Johnson1984; Nogueira, Reference Nogueira2000; San Martín, Reference San Martín and Ramos2003) have followed San Martín's suggestion and the division of Trypanosyllis into subgenera has not been adopted. Furthermore, Imajima (Reference Imajima1966) raised T. (Trypanoseta) to the category of genus, naming it Geminosyllis Imajima, Reference Imajima1966.
MATERIAL EXAMINED
‘Biodiversity of Intertidal Polychaetes (Annelida: Polychaeta) on Rocky Shores off the State of São Paulo’. Ubatuba–Praia do Félix (23°23′S 44°58′W): 9 spec, 4 November 2002 (MZUSP 596); Praia de Perequê-Mirim (23°29′S 45°06′W): 1 spec, 5 January 2003 (ZMUC Pol-1948); Praia de Domingas Dias (23°30′S 45°08′W): 1 spec, 22 July 2002; 1 spec, 2 November 2002. São Sebastião–Praia de São Francisco (23°44′S 45°24′W): 1 spec, 19 April 2003 (MZUSP 597); Praia do Araçá (23°49′S 45°24′W): 1 spec, 3 November 2002; 1 spec, 25 September 2003 (MZUSP 600); Praia Preta (23°49′S 45°25′W): 4 specs, 18 April 2003 (ZMUC Pol-1949); 3 specs, 18 July 2003 (MZUSP 599); Praia de Guaecá (23°49′S 45°28′W): 1 spec, 17 July 2003 (MZUSP 598). Guarujá–Praia Pernambuco (23°58′S 46°10′W): 2 specs, 22 June 2005 (ZMUC Pol-1952). Santos–Ilha das Palmas (24°00′S 46°19′W): 3 specs, 6 March 2004 (ZMUC Pol-1951). São Vicente–Ilha Porchat (23°59′S 46°22′W): 2 specs, 15 June 2003 (ZMUC Pol-1950); 1 spec, 9 December 2003 (MZUSP 601).
‘Project Benthic Marine Biodiversity in the State of São Paulo’. Ubatuba–Ilha dos Porcos Pequena (23°23′S 44°56′W): 4 specs, 18 October 2001 (ZMUC Pol-1947). Caraguatatuba–Ponta do Cambiri (23°37′S 45°23′W): 1 spec, 9 May 2001; 3 specs, 17 October 2001 (MZUSP 595). São Sebastião–Praia da Baleia (23°46′S 45°39′W): 4 specs, 8 April 2001 (MZUSP 594); Praia de Toque–Toque Grande (23°50′S 45°30′W): 2 specs, 10 April 2001 (ZMUC Pol-1946).
COMPARATIVE MATERIAL EXAMINED
São Sebastião–Ilha dos Alcatrazes, Baía do Oratório (24°06′S 45°42′W): 5 species, 4 December 1996, in colonies of the stony coral Mussismilia hispida (Verrill, 1868). Santos–Laje de Santos (24°05′S 46°17′W): 5 species, 17 March 1996, in colonies of the stony coral Mussismilia hispida.
DESCRIPTION
Brazilian specimens with up to 170 chaetigers and measuring up to 32 mm in length and 1.12 mm in width, at proventricular level. Large specimens with characteristic pigmentation, as two transverse dark bars per segment, and antennae, peristomial cirri, dorsal cirri throughout and anal cirri dark red (Figure 1A). Prostomium small, dorsally bilobed, with two pairs of eyes in trapezoidal arrangement; palps shorter than prostomium, kidney-shaped; lateral antennae with 14–19 articles, originating frontally, at anterior border of prostomium, central antenna longer, with 21–25 articles, originating dorsally on prostomium, close to anterior border (Figure 1A,B; Table 1). Peristomium dorsally reduced; dorsal pair of peristomial cirri with up to 29 articles, ventral pair with 9–17 articles (Table 1). Ventrally, segments throughout with two transverse ciliated bands each, one band at midlength of segment, other band less conspicuous, close to posterior border of segment (Figure 1C); dorsally, segments with one conspicuous ciliated band at midlength, continuing to parapodial lobes (Figure 1B,D&E). Dorsal cirri throughout long, distally pointed, those on segment 1 slightly longer than following cirri, usually with ~25 articles, but specimens with dorsal cirri on chaetiger 1 with up to 48 articles were studied (Figure 1A–E; Table 1); from chaetiger 10 onwards, dorsal cirri alternating long and short, long cirri with 14–35 articles until midbody, 6–20 articles on posterior segments, short cirri with 7–20 articles on anterior and midbody parapodia (Figure 1A,B,D&E), 5–15 articles on posterior segments (Table 1). Ventral cirri short, digitiform, not exceeding length of parapodial lobes (Figure 1C). Anterior parapodia with 7–11 compound chaetae each, 7–10 chaetae per parapodium at midbody, 4–8 chaetae per parapodium on posterior chaetigers (Table 1). Compound chaetae as bidentate falcigers, with teeth about same size and rounded space in between; on anterior segments, blades with dorsoventral gradation in length, measuring ~45–22 µm (Figure 1F–H; Table 1); blades shorter from midbody, with less conspicuous dorsoventral gradation in length, measuring ~35–22 µm on midbody parapodia (Figure 1J; Table 1), ~32–12 µm on posterior parapodia (Figure 1K; Table 1). Dorsal and ventral simple chaetae only present on posteriormost chaetigers, dorsal simple chaetae thin, distally bidentate (Figure 1I), beginning posteriorly to beginning of ventral simple chaetae; ventral simple chaetae sigmoid, about as thick as shafts of falcigers, distally bidentate, with distal tooth larger than subdistal tooth, similar to blades of falcigers. Anterior parapodia with up to 4 aciculae each, aciculae thick, one of them slightly irregularly curved distally, remaining aciculae straight, distally sharp; 2–3 aciculae on each midbody parapodium, 1–2 aciculae on posterior parapodia; longest 1–2 aciculae on each parapodium sharper, protruding from parapodial lobe. Body terminating by one pair of anal cirri with 13–17 articles (Table 1). Pharynx extending for 6–14 segments, with anterior trepan with about 10 sharp teeth, and, sometimes, a large, triangular, central tooth (Table 1); proventricle extending through 6–12 segments, with ~40 rows of muscle cells (Table 1).
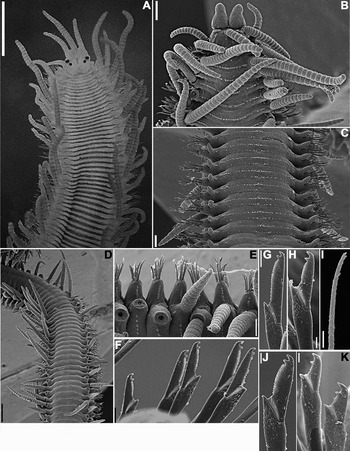
Fig. 1. Trypanosyllis zebra (A) MZUSP 597; (B–K) discarded specimen. (A) Live specimen, anterior end, dorsal view; (B) anterior end, dorsal view; (C) midbody segments, ventral view; (D) midbody segments, dorsal view; (E) midbody parapodia; (F) falcigers, midbody parapodium; (G) superior falciger, anterior parapodium; (H) inferior falciger, anterior parapodium; (I) dorsal simple chaeta; (J) superior falciger, midbody parapodium; (K) inferior falcigers, posterior parapodium. Scale bars: A, 500 µm; B, 150 µm; C, E, 100 µm; D, 400 µm; F, G, 10 µm; H, J, K, 5 µm; I, 4 µm.
Table 1. Variation among the specimens of Trypanosyllis zebra examined for the present study (‘?’ was attributed when the condition of the specimen did not allow to see that character; ‘–’ was attributed when that particular structure was absent in that specimen). Specimens 1–3: Laje de Santos (24°05′S 46°17′W), 17 March 1996; specimen 3 budding female epitokous; specimen 4: Praia da Baleia (23°46′S 45°39′W), 8 April 2001; specimen 5: Ponta do Cambirí (23°37′S 45°23′W), 9 May 2001; specimen 6 (MZUSP 596): Praia do Félix (23°23′S 44°58′W), 4 November 2002; specimen 7 (MZUSP 596): Praia do Félix (23°23′S 44°58′W), 4 November 2002; specimen 8: Praia Domingas Dias (23°30′S 45°08′W), 22 July 2002.

