Introduction
Unusual colour morphs, or colour aberrations, exist across many taxa and species. They can arise from a variety of causes, including dietary imbalances, environmental conditions, disease or genetic mutations (van Grouw Reference Van Grouw2013). The Wright and Haldane models predict that the more deleterious a genetic mutation, the more recessive it tends to be (Huber et al. Reference Huber, Durvasula, Hancock and Lohmueller2018). However, while recessive mutations lie dormant in a given population, the variation they encode may hold important value in different conditions, such as after a sudden environmental change. A classic example is the peppered moth in Britain during the Industrial Revolution. The white-bodied morph of the species was predominant, using its colouration as camouflage from predators; however, as pollution levels increased and tree trunks darkened, the once-rare melanistic individuals rapidly proliferated until they became the common colour morph. When air pollution decreased, the white-bodied morph once again became more abundant (Maderson Reference Maderson1975, Cook et al. Reference Cook, Grant, Saccheri and Mallet2012).
Variation in colour can also signal individual condition and breeding ability (MacDougall & Montgomerie Reference MacDougall and Montgomerie2003, Reudink et al. Reference Reudink, Studds, Marra, Kurt Kyser and Ratcliffe2009). Even in monomorphic species, colour may still be used to select mates. Both male and female king penguins (Aptenodytes patagonicus, Miller) have yellow and orange pigmentation, the intensity of which signals their overall health and is used for mate selection (Nolan et al. Reference Nolan, Dobson, Dresp and Jouventin2006, Jouventin et al. Reference Jouventin, Nolan, Stephen Dobson and Nicolaus2007). In one study, females selected males with larger coloured patches, and when a male's pigmentation was experimentally covered up, they had a much harder time pairing (Jouventin et al. Reference Jouventin, Nolan, Stephen Dobson and Nicolaus2007, Dobson et al. Reference Dobson, Couchoux and & Jouventin2011). However, males appeared to be less selective when choosing mates and paid less attention to the female's manipulated patch (Jouventin et al. Reference Jouventin, Nolan, Stephen Dobson and Nicolaus2007).
Colour variation may also be linked to differences in individual behaviour. For example, dark and light morphs of the Pacific reef heron (Egretta sacra, Gmelin) employ different foraging tactics, with light morphs utilizing flight in their pursuit of prey, whereas dark morphs rely more on land-based attacks. They can even forage in different locations. On Mangaia in the Southern Cook Islands, light morphs favoured foraging on the windward side of the island and dark morphs on the leeward side (Rohwer Reference Rohwer1990). Additionally, there is evidence of colour expression being related to thermoregulation on a genetic level. In a single common murre (Uria aalge, Pontopippidan) population, the bridled morph showed greater survival during colder years, while the unbridled morph did better in warmer years (Reiertsen et al. Reference Reiertsen, Erikstad, Barrett, Sandvik and Yoccoz2012). Because the difference in colouration is slight between these two morphs, this indicates that colour expression could be correlated with specific thermoregulatory genes (https://www.biorxiv.org/content/10.1101/507384v1). As climate change continues to alter the environment, different colour variations or morphs may have an advantage, like with the peppered moth during the Industrial Revolution. Darker morphs with more melanin may become more advantageous because of the increased protection against ultraviolet radiation (Roulin Reference Roulin2014). On the other hand, it may become increasingly difficult for darker-pigmented birds to thermoregulate, leading to overheating (Margalida et al. Reference Margalida, Negro and Galván2008).
