1. Introduction
Dispersed mobile belts provide records of ever-changing continent configurations, especially the assembly and break-up of large continental masses throughout Earth's history (Condie, Reference Condie2005). Ultrahigh-temperature (UHT) metamorphism that followed an isothermal decompression (ITD) path, as evident in some granulite terrains, is possibly a consequence of the existence of particularly large continents (Yoshida et al. Reference Yoshida, Jacobs, Santosh, Rajesh, Yoshida, Windley and Dasgupta2003) and may thus hold clues for the reconstruction of former supercontinents. Such UHT–ITD metamorphism is preserved at several widely dispersed localities in the Indo-Antarctic region, with the Eastern Ghats Mobile Belt (EGMB) on the Indian subcontinent occupying a key position in the reconstruction of Meso- to Neoproterozoic continent configuration. Several occurrences of UHT-diagnostic metamorphic mineral assemblages in the EGMB have attracted petrologists to studying the area over the last decade (Dasgupta & Sengupta, Reference Dasgupta, Sengupta, Yoshida, Windley and Dasgupta2003; Lal, Reference Lal and Mohan2003; Dasgupta, Raith & Sarkar, Reference Dasgupta, Raith and Sarkar2008; Mukhopadhyay & Basak, Reference Mukhopadhyay and Basak2009; Dasgupta, Bose & Das, Reference Dasgupta, Bose and Das2013). One of the diagnostic features of UHT metamorphism is the stable coexistence of sapphirine+quartz, which has been reported from a few localities in the EGMB (Lal, Ackermand & Upadhyay, Reference Lal, Ackermand and Upadhyay1987; Kamineni & Rao, Reference Kamineni and Rao1988; Dasgupta et al. Reference Bhowmik, Dasgupta, Hoernes and Bhattacharya1995; Bose et al. Reference Bose, Fukuoka, Sengupta and Dasgupta2000; Dharma Rao, Santosh & Chmielowski, Reference Dharma Rao, Santosh and Chmielowski2012). Apart from the assemblage sapphirine+spinel+quartz, that of highly aluminous orthopyroxene+sillimanite+quartz as well as the occurrence of osumilite are further indications of UHT metamorphism and have been noted in the EGMB (Lal, Ackermand & Upadhyay, Reference Lal, Ackermand and Upadhyay1987; Dasgupta et al. Reference Dasgupta, Sanyal, Sengupta and Fukuoka1994; Bhattacharya & Kar, Reference Bhattacharya and Kar2002).
In particular, sapphirine-bearing granulites have become the focus of numerous petrological studies in that area (Lal, Ackermand & Upadhyay, Reference Lal, Ackermand and Upadhyay1987; Kamineni & Rao, Reference Kamineni and Rao1988; Sengupta et al. Reference Sengupta, Dasgupta, Bhattacharya, Fukoka, Chakraborti and Bhowmik1990, Reference Sengupta, Karmakar, Dasgupta and Fukuoka1991, Reference Sengupta, Dasgupta, Ehl and Raith1997a ,Reference Sengupta, Sanyal, Dasgupta, Fukoaka, Ehl and Pal b , 1999; Dasgupta et al. Reference Dasgupta, Sanyal, Sengupta and Fukuoka1994, Reference Bhowmik, Dasgupta, Hoernes and Bhattacharya1995; Shaw & Arima, Reference Shaw and Arima1996; Mohan, Tripathi & Motoyashi, Reference Mohan, Tripathi and Motoyashi1997; Mukhopadhyay & Bhattacharya, Reference Mukhopadhyay and Bhattacharya1997; Pal & Bose, Reference Pal and Bose1997; Shaw & Arima, Reference Shaw and Arima1998; Bose et al. Reference Bose, Fukuoka, Sengupta and Dasgupta2000, Reference Bose, Das, Dasgupta, Miura and Fukuoka2006; Gupta et al. Reference Gupta, Bhattacharya, Raith and Nanda2000; Rickers, Mezger & Raith, Reference Rickers, Mezger and Raith2001; Bhattacharya & Kar, Reference Bhattacharya and Kar2002; Bhattacharya et al. Reference Bhattacharya, Kar, Teixeira and Basei2003; Sarkar et al. Reference Sarkar, Santosh, Dasgupta and Fukuoka2003; Bose & Das, Reference Bose and Das2007; Das et al. Reference Das, Bose, Karmakar, Dunkley and Dasgupta2011; Dharma Rao & Chmielowski, Reference Dharma Rao and Chmielowski2011; Korhonen et al. Reference Korhonen, Saw, Clark, Brown and Bhattacharya2011; Dharma Rao, Santosh & Chmielowski, Reference Dharma Rao, Santosh and Chmielowski2012). In addition, petrological studies on calc-silicate granulite (Dasgupta et al. Reference Dasgupta, Sengupta, Mondal and Fukuoka1993; Bhowmik et al. Reference Bhowmik, Dasgupta, Hoernes and Bhattacharya1995) as well as mafic and orthopyroxene-bearing quartzo-feldspathic granulite (Dasgupta et al. Reference Dasgupta, Sengupta, Guha and Fukuoka1991, Reference Dasgupta, Sengupta, Mondal and Fukuoka1993; Sengupta et al. Reference Sengupta, Dasgupta, Bhui, Ehl and Fukoaka1996; Mohan, Singh & Sachan, Reference Mohan, Singh and Sachan2003) have been carried out.
We discovered a hitherto unknown locality of sapphirine–spinel granulite that contains sapphirine+quartz in an area around Diguva Sonoba in the southwestern part of the EGMB (Fig. 1). In this paper we present new petrographic and mineral chemical data, based on which we provide new constraints on the metamorphic evolution of these UHT granulites as derived from well-constrained micro-structural relations, mineral assemblages and forward modelling (isochemical pseudosection). Our new results, in comparison with those from other localities of the EGMB, make it possible to re-evaluate the regional tectonothermal evolution of the EGMB.

Figure 1. (a) Reference map of India showing the position of the Eastern Ghats Mobile Belt. (b) Lithological map of the Eastern Ghats Mobile Belt (after Ramakrishnan, Nanda & Augustine, Reference Ramakrishnan, Nanda and Augustine1998). Abbreviations: WCZ – Western Charnockite Zone; WKZ – Western Khondalite Zone; CMZ – Central Migmatite Zone; EKZ – Eastern Khondalite Zone. (c) Geological map of the area around Diguva Sonaba.
2. Regional geology of the Eastern Ghats Mobile Belt
On the basis of dominant lithological assemblages, Ramakrishnan, Nanda & Augustine (Reference Ramakrishnan, Nanda and Augustine1998) divided the EGMB into four broadly longitudinal zones (Fig. 1), which, from northwest to southeast, are: (i) the Western Charnockite Zone (WCZ), which consists of enderbite, mafic granulites and charnockite with enclaves of mafic–ultramafic rocks and minor amounts of metapelites; (ii) the Western Khondalite Zone (WKZ), which is dominated by khondalite, quartzite, calc-silicate rocks and sapphirine–spinel granulite; (iii) the Central Migmatite Zone (CMZ), which consists of migmatite gneisses and leptynites; and (iv) the Eastern Khondalite Zone (EKZ), which is lithologically similar to the WKZ. The metamorphic rocks of the EGMB display a gradual increase in metamorphic grade from the southwest to the northeast.