REMARKS
Trypanosyllis zebra is known to occur throughout the Atlantic, from the English Channel to South Africa and also in the Mediterranean. According to San Martín (Reference San Martín and Ramos2003), it is possibly a cosmopolitan species. However, very few descriptions of specimens from different localities are available in the literature, making it difficult to make comparisons between specimens from different parts of the world and evaluate whether this is a single species or a complex of sibling species. In addition, the holotype is lost (anonymous referee, personal communication).
Our material matches the description by San Martín (Reference San Martín and Ramos2003) and therefore we consider the Brazilian specimens as belonging to the same species as those from Spain.
MATERIAL EXAMINED
‘Biodiversity of Intertidal Polychaetes (Annelida: Polychaeta) on Rocky Shores off the State of São Paulo’. Peruíbe–Praia do Guaraú (24°22′S 47°01′ W): 23 specs, 5 March 2007.
Type series
Holotype and 4 paratypes deposited at MZUSP, all slide mounted specimens (holotype: MZUSP 589; paratypes: MZUSP 590–593), 3 paratypes deposited at ZMUC (ZMUC Pol 1943–1945). Holotype (MZUSP 589): 113 chaetigers, 10 mm long, 0.6 mm wide; paratype 1 (MZUSP 590): 119 chaetigers, 9.48 mm long, 0.65 mm wide; paratype 2 (MZUSP 591): 66 chaetigers, 5.17 mm long, 0.45 mm wide; paratype 3 (MZUSP 592): 85 chaetigers, 7.25 mm long × 0.57 mm wide; paratype 4 (MZUSP 593): 88 chaetigers; ~11 mm long, 0.7 mm wide, posteriorly budding stolons; paratype 5 (ZMUC Pol 1943): 71 chaetigers (plus posterior achaetous zone of ~10 segments), ~8.2 mm long, 0.5 mm wide; paratype 6 (ZMUC Pol 1944): ~110 chaetigers, ~14 mm long, 0.9 mm wide, posteriorly budding stolons; paratype 7 (ZMUC Pol 1945): ~100 chaetigers, ~13 mm long, 0.7 mm wide, posteriorly budding stolons.
DESCRIPTION
Holotype, with 113 chaetigers, measuring 10 mm in length, 0.6 mm in width, at proventricular level. In life, body yellow to orange, sometimes with two transverse dark red bars per segment, quickly fading after preservation; antennae, peristomial cirri, dorsal cirri throughout and anal cirri dark red, due to inclusions arranged as one pair per article, darker on longer cirri from midbody (Figures 2A & 3A, B, G). Prostomium small, dorsally bilobed, with two pairs of eyes in trapezoidal arrangement, quickly fading after preservation, especially in slide mounted specimens; palps shorter than prostomium, kidney-shaped; lateral antennae with ~14 articles, originating frontally, at anterior border of prostomium, central antenna longer, with 12–19 articles, originating dorsally on prostomium, close to anterior border (Figure 4A–C, F & G; Table 2); nuchal organs as one posterior row of cilia around each prostomial lobe (Figure 4A,B). Peristomium dorsally reduced; dorsal pair of peristomial cirri with 17–24 articles, ventral pair with 7–15 articles (Figures 3A, B & 4A, C, F, G; Table 2). In atokous forms, body ventrally smooth, except on parapodial lobes, which have one row of cilia arranged in irregularly distributed bunches (Figures 4F,G & 5B,C,E), with two transverse ciliated bands per segment dorsally, one near anterior border of segment, other at midlength (Figures 4A,B & 5A,D); cirrophores with an additional row of cilia posteriorly (Figure 5D). Dorsal cirri throughout long, distally pointed, with conspicuous cirrophores, those on segment 1 longer than following cirri, with 17–27 articles; until beginning of proventricle, dorsal cirri, with ~20 articles; from proventricular level, dorsal cirri alternating long and short, long cirri with 13–17 articles, short cirri with 7–11 articles (Figures 2A; 3A, B, G, 4A, C, G & 5A–E; Table 2). Ventral cirri short, digitiform, slightly exceeding parapodial lobes (Figures 4C,D,F,G & 5B,C,E). Anterior parapodia with 3–8 compound chaetae each, 3–6 chaetae per parapodium at midbody, 1–5 chaetae per parapodium on posterior chaetigers (Table 2). Compound