The little blue penguin (Eudyptula minor, Forster) is the only penguin species with an accepted colour morph, the white-flippered penguin, though its status as a morph vs a subspecies (Eudyptula minor albosignata, Finsch) is under debate (Banks et al. Reference Banks, Mitchell, Waas and Paterson2002). Nevertheless, colour variations have been recorded in at least 13 of 17 penguin species (combining the southern rockhopper (Eudyptes chrysocome, Forster) and northern rockhopper (Eudyptes moseleyi, Matthews & Iredale) because data are not detailed enough post-speciation; Stevens Reference Stevens2000, Everitt & Miskelly Reference Everitt and Miskelly2003, Finger et al. Reference Finger, Aver, Koch and Petry2017, Reference Finger, dos Santos, Corrêa, de Brum and Petry2018, https://www.youtube.com/watch?v=NuIczQ4W7oU). Continuing to understand and monitor the occurrence of these aberrations is important as species adapt to environmental changes (Le Corre Reference Le Corre1999).
Adélie penguins (Pygoscelis adeliae, Hombron & Jacquinot) are an abundant species that breed only in Antarctica and are an important indicator of climate change (Ainley Reference Ainley2002, Lynch & LaRue Reference Lynch and LaRue2014). As such, they are potentially useful for understanding the frequency of colour variation, the effects that it has on mate selection and the possible implications in a warming climate. Adélie penguin plumage is sexually monomorphic, with both sexes exhibiting countershading (black-pigmented feathers on the dorsal region, neck, head and upper side of the flipper and white feathers on the ventral sides of their body and flippers). Chicks have dark or light grey down until they fledge ~46–55 days after hatching (Fig. 1) (Ainley et al. Reference Ainley, LeResche and Sladen1983). Any variation from this colouration is considered a colour aberration, and we focus on aberrations that affect the entire body as opposed to ones affecting just a small area, most probably due to some form of trauma (van Grouw Reference Van Grouw2006, Reference Van Grouw2013). Because Adélie populations are well studied, they are among the penguins with the most aberrations reported in the scientific literature. In fact, the first record of a colour aberrant is an ‘isabelline’ or brown (sex-linked) Adélie painted by E.A. Wilson from R.F. Scott's early polar expeditions (Wilson Reference Wilson1907). Since then, at least five additional varieties have been recorded (leucism, melanism, brown, dilute and ino: Falla Reference Falla1937 as cited in Finger et al. Reference Finger, Aver, Koch and Petry2017, Araya & Arrieta Reference Araya and Arrieta1971, Forrest & Naveen Reference Forrest and Naveen2000, Juáres et al. Reference Juáres, Negrete, Mennucci, Longarzo and Coria2011, Finger et al. Reference Finger, dos Santos, Corrêa, de Brum and Petry2018). However, nomenclature for the aberrations is diverse. For this paper, we follow van Grouw's (Reference Van Grouw2013, Reference Van Grouw2018) definitions and descriptions.

Fig. 1. Two normal-coloured Adélie penguins with the traditional black and white countershading standing next to two normal-coloured (although muddy), downy Adélie chicks. Photograph credit: Parker M. Levinson.
To date, little has been done to understand the frequency of various colour mutations or how they might affect breeding behaviour. In this paper, we aim to document the variety, frequency and sex of colour-aberrant Adélie penguins at the southern edge of the species range. This study was conducted at one of the largest colonies of the species at Cape Crozier with > 300,000 pairs (Anderson & Shanhun Reference Anderson and Shanhun2020). We describe their breeding behaviour and compare it to that of normal-coloured penguins at Cape Crozier. We hypothesize that colour aberrations have a deleterious effect on breeding. We predict the following: 1) colour aberrants are less likely to breed than normal-coloured penguins, and 2) if they do breed, they are less successful.
Methods
The Cape Crozier Adélie penguin colony is located on the north-eastern corner of Ross Island, Antarctica (77.5294°S, 167.2123°E). It is among the five largest colonies in the world, with current estimates exceeding 300,000 breeding pairs (Lynch & LaRue Reference Lynch and LaRue2014, Anderson & Shanhun Reference Anderson and Shanhun2020). The colony is divided into two portions, East and West Crozier, with hundreds of smaller groups or sub-colonies (Fig. 2). East Crozier (~30,000 pairs) is an order of magnitude smaller than West Crozier (~280,000 pairs), where the majority of the colony breeds. As part of a long-term demographic study, researchers have been present at the colony each summer since 1996. A by-product of this work is that penguins with abnormally coloured plumage were incidentally recorded and peripherally monitored.