The ages of both the protoliths and metamorphic events in the EGMB remain poorly constrained. Whole-rock Rb–Sr (3100±20 Ma in khondalite from Puri, Vinogradov et al. Reference Vinogradov, Tugarinov, Zhykov, Stapnikova, Bibikova and Korre1964; 2600±63 Ma in charnockite, Paul et al. Reference Paul, Berman, Menaughton, Fletcher, Potts, Ramakrishnan and Augustine1990), Sm–Nd (2900±36 Ma in charnockites and mafic granulites, Paul et al. Reference Paul, Berman, Menaughton, Fletcher, Potts, Ramakrishnan and Augustine1990) and inherited U–Pb zircon ages of 2580–2600 Ma (Vinogradov et al. Reference Vinogradov, Tugarinov, Zhykov, Stapnikova, Bibikova and Korre1964) point to an Archaean crustal component in the protoliths of the EGMB metamorphites.
Based on field relationships and petrography, three major deformational and metamorphic events can be distinguished. During an earlier granulite-facies metamorphism (M1), the supracrustal rocks were deformed under UHT conditions as evidenced by sapphirine–spinel–quartz-bearing parageneses found in various domains within the EGMB. Later, the EGMB was intruded by voluminous enderbitic charnockites that subsequently underwent deformation again under granulite-facies conditions (M2). This M2 event is widely recognized throughout the EGMB. Vast granite and anorthosite bodies that were emplaced post-M2 show evidence of further, retrograde metamorphic overprint (M3).
The absolute ages of the various metamorphic events remain enigmatic. Mezger & Cosca (Reference Mezger and Cosca1999) distinguished between two segments in the EGMB, separated by the Godavari Rift. The northern segment is dominated by age data around 1.0 to 0.5 Ga and Nd model ages of 3.9 to 3.2 Ga, whereas the southern segment is distinguished by a major thermal event around 1.6 Ga and Nd model ages between 2.3 and 2.8 Ga (Rickers, Mezger & Raith, Reference Rickers, Mezger and Raith2001).
The age of the earlier UHT metamorphism (M1) preserved locally in Mg–Al-rich granulites remains open to debate. Both pre-Grenvillian and syn-Grenvillian ages have been suggested (Mezger & Cosca, Reference Mezger and Cosca1999). Possible pre-Grenvillian ages are 1630 Ma (Upadhyay, Gerdes & Raith, Reference Upadhyay, Gerdes and Raith2009) and c. 1760 Ma (Bose et al. Reference Bose, Dunkley, Dasgupta, Das and Arima2011), the age of mafic magmatism (Sengupta et al. Reference Sengupta, Dasgupta, Raith, Bhui and Ehl1999). Most recent U–Pb age determinations on in situ zircon and monazite grains indicate UHT metamorphic conditions having lasted for at least 50 Ma, possibly as long as 200 Ma, from c. 1130 to 930 Ma (Korhonen et al. Reference Korhonen, Clark, Brown, Bhattacharya and Taylor2013), supporting previous age data of 1030 to 990 Ma from Bose et al. (Reference Bose, Dunkley, Dasgupta, Das and Arima2011).
In Pan-African times, the EGMB experienced a further major thermal overprint at amphibolite-facies conditions. This is indicated by a U–Th–Pb age of 573±2 Ma obtained on syntectonic monazite in garnet–sillimanite gneiss (Upadhyay et al. Reference Upadhyay, Raith, Mezger, Bhattacharya and Kinny2006) and emplacement of nepheline syenite, pegmatite and megacrystic granite at around the same time (Paul et al. Reference Paul, Berman, Menaughton, Fletcher, Potts, Ramakrishnan and Augustine1990; Aftalion et al. Reference Aftalion, Bowes, Dash and Fallick2000; Upadhyay et al. Reference Upadhyay, Raith, Mezger, Bhattacharya and Kinny2006). No evidence of Pan-African granulite-facies metamorphism exists, however, in the EGMB.
3. Geological setting of the study area
Diguva Sonaba is situated at about 120 km northwest of the Vishakhapatnam district, Andhra Pradesh, within the granulite terrain of the EGMB. The study area (longitude 82°50′E to 82°58′25″E and latitude 18°10′N to 18°13′29″N) comprises a variety of granulitic rocks, mainly charnockites, mafic granulites, leptynite, khondalite, sapphirine–spinel granulite, calc-granulite and migmatitic gneisses (Fig. 1). All of these rocks were subjected to polyphase deformation and metamorphism. Garnet-bearing charnockite is coarse to medium grained and dark grey in colour (Fig. 2a). Mafic granulite is coarse to medium grained and dark (Fig. 2b). Leptynite is characterized by a massive fabric due to a predominance of K-feldspar and quartz (Fig. 2c). Veins of perthite and quartz are present. Evenly distributed small garnet grains are common to many of these rocks (Fig. 2c). Garnet porphyroblasts of up to 4×3 cm in size (Fig. 2d) are present in the leptynite. Pale to pinkish coloured khondalite is the dominant rock type in the study area (Fig. 2e). The oldest magmatic rocks in the area are the protoliths of mafic granulite that occur intercalated with khondalite and charnockite. The migmatites show layers of parallel leucosomes with thicker melanosomes (Fig. 2f), suggesting formation by grain-scale distribution of melt, because grain-scale flow is probably ineffective at transferring melt over large distances (Rutter, Reference Rutter and Holness1997). Sapphirine–spinel-bearing granulite is coarse to medium grained and dark in colour. Blue crystals of sapphirine and dark green spinel are recognized in hand specimen (Fig. 2g). The calc-granulite is interbanded with khondalite and is usually banded, and it is marked by grooved surfaces on weathered outcrops (Fig. 2h). The most abundant planar features of the various metamorphic rocks in the study area are foliations. From the orientation pattern of the foliations in three structurally similar sub-areas and corresponding β-maxima in stereograms (Fig. 3), refolding of the syn-metamorphic foliation during a later deformational event is evident.

Figure 2. Field photographs of the principal rock types in the study area: (a) Massive charnockite exposure with greasy look due to predominance of grey quartz with waxy lustre and dark brown garnet; (b) black to dark grey mafic granulite; dark patches are rich in ferromagnesian minerals; (c) grey leptynite with high proportion of finely dispersed, small garnet grains; (d) yellowish weathered leptynite with large garnet accumulations (dark red-brown); (e) medium- to coarse-grained, yellowish-greyish weathered khondalite; (f) banded migmatite with a separation into restitic and coarse-grained quartzo-feldspathic leucosome layers; (g) sapphirine–spinel-bearing granulite with bluish crystals of sapphirine and dark green spinel; (h) calc-silicate rock with serrated pattern due to differential weathering.