chaetae as bidentate to sub-bidentate falcigers, shafts of unequal lengths and thicknesses, with few subdistal spines, blades with sub-distal tooth resembling an enlarged spine, with short spines on cutting edge; blades slightly diminishing in length towards posterior end, measuring ~30–16 µm on anterior chaetigers, ~30–15 µm on midbody parapodia, ~20–10 µm on posterior chaetigers (Figures 2B–D, 3D, E, H, I & 5F–I; Table 2); nearly on all chaetigers, falcigers in formation present inside parapodial lobes, not protruding yet (Figure 3D,G). Dorsal simple chaetae absent in all specimens examined; ventral simple chaetae present in some specimens including the holotype, chaetae sigmoid, slightly thinner than shafts of falcigers, sub-bidentate, sub-distal tooth resembling an enlarged spine (Figures 2E & 3I; Table 2). Anterior body with up to 3 aciculae per parapodium, midbody parapodia with 2 aciculae each; aciculae thick, distally sharp, tips protruding from parapodial lobes, one of them slightly curved distally; posterior parapodia with single acicula, with same morphology as on anterior and midbody chaetigers (Figures 2F–H & 3F–H). Several specimens with up to 8 achaetous segments in the posterior end, followed by large unsegmented zone with chaetigers in formation (Figures 2I & 3C). Body terminating by one pair of anal cirri, with ~14 articles (Figures 2I & 3C; Table 2). Pharynx extending for 9–13 chaetigers, with 10 rounded papillae at anterior end and trepan with 10 small, triangular teeth, central tooth absent (Figure 4D,E); proventricle through 7–8 chaetigers, with ~28 rows of muscle cells (Figures 2A & 3A; Table 2).

Fig. 2. Trypanosyllis aurantiacus sp. nov. (A–H) holotype (MZUSP 589); (I) paratype 2 (MZUSP 591). (A) Anterior end, dorsal view; (B) falcigers, anterior parapodium; (C) falcigers, midbody parapodium; (D) falcigers, posterior parapodium; (E) ventral simple chaeta; (F) aciculae, anterior parapodium; (G) aciculae, midbody parapodium; (H) acicula, posterior parapodium; (I) posterior end, dorsal view. Scale bars: A, 500 µm; B–D, F–H, 10 µm; E, 5 µm; I, 150 µm.

Fig. 3. Trypanosyllis aurantiacus sp. nov. (A, C) holotype (MZUSP 589); (B) paratype 5 (ZMUC Pol 1943); (D–I) slide mounted detached parapodia, paratype 4 (MZUSP 593). (A) Anterior end, dorsal view, slide mounted specimen, arrows point to beginning and ending of proventricle; (B) anterior end, dorsal view, recently preserved specimen; (C) posterior end, dorsal view, slide mounted specimen; (D, E), falcigers, anterior parapodium; (F) aciculae, midbody parapodium; (G) midbody parapodia; (H) chaetae and aciculae, midbody parapodium; (I) ventral simple chaetae and inferior falcigers, posterior parapodia. Scale bars: A–C, 250 µm; D, 20 µm; E, I, 10 µm; F, 7 µm; G, 50 µm.

Fig. 4. Trypanosyllis aurantiacus sp. nov. (A–E) specimen 1; (F–G) specimen 2. (A) Anterior end, dorsal view; (B) detail, anterior end, dorsal view; (C) anterior end, lateral view (pharynx everted); (D) anterior end, ventral view (pharynx everted); (E) detail, pharynx opening; (F) anterior end, ventral view; (G) anterior end, antero-lateral view. Scale bars: A, 90 µm; B, 30 µm; C, 150 µm; D, E & G, 60 µm; F, 150 µm.

Fig. 5. Trypanosyllis aurantiacus sp. nov. (A–G) specimen 2; (H, I) specimen 1. (A) Midbody segments, dorsal view; (B) midbody segments, ventral view; (C) midbody parapodium, ventral view; (D) cilliation on midbody segments, dorsal view; (E) midbody parapodia, lateral view; (F) falcigers, anterior parapodium; (G) detail, same parapodium; (H) falciger, midbody parapodium; (I) falciger, posterior parapodium. Scale bars: A, B, 200 µm; C, 50 µm; D, 60 µm; E, 90 µm; F, 20 µm; G, 9 µm; H, I, 6 µm.
Table 2. Variation among the slide mounted specimens of the type-series of Trypanosyllis aurantiacus sp. nov. (‘?’ was attributed when the condition of the specimen did not allow to see that character; ‘–’ was attributed when that particular structure was absent in that specimen).