Fig. 2. Location of Adélie penguin colour aberrants in blue stars overlaid on yellow sub-colony boundaries with inset of Cape Crozier location (noted by red star) on Ross Island, Antarctica. Note higher concentration of blue stars (i.e. colour aberrants) towards the middle of West Crozier. Satellite image from WorldView-3, 20 November 2014 (copyright 2014 DigitalGlobe, NextView License). Ross Island image acquired by moderate-resolution imaging spectroradiometer (MODIS) on board Aqua satellite on 29 November 2011 (courtesy of Rapid Response Imagery from Land, Atmosphere Near Real-Time Capability for Earth Observing System operated by NASA/GSFC/Earth Science Data and Information System with funding provided by NASA/HQ).
During the summer of 2019–2020 (hereafter ‘2019 season’), we conducted a targeted study to document and monitor all colour-aberrant penguins present at the Cape Crozier colony. We defined abnormal colouration as any noticeable colour variation from the typical black and white patterning that is not a scar (Fig. 1). A core study area in West Crozier was searched every 4 days (described below) for abnormally coloured penguins, and the remainder of West Crozier was searched every 7–14 days from late October to early January. East Crozier was searched roughly once a month. Additionally, we conducted weekly targeted checks at known locations of past colour aberrants in West Crozier to determine whether the individuals were present.
We searched for colour aberrants in pairs, starting at the same point and walking in opposite directions ~5–10 m from the edge of each sub-colony until meeting on the other side of the sub-colony while visually scanning for any unusual penguins. With this method, we searched every sub-colony extremely thoroughly.
Upon discovery, the territory of a colour-aberrant individual was marked by hammering a uniquely numbered plastic tag attached to an 8 inch stake into the ground ~6 inches from the territory edge. Territories were then monitored weekly for the rest of the season (with the exception of one individual located in East Crozier whose territory was checked bi-monthly). At each visit, we recorded the following: 1) whether the colour aberrant penguin was present, alone or paired, 2) the nest position relative to the sub-colony edge, and 3) the breeding status.
To determine nest position, we counted occupied territories from the sub-colony edge to the nest in question in as straight a line as possible. If the individual was breeding, the number of eggs or chicks seen was recorded, as well as an estimate of chick size. An attempt to sex the penguin was made using visual and behavioural cues (Ainley & Emison Reference Ainley and Emison2008). Because Adélie penguins are slightly size-dimorphic, with males larger than females, relative body size, bill size and head size were used to sex birds. Behavioural cues used included copulatory position, if observed, and muddy footprints left on the back of females post-copulation. Additionally, males perform a display behaviour known as ecstatic vocalization in which they point their head to the sky and flap their flippers while vocalizing, and they tend to take the first incubation shift (Ainley Reference Ainley2002). A combination of these cues were used to sex birds.
In order to determine the type of colour aberration, we took at least one complete set of photographs of each individual, including photographs of their face and their feet. We compared these pictures to van Grouw's (Reference Van Grouw2013) descriptions of each colour aberration and classified individuals accordingly.
Because Adélie penguins occupy a single territory for the entire breeding season, it was possible to differentiate penguins with similar colour aberrations (i.e. two dark brown penguins) when they were seen at their nest site. However, it was not possible to uniquely identify all colour-aberrant penguins not on a territory; therefore, only observations of individuals on identified territories were used in these analyses.
We estimated frequency of colour aberrations by utilizing the most recent count of 282,381 breeding pairs in West Crozier, excluding East Crozier because the area was not sampled as thoroughly (Anderson & Shanhun Reference Anderson and Shanhun2020).