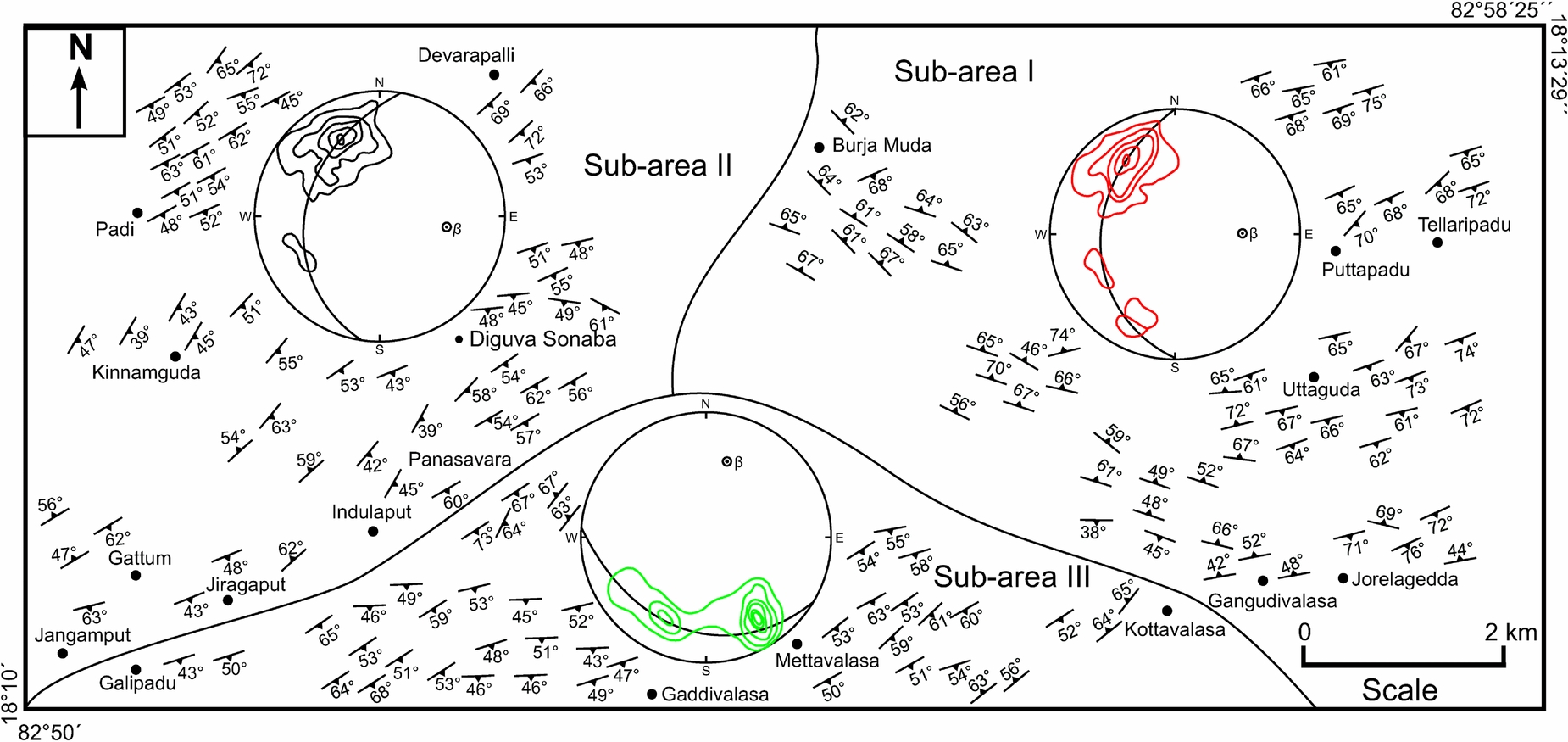
Figure 3. Structural map of the study area showing prominent S-surfaces (foliation) along with lower hemisphere of equal area projection of poles (N = 161) to foliation (contour interval: 3, 6, 9 and 12% per 1% area). The blocks demarcated by continuous lines correspond to sub-areas.
4. Petrography
4.a. Charnockite, enderbite and mafic granulite
All of these rocks are characterized by a granoblastic texture formed by a mosaic of garnet, orthopyroxene, plagioclase, quartz and K-feldspar. Garnet occurs as coarse- to fine-grained anhedral to euhedral crystals. Inclusions of quartz, biotite, plagioclase and ilmenite are present in garnet (Fig. 4a), suggesting the prograde reaction
 \begin{eqnarray}
&&\textrm{2 biotite + 6 quartz = garnet + orthopyroxene} \nonumber\\
&&\quad \textrm{ +\, 2 K - feldspar + 2 H}_{\rm 2} {\rm O}
\end{eqnarray}
\begin{eqnarray}
&&\textrm{2 biotite + 6 quartz = garnet + orthopyroxene} \nonumber\\
&&\quad \textrm{ +\, 2 K - feldspar + 2 H}_{\rm 2} {\rm O}
\end{eqnarray}
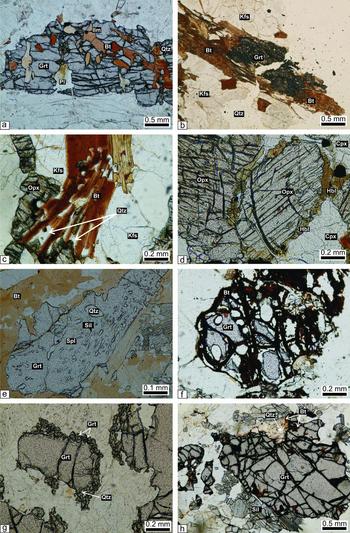
Figure 4. Photomicrographs of charnockite, mafic granulite, khondalite and leptynite examined in this study. (a) Quartz, biotite, plagioclase and ilmenite occurring as inclusions in garnet porphyroblasts (sample no. 1113); (b) biotite rims around corroded garnet (sample no. 1113); (c) biotite–quartz symplectite around orthopyroxene and K-feldspar (sample no. 1113); (d) hornblende around orthopyroxene and clinopyroxene (sample no. 1101); (e) poikiloblastic garnet containing inclusions of quartz, sillimanite and spinel (sample no. 1100); (f) biotite along fractures and rims of relict garnet (sample no. 1105); (g) presence of comparatively small second-generation garnet with quartz around xenoblasts of garnet (sample no. 1100); (h) development of sillimanite and biotite–quartz symplectites around corroded garnet porphyroblast (sample no. 1100).
Secondary biotite rimming garnet, orthopyroxene and K-feldspar in the garnet enderbite and charnockite indicates that Reaction (1) also took place in the retrograde direction.
Hornblende occurs as both prograde and retrograde generation. In the mafic granulite relict hornblende occurs as inclusions within pyroxenes, suggesting the prograde reaction
 \begin{eqnarray}
&&\textrm{ 2 hornblende + 8 quartz = 3 orthopyroxene}\nonumber\\
&&\quad +\, \textrm{ 2 clinopyroxene + 4 plagioclase + 2 H}_{\rm 2} {\rm O}\quad
\end{eqnarray}
\begin{eqnarray}
&&\textrm{ 2 hornblende + 8 quartz = 3 orthopyroxene}\nonumber\\
&&\quad +\, \textrm{ 2 clinopyroxene + 4 plagioclase + 2 H}_{\rm 2} {\rm O}\quad
\end{eqnarray}
whereas retrograde hornblende occurs as rims around ortho- and clinopyroxene (Fig. 4d), indicating that Reaction (2) was partially reversed during retrograde overprint.
Accessory minerals include magnetite, ilmenite, apatite, titanite, spinel and pyrrhotite. Magnetite occurs as elongated grains in the matrix and also along the cleavage planes in pyroxenes and biotite.
4.b. Leptynite and khondalite
Leptynite and garnet-leptynite exhibit a mostly granoblastic texture defined by an interlocking arrangement of quartz, K-feldspar, plagioclase and garnet. Myrmekitic intergrowths of quartz and plagioclase, and perthitic and antiperthic intergrowths in feldspars are common. With gradually increasing content of biotite, leptynite changes into biotite-gneisses (±garnet).
Garnet typically occurs as medium- to coarse-grained xenoblasts in khondalite. Poikiloblastic garnet contains inclusions of quartz, sillimanite and spinel (Fig. 4e). Retrograde biotite occurs along fractures and rims of garnet (Fig. 4f). Symplectites of secondary garnet II+quartz form rims around xenoblasts of garnet I (Fig. 4g).
In both the khondalite and leptynite, rounded blebs of biotite and subangular quartz grains occur within garnet and also within perthite that is present in the matrix. This textural relationship may be due to the reaction
In the sillimanite-bearing assemblages, sillimanite occurs, together with biotite and quartz, also as inclusions in garnet and K-feldspar, which may be attributed to the reaction
 \begin{eqnarray}
&&\textrm{ biotite + sillimanite + 2 quartz = garnet}\nonumber\\
&&\quad \textrm{ +\, K - feldspar + H}_{\rm 2} {\rm O}\end{eqnarray}
\begin{eqnarray}
&&\textrm{ biotite + sillimanite + 2 quartz = garnet}\nonumber\\
&&\quad \textrm{ +\, K - feldspar + H}_{\rm 2} {\rm O}\end{eqnarray}
Coarse-grained biotite and biotite–quartz symplectites and sillimanite rimming xenoblasts of garnet (Fig. 4h) presumably formed owing to retrograde hydration through reversal of reactions (3) and (4).