BIOLOGY
Six of the specimens examined were budding Tetraglene stolons from the posterior part of the body. Atokous forms budding up to 9 epitokous simultaneously were observed. Epitokous forms conspicuously ciliated, with one irregular row of cillia dorsally, arranged in circular bunches (Figure 6B,D), two continuous rows of cilia ventrally, one of which near anterior border of segment, the other at midlength (Figure 6A,C&E). Epitokous forms with 1–3 falcigers per parapodium, notopodial capillary chaetae absent in all specimens examined.
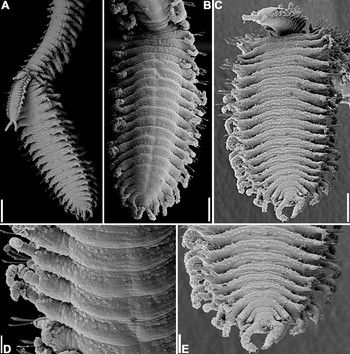
Fig. 6. Trypanosyllis aurantiacus sp. nov. (specimen 2). (A) posterior end, lateral view—three epitokous specimens budding from atokous specimen; (B) larger epitokous specimen, dorsal view; (C) same epitokous specimen, ventral view; (D) same epitokous specimen, detail, dorsal view; (E) same epitokous specimen, posterior end, ventral view. Scale bars: A, 300 µm; B,C, 200 µm; D, 60 µm; E, 40 µm.
REMARKS
This species is particularly tricky because several of its characters fade very quickly after preservation, making it difficult to identify from preserved specimens. Not only body pigmentation, except for dorsal cirri, disappears in preserved material, but also the eyes fade and disappear in a matter of days in slide mounted specimens, the same happening with proventricular rows muscle cells (Figure 3A,B). Therefore, the colour of the specimens is useful for the identification of this species, but only on live or freshly collected material; on the other hand, the absence of pigmentation on preserved material, especially on slide mounted specimens, is also characteristic, as the species of Trypanosyllis usually have conspicuous dorsal pigmentation.
Trypanosyllis aurantiacus sp. nov., is part of a small group of species of Trypanosyllis with falcigers with bidentate blades, with subdistal tooth distinctly shorter than distal one, condition sometimes referred to as ‘sub-bidentate’. To this group also belong T. aeolis Langerhans, 1879, T. parazebra Hartmann-Schröder, Reference Hartmann-Schröder1965, T. savagei Perkins, Reference Perkins1980, Trypanosyllis sp. B sensu Uebelacker, Reference Uebelacker, Uebelacker and Johnson1984, Trypanosyllis (Trypanosyllis) sp. B sensu Kudenov & Harris, Reference Kudenov, Harris, Blake, Hilbig and Scott1995, and T. sanmartini Çinar, Reference Çinar2007.
Trypanosyllis aurantiacus sp. nov., differs from T. aeolis by having smaller body, by the absence of rounded papillae forming two transverse lines on the dorsum of each segment, by the presence of ventral simple chaetae, by the absence of a thinner acicula on parapodial lobes and by having a longer pharynx, extending through 9–12 segments, instead of only through 4 segments as in T. aeolis (San Martín, Reference San Martín and Ramos2003).
Trypanosyllis parazebra possesses pigmentation as a single transverse brown bar per segment dorsally, antennae and cirri throughout with fewer articles (central antenna: 10 articles; lateral antennae: 8–9 articles; peristomial dorsal cirri: 9–11 articles; peristomial ventral cirri: 7 articles; dorsal cirri of anterior parapodia: 9–11 articles; dorsal cirri of posterior parapodia: 6–7 articles), peristomium not reduced dorsally as in T. aurantiacus sp. nov., just slightly shorter than following chaetigers, aciculae straight, needle-like, and anterior parapodia with 2 aciculae each at most. Finally, T. parazebra has a large central tooth in the pharynx and smaller proventricle, extending through ~4 segments, with 17 rows of muscle cells (Hartmann-Schröder, Reference Hartmann-Schröder1965).
Trypanosyllis savagei is a smaller species, less than 5 mm long and with 80 chaetigers at most. It also differs from our new species by possessing antennae, tentacular cirri and dorsal cirri throughout with 4–8 articles only, blades of falcigers ~12 µm long throughout, with smooth shafts, shorter anal cirri, with 3 articles each, and smaller proventricle, occupying 3–4 segments, with ~17 rows of muscle cells (Perkins, Reference Perkins1980).