In order to compare colour aberrants to normal-coloured penguins, we used the demographic data collected from an existing population of individually marked birds. Beginning in 1996, 500–1000 chicks were banded annually on the left flipper with a unique numbered stainless steel band (see Dugger et al. Reference Dugger, Ballard, Ainley and Barton2006 for details on band design). Every time a banded bird was sighted, the band number and the breeding status or behaviour of the penguin were recorded. To compare the likelihood of a colour-aberrant bird breeding with the likelihood of a normal-coloured penguin breeding, we only used banded birds seen on a territory in the 2019 season. In order to compare ‘success’ of breeding, we chose a chick size threshold of at least ~20% the size of an adult penguin (~1000 g) when last seen in the territory before they disappeared. This size was chosen because a majority of chicks that reach this size are able to survive once entering the crèche (when both parents leave to forage and young chicks enter into groups for predator protection; A. Schmidt, unpublished data 2012–2020).
To test whether colour aberrants bred less frequently relative to normal-coloured Adélie penguins, we compared the proportion of all banded individuals that were seen breeding in the 2019 season to the proportion of colour aberrants breeding in the 2019 season with a one-sided Pearson's χ2 test. Breeding was defined as having laid at least one egg. All birds seen occupying a territory, regardless of sex, were included in this analysis. To understand the effect size of our statistics, we ran power analyses for proportional tests with two different sample sizes. All analyses were conducted in R 3.6.1 (R Development Core Team 2019) with the ‘stats’ package.
We also reviewed daily activity logs from Cape Crozier from 2002 to 2019 where interesting, unusual or unique observations were noted in order to determine the relative age of certain individuals present for the study and to identify other types of colour aberrations not observed during the 2019 season. To corroborate these data, we surveyed former researchers stationed at Cape Crozier between 2002 and 2019 for descriptions and photographs of aberrant penguins.
Results
Type and sex
In the 2019 season, we located 12 penguins with colour aberrations: seven dark brown, one dilute (grey colour), three progressive greying and one brown (Fig. 3). Dark brown penguins had brown to dark brown plumage instead of black with lighter brown to blonde feathers on the lower back that got progressively lighter as the season went on (Fig. 4). The dilute individual had light grey or platinum-coloured plumage in place of black. One progressive greying individual had what appeared to be partially pigmentless (i.e. partially white) feathers randomly interspersed on the dorsal region, the topside of the flippers and on the head, concentrated around the eyes and bill and is hereafter referred to as the speckled progressive greying bird. The other two progressive greying individuals had white splotches somewhat bilaterally symmetrical around the eyes, bill and on the flippers but not on the dorsal region (Fig. 3). Finally, the brown individual had tan-coloured plumage instead of black plumage with pink feet, brown claws and a lighter-coloured bill (for more information on underlying genetic conditions, see van Grouw Reference Van Grouw2013).