4.c. Sapphirine and/or spinel-bearing granulite
4.c.1. Sapphirine-bearing granulite without spinel
Aggregates of sillimanite (up to 0.8 mm in length), which mimic the shape and simple twinning of coarse-grained kyanite blades (Fig. 5a), have been interpreted as pseudomorphs after kyanite (Lal et al. Reference Lal, Ackermand, Raith, Raase and Seifert1984; Raith, Karmakar & Brown, Reference Raith, Karmakar and Brown1997; Brandt et al. Reference Brandt, Schenk, Raith, Appel, Gerdes and Srikantappa2011). This implies a prograde assemblage of biotite–orthopyroxene–garnet–plagioclase–kyanite, and a peak metamorphic assemblage of orthopyroxene–garnet–sillimanite–alkali feldspar–rutile–quartz.

Figure 5. Photomicrographs of sapphirine-bearing granulite without spinel: (a) polycrystalline aggregate of sillimanite pseudomorphs after coarse blades of earlier kyanite (sample no. 1111); (b) sapphirine rimmed by sillimanite in matrix of cordierite (sample no. 1111); (c) radial replacement of orthopyroxene by sapphirine mantled by cordierite (sample no. 1111); (d) sapphirine separated from orthopyroxene by cordierite (sample no. 1111); (e) sapphirine separated from quartz by a moat of sillimanite and orthopyroxene (sample no. 1112); (f) sapphirine in a matrix of symplectitic intergrowth of orthopyroxene and cordierite (sample no. 1107); (g–h) orthopyroxene–cordierite/sillimanite–K-feldspar–quartz symplectite, which possibly formed from the breakdown of osumilite (sample no. 1112).
The post-peak mineral assemblages are represented by various types of reaction coronas. These coronas occur within a matrix of cordierite, quartz, K-feldspar and plagioclase. The corona includes the following types (minerals arranged from core to rim): (a) sapphirine–sillimanite–cordierite (Fig. 5b); (b) sapphirine–cordierite–orthopyroxene (Fig. 5c, d); (c) sapphirine–sillimanite–orthopyroxene (Fig. 5e), in which sapphirine is separated from quartz by successive reaction coronas of sillimanite and orthopyroxene; and (d) sapphirine–sillimanite–cordierite–orthopyroxene. Other assemblages include: sapphirine–cordierite symplectites, orthopyroxene–cordierite symplectites (Fig. 5f), cordierite–orthopyroxene–K-feldspar–quartz (Fig. 5g), and coarse-grained orthopyroxene–sillimanite–K-feldspar–quartz (Fig. 5h), which form symplectitic intergrowths.
4.c.2. Spinel-bearing granulite without sapphirine
This rock type can contain three different mineral assemblages, each characterized by specific types of corona.
The paragenesis spinel–sillimanite–cordierite–quartz–orthopyroxene–magnetite displays, in places, spinel–magnetite–sillimanite–orthopyroxene coronas in which spinel with exsolved magnetite is successively mantled by sillimanite and orthopyroxene in a matrix of quartz (Fig. 6a). Where the peak metamorphic mineral assemblage is spinel–magnetite–sillimanite, spinel is isolated from cordierite (Fig. 6b). Other minerals that form symplectites around spinel include orthopyroxene–sillimanite–cordierite (Fig. 6c).
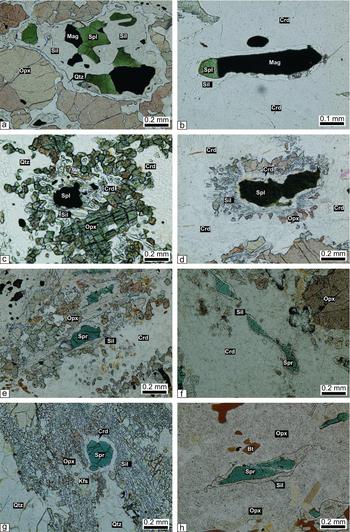
Figure 6. Photomicrographs of spinel-bearing granulite without sapphirine (a–c) and of sapphirine–spinel-bearing granulite (d–h): (a) spinel and successively rimmed by sillimanite, quartz and orthopyroxene (sample no. 1122); (b) spinel and magnetite rimmed by sillimanite in the matrix of cordierite (sample no. 1122); (c) spinel and quartz are isolated from each other by orthopyroxene–cordierite–sillimanite symplectitic intergrowth (sample no. 1122); (d) spinel is successively rimmed by cordierite, sillimanite and orthopyroxene (sample no. 1108); (e) sapphirine is successively rimmed by sillimanite and fine-grained orthopyroxene aggregates in a matrix of cordierite (sample no. 1102); (f) sapphirine is completely rimmed by sillimanite in the matrix of cordierite (sample no. 1102); (g) sapphirine rimmed by cordierite in a matrix of symplectitic intergrowth of orthopyroxene–sillimanite–K-feldspar–quartz (sample no. 1102); (h) sapphirine completely rimmed by sillimanite within orthopyroxene (sample no. 1102).
In the assemblage spinel–garnet–K-feldspar±phlogopite±sillimanite, garnet and K-feldspar occur as inclusions in spinel.
4.c.3. Sapphirine–spinel-bearing granulite
Two types of this rock are distinguished:
(1) A granulite with the paragenesis sapphirine, spinel, orthopyroxene, cordierite, quartz, sillimanite, (cordierite–orthopyroxene–K-feldspar–quartz)-bearing symplectite±plagioclase, magnetite, rutile, zircon and lamellar intergrowths of haematite–ilmenite.
This rock displays a number of reaction coronas and the minerals present in them constitute the following mineral assemblages: (a) spinel–sillimanite–cordierite–orthopyroxene (Fig. 6d); (b) sapphirine–sillimanite–cordierite–orthopyroxene (Fig. 6e); (c) sapphirine–sillimanite–cordierite (Fig. 6f); (d) sapphirine–cordierite mantled by symplectites of orthopyroxene, sillimanite, quartz and K-feldspar (Fig. 6g); (e) sapphirine–sillimanite–orthopyroxene (Fig. 6h); (f) sapphirine–spinel–magnetite–rutile–ilmenite–haematite–sillimanite–orthopyroxene (Fig 7a); (g) spinel–sapphirine–sillimanite–orthopyroxene–quartz–K-feldspar (Fig. 7b); (h) (spinel–sapphirine)–cordierite–orthopyroxene (Fig. 7c). These coronas commonly occur in the matrix of quartz and symplectites of cordierite–orthopyroxene–K-feldspar–quartz. In places, coronas are present within the matrix of this symplectite only.

Figure 7. Photomicrographs of sapphirine–spinel-bearing granulite: (a) rutile–ilmenite–haematite lamellae within spinel (sample no. 1104); (b) sapphirine, magnetite and spinel rimmed by sillimanite in the matrix of quartz (sample no. 1104); (c) spinel and sapphirine successively rimmed by cordierite and orthopyroxene (sample no. 1110); (d) spinel rimmed by sapphirine within garnet (sample no. 1110); (e) spinel in grain contact with quartz (both in plain-polarized light and cross-polarized light) (sample no. 1108); (f) sapphirine and spinel inclusion within sillimanite (sample no. 1108); (g) sapphirine in grain contact with quartz (both in plain-polarized light and cross-polarized light) (sample no. 1108); (h) calc-granulite showing granoblastic microstructure. Grossular forms a reaction rim between calcite, clinopyroxene and scapolite (sample no. 1109); Bt – biotite; Cal – calcite; Cpx – clinopyroxene; Crd – cordierite; Grt – garnet; Hbl – hornblende; Ilm – ilmenite; Kfs – K-feldspar; Ky – kyanite; Mag – magnetite; Opx – orthopyroxene; Pl – plagioclase; Qtz – quartz; Rt – rutile; Scp – scapolite; Sil – sillimanite; Spl – spinel; Spr – sapphirine.