Trypanosyllis sp. B sensu Uebelacker, Reference Uebelacker, Uebelacker and Johnson1984 also has antennae, tentacular cirri and dorsal cirri throughout shorter than T. aurantiacus sp. nov., with 8 articles at most. Furthermore, Trypanosyllis sp. B sensu Uebelacker differs from T. aurantiacus sp. nov., by having shorter proventricle, extending through 4–5 segments, with ~21 muscle cell rows, and shorter pharynx, extending for 3–4 segments (Uebelacker, Reference Uebelacker, Uebelacker and Johnson1984).
Trypanosyllis sp. B sensu Kudenov & Harris, Reference Kudenov, Harris, Blake, Hilbig and Scott1995, is distinguished from T. aurantiacus sp. nov., by possessing a tuft of cilia on the last article of antennae, peristomial and dorsal cirri, at least on anteriormost chaetigers, according to the figure provided by Kudenov & Harris (Reference Kudenov, Harris, Blake, Hilbig and Scott1995: figure 1.30A). Furthermore, Trypanosyllis sp. B sensu Kudenov & Harris, Reference Kudenov, Harris, Blake, Hilbig and Scott1995 has the superiormost falcigers with nearly smooth blades, becoming progressively more conspicuously spinulated inferiorly, blades with somewhat stronger subdistal tooth, according to the figures provided (Kudenov & Harris, Reference Kudenov, Harris, Blake, Hilbig and Scott1995: figure 1.30F–H), and aciculae with hook-like subdistal collar (Kudenov & Harris Reference Kudenov, Harris, Blake, Hilbig and Scott1995: figure 1.30J–K).
Finally, a recently described species from Turkey, eastern Mediterranean, is the most similar species to T. aurantiacus sp. nov. Trypanosyllis sanmartini presents pattern of pigmentation, distribution of chaetae throughout, morphology of pharynx and proventricle, and stolonization very similar to T. aurantiacus sp. nov. However, it differs from T. aurantiacus sp. nov., in having each anterior segment with 2 rows of papillae on dorsum, more articles on antennae and cirri throughout (central antenna: 28 articles; lateral antennae: 20 articles; peristomial dorsal cirri: 30 articles; peristomial ventral cirri: 20 articles; anterior dorsal cirri: 18–38 articles; posterior dorsal cirri: 12–26 articles), cirrophores with papillae but without cilia, longer blades of falcigers, measuring ~40–27.5 µm on anterior body, ~42.5–30 µm on midbody, and ~40–25 µm on posterior chaetigers, and pharynx and proventricle densely pigmented (Çinar, Reference Çinar2007). As the Brazilian specimens studied for this paper were adults, many of them budding Tetraglene stolons, differences in size cannot be attributed to different ontogenetic stages. In addition to the geographical distribution, we consider those differences enough to describe T. aurantiacus sp. nov., as a new species.
ETYMOLOGY
The specific name aurantiacus is attributed to this species due to the colour of specimens in life (aurantiacus = Latin for ‘orange colour’).
ACKNOWLEDGEMENTS
This study was funded by FAPESP—Fundação de Amparo à Pesquisa no Estado de São Paulo (proc. 04/02774-4). In addition, J.M.M.N. receives a productivity fellowship from CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico and M.V.F. receives a PhD fellowship from FAPESP (proc. 07/53040-9). We are grateful to Cecília Amaral, coordinator of the project ‘BIOTA/FAPESP/Benthic Marine Biodiversity’, for all of her support; to the team of the project ‘BIOTA/FAPESP/Benthic Marine Biodiversity’, for collecting part of the material; to Nilcea Aparecida Veronesi, for providing accommodation in Praia Grande. We are also grateful to Professor Sergio Antonio Vanin, from Departamento de Zoologia, IB–USP, for the help with Latin adjectives; to Enio Mattos and Eduardo Mattos, from Departamento de Zoologia, IB–USP, for preparing the specimens for the study under SEM; and to Lara Guimarães, from Laboratório de Microscopia Eletrônica, MZUSP, and Adriane Sprogis and Antônia Lima, from Laboratório de Microscopia Eletrônica, IB–UNICAMP for the SEMs included in the present paper.