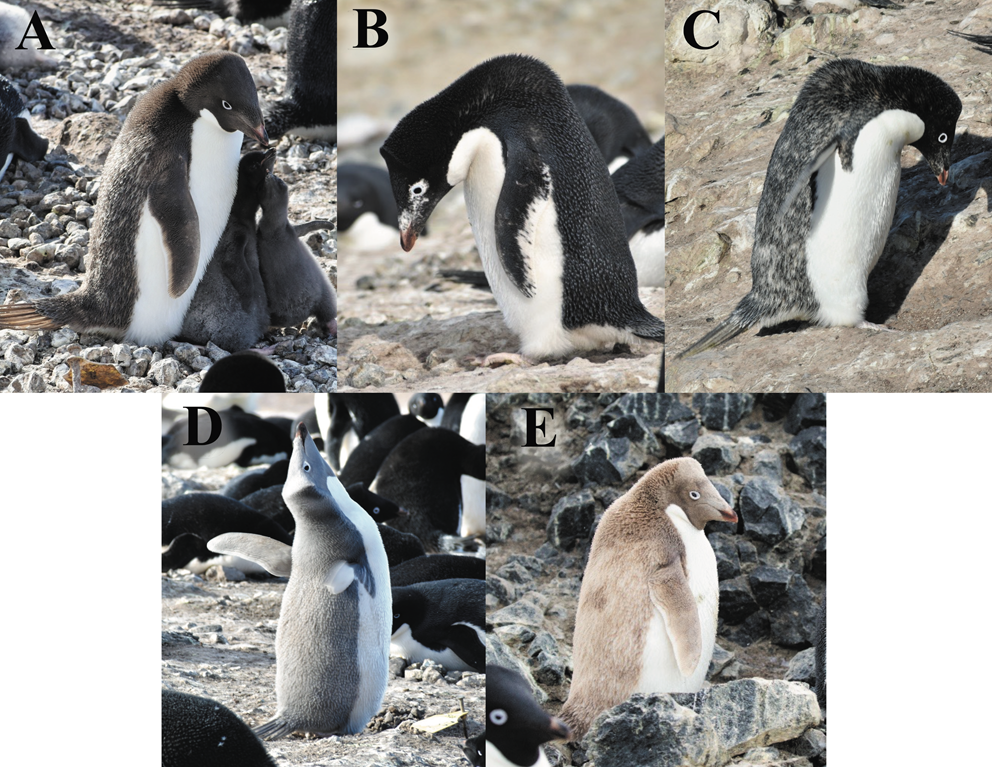
Fig. 3. Photographs of each variety of Adélie penguin colour aberration seen during the 2019 season at Cape Crozier, Ross Island, Antarctica: a. dark brown adult; b. progressive greying male; c. progressive greying female; d. dilute male; e. brown female. Photograph credits: Parker M. Levinson (a.–d.) and Annie E. Schmidt (e.).

Fig. 4. A selection of Adélie penguins at Cape Crozier, Ross Island, Antarctica, exhibiting different shades of dark brown colouration with varying degrees of feather bleaching limited to the lower dorsal region. Photograph credits: Parker M. Levinson and Annie E. Schmidt.
In our sample of colour-aberrant individuals, there were five females, three males and four of unknown sex. Three of the seven dark brown penguins were sexed female by size. We were unable to sex four of the dark brown penguins by behaviour or size in the field. The speckled progressive greying bird was sexed female by copulatory position. The other two progressive greying individuals as well as the dilute penguin were sexed male by ecstatic vocalization and size. The brown bird was sexed female by size and timing of incubation shift. Additionally, because the genetic mutation causing brown or ino colouration is a recessive, sex-linked mutation found on the Z chromosome, it almost exclusively occurs in females (van Grouw Reference Van Grouw2013). One ino chick was also observed. It had normal-coloured parents and a normal-coloured sibling but was not included in the analyses.
Colour aberrants were found throughout the entire colony at Cape Crozier at a low frequency of 1:50,000. There was a somewhat higher concentration of aberrants in the middle of the colony where researchers spent more time for the unrelated demographic work (Fig. 2).
Breeding
Of the 11 individuals routinely monitored, eight were seen with a normal-coloured partner at some point in the season. The remaining three were never seen with a partner.
To compare normal-coloured penguins with aberrant penguins, we can detect a medium to large effect (h = 0.65) with our sample size of 306 banded birds seen on territories and 11 aberrants on territories, setting P to 0.1 and statistical power to 0.8. As evident by these power analyses, we do not have a lot of statistical power to test for small or medium effects; thus, we present the estimated proportions with 95% confidence intervals (CIs) to aid with visualization and understanding of aberrant breeding behaviour.
No male aberrants bred, three of the five females bred (one brown, one dark brown and one speckled progressive greying) and three dark brown birds of unknown sex bred.
There was no detectable difference in the percentage of colour aberrants (55%; n = 11; 95% CI: 23–83%) and the percentage of normal-coloured banded birds (65%; n = 306; 95% CI: 60–71%) breeding in the 2019 season (one-sided Pearson χ2 test, P = 0.34, χ2 = 0.174).