(2) The second variety is made up of sapphirine, spinel, garnet, sillimanite, phlogopite, quartz and K-feldspar, with accessory ilmenite and zircon. The rock contains the following types of coronas: (a) spinel–sapphirine in garnet (Fig. 7d); (b) spinel–sapphirine inclusions in quartz (Fig. 7e); (c) spinel–sapphirine inclusions in sillimanite (Fig. 7f); and (d) spinel–sapphirine–cordierite–garnet.
In the assemblage spinel–garnet–phlogopite–quartz–magnetite–ilmenite, spinel/sapphirine occurs in contact with quartz (Fig. 7e, g).
The textural relationships of the different minerals present in the above rock types are summarized as follows:
Two textural types of orthopyroxene are present. An older generation of orthopyroxene I is coarse grained and anhedral to subhedral (up to 2 cm in length). Prograde corroded biotite (phlogopite) occurs as inclusions in this orthopyroxene. The second generation of orthopyroxene is fine to medium grained, anhedral and forms coronas with spinel, sapphirine, sillimanite and cordierite. It also occurs as finger-like trails in symplectitic intergrowths of cordierite–orthopyroxene–K-feldspar–quartz and orthopyroxene–sillimanite–quartz±K-feldspar (Fig. 5g, h).
Cordierite occurs in various types of coronas around sillimanite, sapphirine, spinel and orthopyroxene. Cordierite also forms symplectitic intergrowths with orthopyroxene, K-feldspar and quartz (Fig. 5g).
Like cordierite and orthopyroxene, two generations of sillimanite are present. Sillimanite I is not involved in the formation of corona structures and the symplectitic intergrowths. It occurs as coarse prismatic grains or needles in association with coarse-grained orthopyroxene. Sillimanite II is observed in coronas around sapphirine and spinel (Figs 5b, 7b). In some samples sillimanite II also forms symplectitic intergrowths with orthopyroxene, K-feldspar and quartz (Fig. 5h).
Sapphirine occurs in grain contact with quartz, which indicates that sapphirine+quartz was stable during the thermal peak of metamorphism (Fig. 7g). In other samples, sapphirine and quartz are separated by retrograde corona rims. The presence of ilmenite–haematite intergrowths within spinel, particularly where it mantles sapphirine (Fig. 7a, b), suggests high oxygen fugacity during the formation of the reaction coronas involving sapphirine, spinel, sillimanite and orthopyroxene.
In most of the samples, spinel is fine grained, green in colour and anhedral. In a few samples it is intergrown with magnetite suggesting mutual exsolution (Fig. 6a, b). In most of the samples, spinel occurs in the cores of sapphirine and forms intergrowths with magnetite and K-feldspar (Fig. 7b).
In one sample (no. 1110), garnet occurs in the corona where spinel is successively rimmed by sapphirine and garnet in a matrix of quartz. This textural relationship may be explained by the reaction
Although osumilite could not be identified in the sapphirine–spinel-bearing rocks, its crystallization during the thermal peak of metamorphism is suggested by the common occurrence of symplectitic intergrowth of cordierite–orthopyroxene–K-feldspar–quartz, which is a breakdown product of osumilite through the reaction
 \begin{eqnarray}
&&\textrm{ 3 osumilite = 2 cordierite + orthopyroxene}\nonumber\\
&&\quad \textrm{ +\, 3 K - feldspar + 7 quartz}
\end{eqnarray}
\begin{eqnarray}
&&\textrm{ 3 osumilite = 2 cordierite + orthopyroxene}\nonumber\\
&&\quad \textrm{ +\, 3 K - feldspar + 7 quartz}
\end{eqnarray}
The breakdown of osumilite to this type of symplectite was first suggested by Schreyer & Seifert (Reference Schreyer and Seifert1967) and has since been reported by several workers (Berg & Wheeler, Reference Berg and Wheeler1976; Ellis, Reference Ellis1980; Grew, Reference Grew1982; Lal, Ackermand & Upadhyay, Reference Lal, Ackermand and Upadhyay1987; Nowicki, Frimmel & Waters, Reference Nowicki, Frimmel and Waters1995; Bhattacharya & Kar, Reference Bhattacharya and Kar2002; Adjerid et al. Reference Adjerid, Godard, Ouzeganes and Kienast2013). In some samples the symplectite pseudomorphs after osumilite are devoid of cordierite and contain orthopyroxene–K-feldspar–sillimanite–quartz, which may be related to the reaction
 \begin{eqnarray}
&&\textrm{ osumilite = orthopyroxene + K - feldspar}\nonumber\\
&&\quad \textrm{ +\, 2 sillimanite + 3 quartz}
\end{eqnarray}
\begin{eqnarray}
&&\textrm{ osumilite = orthopyroxene + K - feldspar}\nonumber\\
&&\quad \textrm{ +\, 2 sillimanite + 3 quartz}
\end{eqnarray}
4.d. Calc-granulite
This rock consists mainly of diopside, calcite, scapolite, wollastonite and plagioclase. Diopside occurs as medium to coarse grained, in places poikiloblastic, grains that contain numerous inclusions of quartz, plagioclase, scapolite, titanite, spinel, magnetite and biotite. Calcite is mostly coarse grained, subidioblastic to xenoblastic. Fine-grained xenoblastic calcite occurs with fine-grained quartz aggregates. Fine-grained calcite occurs as inclusions in diopside and vesuvianite. The association of wollastonite with calcite or quartz is explained by the reaction
Grossular occurs as medium- to coarse-grained xenoblasts. It mostly forms a corona rim along with quartz between diopside and scapolite (Fig. 7h), which suggests the following reaction
Grossular also forms rims between calcite and scapolite (Fig. 7h) suggesting the reaction
5. Mineral chemistry
Electron microprobe analyses (EMPA) were carried out on an automated energy-dispersive electron microanalyser JEOL JSM-5300 and LINK QX2000J system, operated at an acceleration voltage of 15 kV and a specimen current of 15 nA at the Geological Institute, Yokohama National University, Japan. Analyses were refined using the LINK ZAF-4/FLS correction program. Eleven representative samples were chosen for detailed mineral chemical analyses. Routine detection limits are in the order of 0.008 wt% for most elements. The representative microprobe analyses of the analysed minerals are listed in Tables S1–10 in the online Supplementary Material available at http://journals.cambridge.org/geo. Mineral abbreviations used are taken from Kretz (Reference Kretz1983).