Half of the colour-aberrant breeders nested on the periphery of the sub-colony and the other half had interior nest sites. The speckled progressive greying individual failed within 2 weeks of egg-laying, but the other five breeders (four dark brown and one brown) hatched chicks that had normal down coloration. Two dark brown penguins hatched just one chick, while the remaining breeders hatched two (average chicks/pair = 1.33). All five nests raised at least one chick to the size threshold we set for ‘success’ (5 of 6 breeders = 83%; 95% CI: 36–100%), and one dark brown penguin was seen feeding a large normally pigmented chick in crèche. In comparison, 73% (95% CI: 66–79%) of the breeding banded-bird population at Cape Crozier raised at least one chick to the size threshold in the 2019 season.
There was one dark brown-coloured aberrant located in East Crozier that had an unknown breeding status as a result of limited observations due to difficulty of researcher access and was therefore not used in breeding analyses.
Survival
The dilute penguin was first seen in 2009 as an adult and is therefore at least 12 years old. To our knowledge, he has never initiated breeding (defined as at least one egg being laid within a pair) but consistently holds a territory. One of the dark brown penguins was first seen in 2011 at the same territory she currently holds and is therefore at least 10 years old at the time of this study and has bred at least one other time.
Previous sightings
Since 2002, at least 20 additional colour-aberrant Adélie penguins have been sighted at least once at Cape Crozier: seven partially or fully melanistic, eight partially or fully leucistic or progressive greying and three ino (Fig. 5). Some individuals have been seen during multiple seasons, but records are not detailed enough to extrapolate their age. Each aberrant can be individually identified because of their unique pigmentation and their fidelity to nest locations. Interestingly, there is a wide range of partially melanistic and progressive greying colouration. For example, one progressive greying individual had a completely black head, but the remainder of the body was mostly white with some black feathers interspersed in the dorsal region (Fig. 5j). Another individual had white feathers almost everywhere except for the flippers and chin (Fig. 5m). There are typically one to two light ino chicks born to normal-coloured parents every year at Cape Crozier, frequently in the same location (Fig. 5p & q). Some of these chicks may have been albino or leucistic, although it is unlikely and difficult to tell from the photograph records, and these chicks were documented during the down stage when adult plumage is unknown.

Fig. 5. Photographs of select colour-aberrant Adélie penguins seen in previous seasons at Cape Crozier, Ross Island, Antarctica: a.–f. partially or fully melanistic; g. partially leucistic; h.–m. progressive greying; n. ino light; o. ino dark; p. & q. ino chicks. Photograph credits: Megan Elrod (o.), Dennis Jongsomjit (f.), Scott Jennings (n.), Vijay Patil (e.), Ben Saenz (k.), Annie E. Schmidt (b., g.–j., m.), Noah Strycker (a., l., p.) and Viola Toniolo (c., d., q.).
Discussion
To our knowledge, this is the first study to have followed colour-aberrant penguins throughout an entire breeding season. It also appears to be the first to document such a large number and variety of colour aberrations in Adélie penguins. Our findings are consistent with previous observations of colour aberrations in penguin species in both frequency and type of colour aberration (macaroni penguins, Carpenter-Kling et al. Reference Carpenter-Kling, Dyer, Makhado and Pistorius2017; Adélie penguins, Forrest & Naveen Reference Forrest and Naveen2000; African penguins, Traisnel et al. Reference Traisnel, Pichegru, Visser and Edwards2018). This study supports the idea that some form of brown is the most common colour aberration seen in birds, but given the limited reports of brown Adélie penguins in the scientific literature (four in the past 100 years), it also appears to be the colour aberration most likely to be underreported or misidentified as isabelline (Finger et al. Reference Finger, dos Santos, Corrêa, de Brum and Petry2018).
In the 2019 season, 4 of the 12 individuals (1/3) were located in the area of the colony that researchers visit more frequently (an area ~7% the size of West Crozier), indicating that colour aberrations may occur at a rate higher than our estimate of 1:50,000. Because we only recorded individuals that held territories, it is probable that there are more colour aberrants that visited the colony briefly or that were not able to hold a territory. Thus, the prevalence of colour aberrants is probably higher than we report.
In general, the likelihood of colour aberrants breeding was comparable to that of normal-coloured penguins (55% vs 65%), and aberrants raised normal-coloured chicks to a large size at a rate similar to the banded-bird population (83% vs 73%). We recognize that banded individuals may not be representative of the entire Cape Crozier population as they can experience lower breeding success than unbanded individuals (Dugger et al. Reference Dugger, Ballard, Ainley and Barton2006). Additionally, as we only included individuals that had territories, our sample is skewed towards penguins that held territories long enough that we encountered them in our intermittent searches, thus selecting for individuals that may have more breeding experience required to hold this territory (Penney Reference Penney1968). As such, our sample of colour aberrants may inadvertently be ‘higher quality’ than the average penguin at Cape Crozier, which could explain the higher breeding success. However, given the high degree of success of colour aberrants, it appears that having a colour aberration is not detrimental to raising chicks.
It is difficult to accurately classify many of these wild aberrants due to the inability to study feather samples and follow individuals across multiple years. Plus, the nomenclature is continually evolving. Some of the individuals we have called progressive greying may in fact be partially leucistic or differently coloured (grizzled). Specifically, the speckled progressive greying female appeared to have some ‘scaling’ on the feathers, which is indicative of the differently coloured (grizzled) aberration, but without repeated observations across multiple years or feather samples, we cannot definitively know this, and progressive greying is a more common aberration (van Grouw Reference Van Grouw2018). Additionally, although unlikely, some of the ino chicks or more white ino birds seen in previous years, such as the individual in Fig. 5n, may be leucistic.
There were also varying shades of brown pigmentation, and there may be some overlap between the appearance of penguins with the sex-linked brown mutation and those with the non-sex-linked dark brown mutation (Fig. 4). For example, the sex-linked brown penguin at Cape Crozier (Fig. 3e) is lighter than the sex-linked brown Adélie penguin recorded at Turet Point, King George Island (Finger et al. Reference Finger, dos Santos, Corrêa, de Brum and Petry2018). Given this variation, it is possible that some of the dark brown birds at Cape Crozier may be a darker version of the sex-linked brown mutation. However, the dark brown individuals only had feather bleaching on the lower dorsal region as is expected of normal feather wear, whereas the plumage of sex-linked brown birds tends to bleach uniformly (van Grouw Reference Van Grouw2006). Additionally, there is at least one record of a male dark brown penguin at Cape Crozier seen in multiple seasons in the early 2000s, which adds evidence to the notion that this mutation is not the same as the sex-linked lighter brown mutation (https://www.youtube.com/watch?v=6Go5y6xyem0). Thus, it is improbable that any of the dark brown birds were of the sex-linked brown type, but without feather samples, it is impossible to know this. Although some aberrations may be misclassified, for the purposes of understanding how colour aberrations affect survival and breeding behaviour, what is important is that their plumage is visually different from the normal countershading, regardless of what causes that difference.
Although the dark brown aberration appears to be the most common colour aberration in Adélie penguins, little is known about the genetic or physiological causes of it. It could occur during the synthesis of melanin, be caused as a result of different melanin placement within the feather structure or be due to a reduction in melanin production (H. van Grouw, personal communication 2020). Although it is somewhat common in adult Adélie penguins, there are no records of chicks with brown down at Cape Crozier, although one has been documented at Turet Point, King George Island, Maritime Antarctica (J.V.G. Finger, personal communication 2021). This suggests that one mutation could affect the pigmentation deposition of adult plumage while another could affect chick down. It may also be an indication that dark brown is a recessive trait.