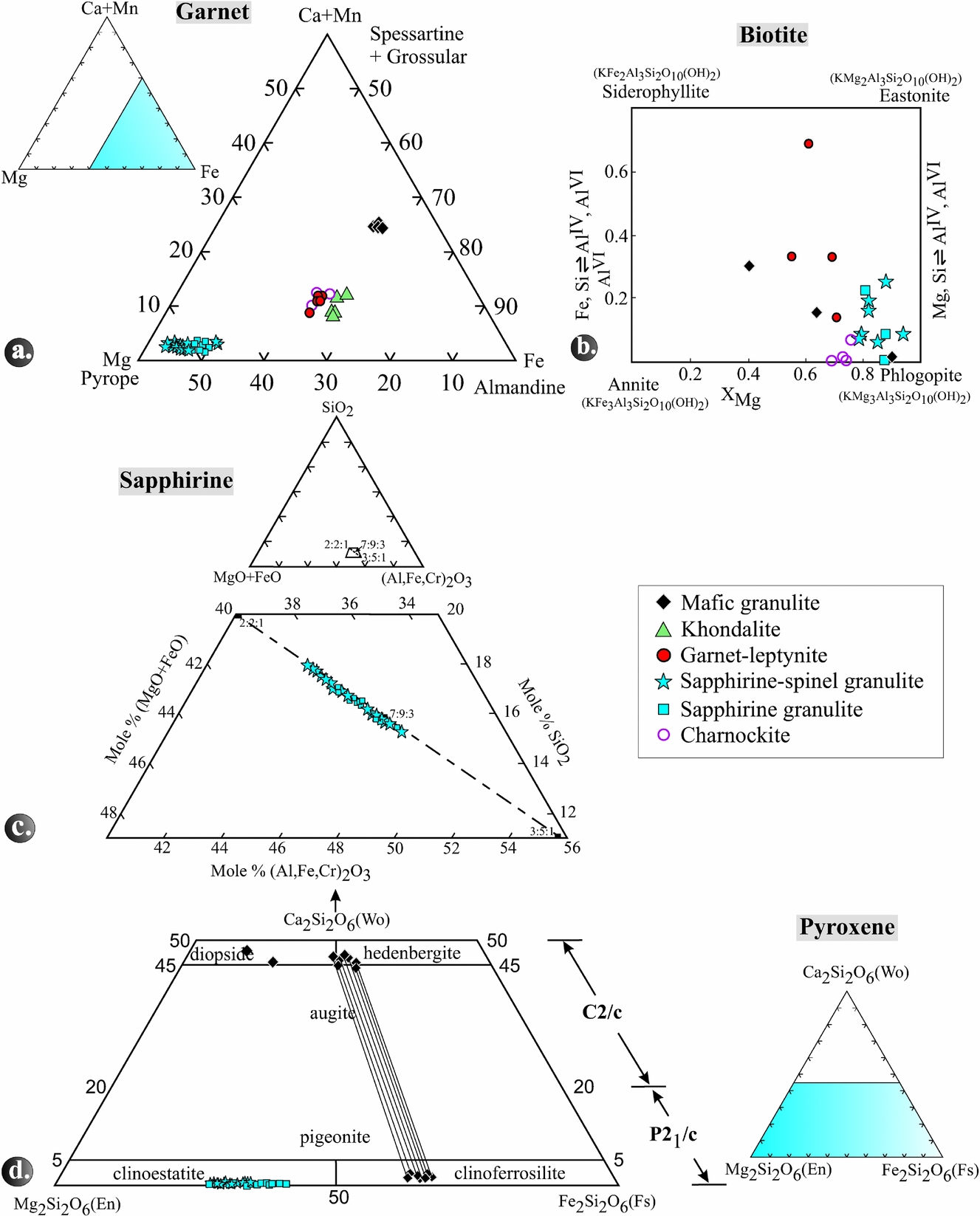
5.a. Garnet
Garnet in the different rock types is essentially a solid solution of almandine, pyrope and grossular, with minor spessartine (Fig. 8a; Table S1 in the online Supplementary Material available at http://journals.cambridge.org/geo).The pyrope content in the garnet is highest in sapphirine–spinel granulite, followed by garnet-leptynite, khondalite and mafic granulite. The XMg ranges from 0.30 to 0.54 in Grt–Spr–Spl–Sil-Qtz coronas, 0.48 to 0.51 in Grt–Opx–Sil–Qtz coronas and 0.47 to 0.50 in Grt–Phl–Crd–Qtz coronas.
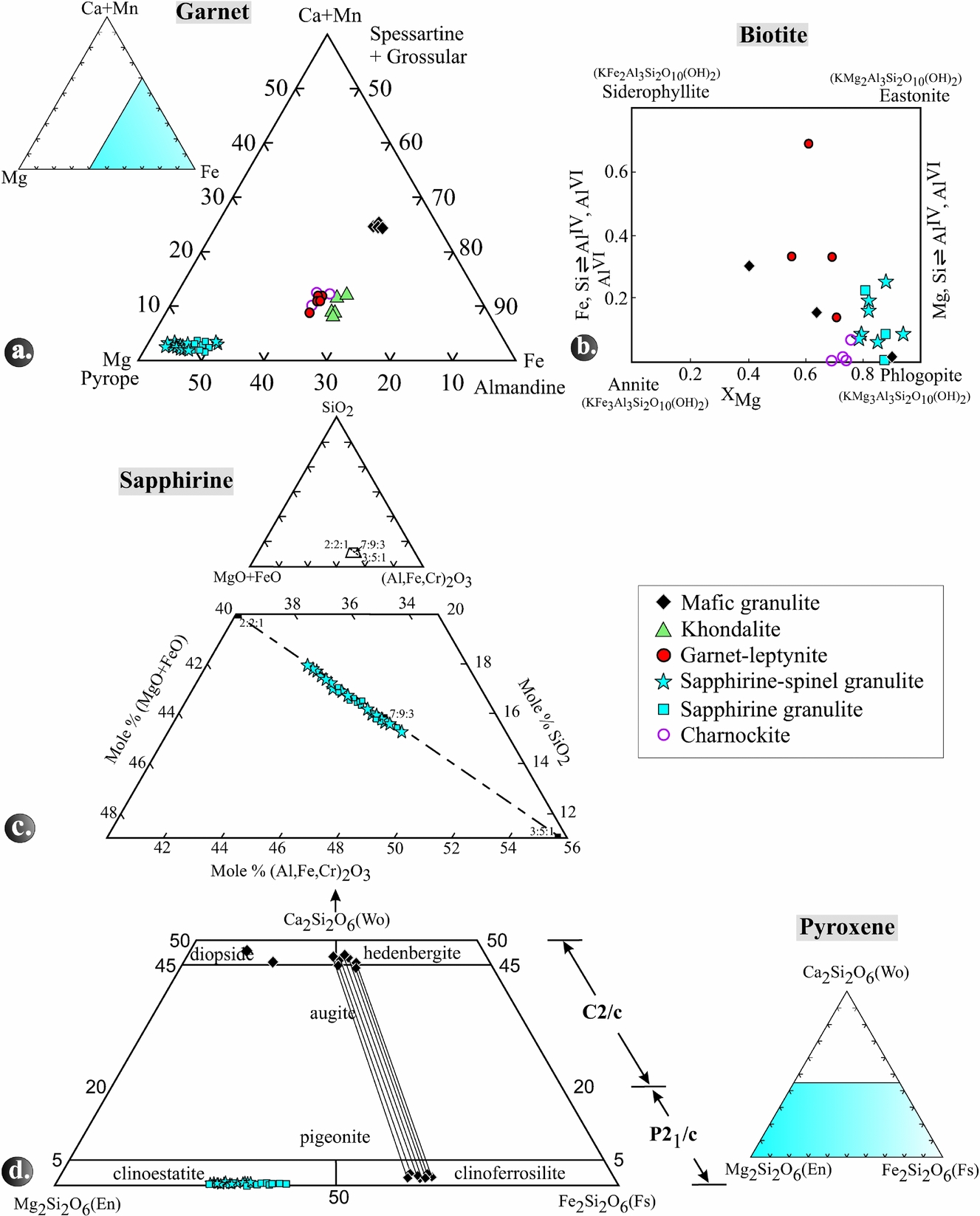
Figure 8. (a) Triangular diagram showing the variation in spessartine+grossular–almandine–pyrope end-member proportions in garnet in the various rock types. (b) Quadrangular diagram showing the variation in annite–phlogopite–siderophyllite–eastonite end-member proportions in biotite in the various rock types. (c) A part of the system SiO2–(FeO+MgO)–(Al,Fe,Cr)2O3 showing plots of the analysed sapphirine. Solid rectangles with numbers 2:2:1, 7:9:3 and 3:5:1 give molar ratios for the ternary composition in the order (FeO+MgO)+(Al,Fe,Cr)2O3+SiO2. (d) Pyroxene quadrilateral showing the composition of ortho- and clinopyroxenes from different rock types. Tie lines represent pairs of coexisting pyroxenes.