This study illustrates that some colour-aberrant penguins are not only able to survive in the wild, but also can successfully breed despite not exhibiting the traditional countershading. The dilute individual, although a non-breeder, has survived for over a decade. Other aberrant individuals, including the brown individual and a dark brown penguin, have bred and survived across many seasons, suggesting that certain colour aberrations may not affect survival, although they may have sub-lethal impacts (Forrest & Naveen Reference Forrest and Naveen2000).
When looking at previous records of aberrants at Cape Crozier, our finding of 20 different colour aberrants over 18 seasons is a conservative estimate of the variety and frequency of aberrants, as only extreme deviations were noted. For example, dark brown penguins were regularly seen but rarely recorded until 2019, except in a couple of instances (G. Ballard, personal communication 2019). That being said, studying these individuals illustrates the wide variety of colour aberrations that can and do survive and breed. It is interesting that melanism was less prevalent than mutations that make the penguin lighter (progressive greying, brown, dilute, etc.), especially because increased melanin levels have been linked to differing metabolism rates and stronger responses to stressors (Roulin & Ducrest Reference Roulin and Ducrest2011, Corbel et al. Reference Corbel, Legros, Haussy, Jacquin, Gasparini, Karimi and Frantz2016). This could be because dark individuals may not forage as efficiently during the summer with increased light levels, as evidenced in the black sparrowhawk (Accipiter melanoleucus, Smith), where dark morphs decreased their foraging activity when light levels increased (Tate & Amar Reference Tate and Amar2017). The limited number of melanistic individuals could also be a result of differing abilities to thermoregulate, such as with bearded vultures (Gypaetus barbatus, Linnaeus), where the darker varieties had more trouble thermoregulating and got warmer than their lighter counterparts (Margalida et al. Reference Margalida, Negro and Galván2008). Breeding success has been linked to colouration, with darker passerines breeding more successfully in colder environments but lighter birds performing better when it was warmer (Sirkiä et al. Reference Sirkiä, Virolainen and Laaksonen2010). If Antarctica continues to warm, these lighter varieties may hold an advantage.
In conclusion, colour aberrations are diverse in Adélie penguins, like other penguin species, and do not appear to affect the likelihood of breeding. The prevalence of colour aberrations is extremely low, perhaps even lower than suggested by previous studies, although it is difficult to know this given the lack of literature on the sightings. We recognize that these analyses were conducted with a small sample size and are unable to detect small or medium effects within the data. However, given that Cape Crozier is one of the largest Adélie penguin colonies in the world, it may be difficult to find a larger dataset of colour aberrations in a wild population. With more reporting by the scientific community and others (e.g. tourists), we can better estimate the prevalence and variety of colour aberrations in Adélie penguins in order to monitor for any changes in frequency due to environmental variables or inbreeding within a population. Additionally, by collecting feather samples from aberrant individuals, we can more confidentially identify the type of colour aberrations and the sex of the bird, allowing us to investigate how colour aberrations affect males and females. More data must be collected by following these individuals across many years in order to better understand survival rates of colour-aberrant Adélie penguins, how the lack of countershading affects their ability to forage and the visual cues used in mate selection.
Acknowledgements
We are incredibly appreciative of all previous biologists stationed at Cape Crozier for their data collection on and photographic records of colour-aberrant birds. We want to especially thank Amélie Lescroёl for assistance with data collection during the 2019 season. Thank are given to H. van Grouw, J.V.G. Finger and the anonymous reviewers for providing important guidance in correctly identifying and understanding each colour aberration, as well as for their comments and revisions that made the manuscript better. This is Point Blue Conservation Science contribution #2345.
Financial support
We appreciate writing support from D.G. Ainley. We thank the United States Antarctic Program for logistical support while in the field. We also thank the National Science Foundation for financial support through grants 1543498 and 1834986.
Author contributions
PML, VM and AES designed the experiment. All authors collected the data. PML and AES ran the analyses. All authors contributed to writing and editing the manuscript.