5.b. Biotite
In general, biotite is most magnesian (XMg = 0.78–0.94) in sapphirine–spinel granulite whereas in mafic granulite and garnet-leptynite it has lower XMg of 0.40–0.89 and 0.55–0.71, respectively (Table S2 in the online Supplementary Material available at http://journals.cambridge.org/geo). The TiO2 contents of biotite in the sapphirine–spinel granulites are between 1.13 and 5.23 wt%, in the garnet-leptynites between 2.57 and 4.99 wt%, and in the mafic granulites between 0.67 and 3.64 wt%.
5.c. Sapphirine
In the triangular diagram (Al,Fe,Cr)2O3–MgO+FeO–SiO2 (Fig. 8c), the analysed sapphirine grains (Table S3 in the online Supplementary Material available at http://journals.cambridge.org/geo) plot between the 7:9:3 and 2:2:1 but close to the 7:9:3 position. Those analyses that plot between 7:9:3 and 3:5:1 of the solid solution series are peraluminous. The XMg ratio of sapphirine ranges from 0.72 to 0.83. The number of silicon atoms related to 18 oxygens varies in different coronas, i.e. 0.69–0.76 p.f.u. in the Spl–Spr–Qtz coronas, 0.81–0.84 in the Grt–Spr–Spl–Qtz and 0.81–0.87 in the Spl–Spr–Sil–Opx–Qtz coronas. Correspondingly, AlVI is highest in Spr–Spl–Qtz and lowest in Spl–Spr-Sil–Opx–Qtz coronas. Moreover, the cores of sapphirine grains are less aluminous than their rims. Total FeO varies from 7.8 to 12.9 wt% and the Fe3+ content of sapphirine increases from the Spl–Spr–Qtz (0.05 p.f.u.) via the Spl–Spr–Opx–Sil (0.07 p.f.u.) to the Grt–Spr–Spl–Qtz coronas (up to 0.15 p.f.u.). With few exceptions, TiO2 in sapphirine is below the detection limit, while Cr2O3 can reach as much as 0.87 and MnO as much as 0.36 wt%.
5.d. Pyroxenes
Most of the mafic granulite samples (nos 1101, 1103, 1106) contain pairs of coexisting clinopyroxene (hedenbergite to diopside with XMg 0.44–0.58) and orthopyroxene (ferrosilite with XMg 0.33–0.38) and Al2O3 contents of 0.48–0.80 wt% (Table S4 in the online Supplementary Material available at http://journals.cambridge.org/geo; Fig. 8d). Samples no. 1101 and 1106 contain diopside with XMg of 0.50–0.58 that does not coexist with orthopyroxene. In contrast, enstatite is the only pyroxene in the other granulite types. Sapphirine-bearing granulite contains orthopyroxene with high Al2O3 contents of 5.39–9.78 wt%, whereas, in charnockites, the Al2O3 content is distinctly lower, ranging from 1.25 to 1.54 wt%. The trend of XMg of orthopyroxene in the rock types is as follows: sapphirine granulite (0.59–0.72), sapphirine–spinel granulite (0.55–0.72) and mafic granulite (0.33–0.38). The highest XMg is observed in orthopyroxene from garnet–orthopyroxene domains (0.62–0.72), followed by sapphirine–spinel–orthopyroxene–sillimanite domains (0.66–0.68) and orthopyroxene–cordierite–spinel domains (0.53–0.69).
5.e. Cordierite
The majority of the analysed cordierite grains (Table S5 in the online Supplementary Material available at http://journals.cambridge.org/geo) are highly magnesian (XMg = 0.80–0.92). No significant zoning was observed. Insignificant amounts of CaO and K2O are present (up to 0.4 and 0.6 wt%, respectively), whereas Na2O is below the detection limit. Except in a few cases, the totals are close to 100 wt%, which indicates that these are essentially anhydrous cordierite grains. Some analyses yielded lower totals, and this is likely due to the presence of some H2O and/or CO2 (Hörmann et al. Reference Hörmann, Raith, Raase, Ackermand and Seifert1980; Lonker, Reference Lonker1981).
5.f. Plagioclase
Judging from the Ca/(Ca+Na+K)×100 values, the An contents of plagioclase (Table S6 in the online Supplementary Material available at http://journals.cambridge.org/geo) range from 43 to 75 mol% in the mafic granulites, from 35 to 54 mol% in garnet-leptynite and from 35 to 43 mol% in the sapphirine–spinel granulites.
5.g. K-feldspar
The albite content ((Na/(Ca+Na+K))×100) ranges between 10 and 22 mol% in the sapphirine–spinel granulite, between 8 and 13 mol% in the khondalite, and it is approximately 10% in the garnet-leptynite and 8% in the mafic granulite (Table S7 in the online Supplementary Material available at http://journals.cambridge.org/geo). Iron (calculated as Fe2O3) may be present up to 0.18 wt%, but is in most cases below the detection limit. The same holds true for TiO2, Cr2O3, MnO and MgO.
5.h. Sillimanite
Most of the sillimanite grains recorded in a few samples contain considerable amounts of Fe2O3 ranging up to 0.86 wt% in sapphirine granulites and from 0.76 to 1.51 wt% in sapphirine–spinel-orthopyroxene–quartz granulites (Table S8 in the online Supplementary Material available at http://journals.cambridge.org/geo). This variation in Fe2O3 suggests differences in oxidizing conditions during metamorphism. With one exception, Cr2O3 is below the detection limit; TiO2, MnO and MgO could not be detected.
5.i. Spinel
Spinel is largely a solid solution between spinel (MgAl2O4) and hercynite (Fe2+Al2O4) with XMg ranging from 0.29 to 0.60 and also some Al–Fe3+ substitution (Table S9 in the online Supplementary Material available at http://journals.cambridge.org/geo). Cr2O3 and MnO are present in minor amounts and, with few exceptions, TiO2 and ZnO are below the detection limit. XMg is at 0.43–0.56 in Spl–Spr–Sil–Opx coronas, 0.44–0.56 in Spl–Spr–Grt coronas, 0.29–0.48 in Spl–Opx–Sil–Crd coronas and 0.36 in spinel–biotite associations. Spinel coexisting with magnetite shows low Al2O3 and, consequently, higher Fe3+ contents (cf. Ackermand et al. Reference Ackermand, Herd, Reinhandt and Wndley1987).
5.j. Ilmenite, Ilmeno-haematite and haematite
The opaque minerals recorded in the granulites investigated include magnetite, ilmenite, ilmenite–haematite solid solutions, haematite and rutile. The sapphirine–spinel granulites contain ilmenite with about 5 mol% Fe2O3, but also ilmeno-haematite with 55–70 mol% Fe2O3, as well as nearly pure haematite (Table S10 in the online Supplementary Material available at http://journals.cambridge.org/geo).
6. Chemographic relationships and metamorphic reactions
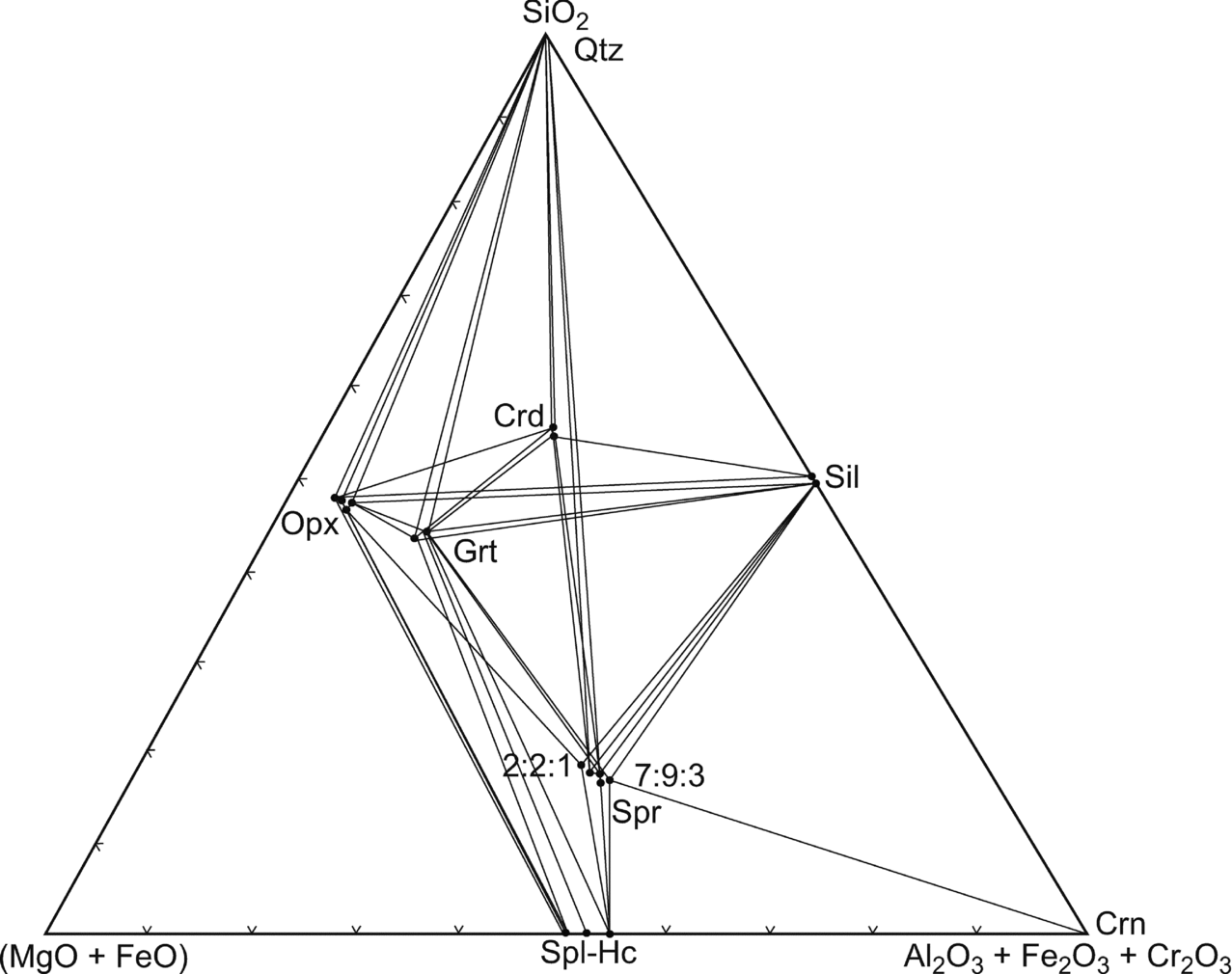
The sapphirine–spinel granulite (as exemplified by sample no. 1110) is particularly useful for petrological modelling because it contains the three-phase assemblage orthopyroxene–sapphirine–spinel in the (MgO+FeO)–(Al2O3+Fe2O3+Cr2O3)–SiO2 model system (Fig. 9).
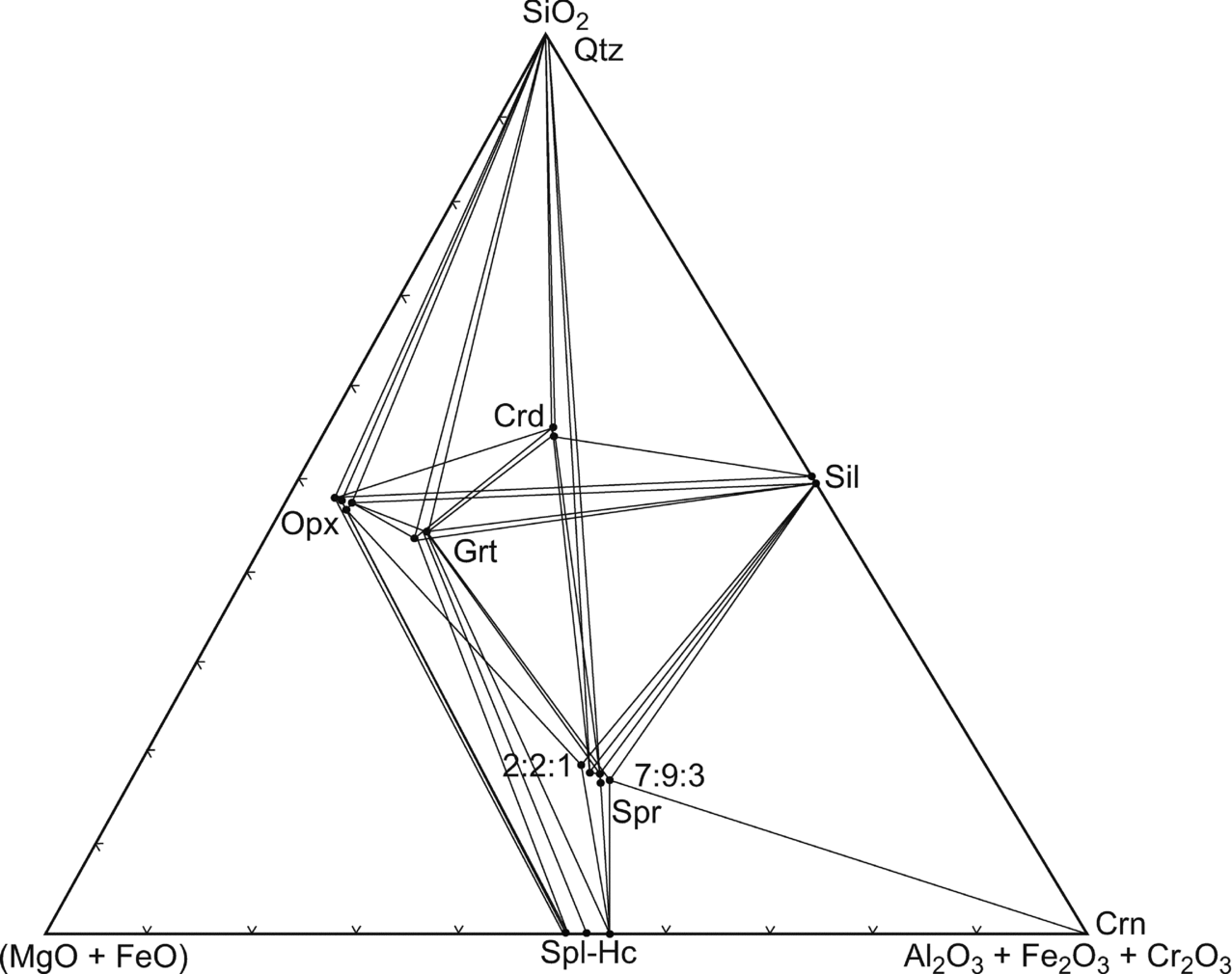
Figure 9. (Mg–Fe2+)O–(Al,Fe3+,Cr)2O3–SiO2 diagram showing the composition of coexisting minerals in the sapphirine–spinel-bearing granulites. Crossed tie lines reflect the higher variance of the assemblages due to the fractionation of Mg and Fe2+ as well as Al, Cr, Fe3+ among the phases.
6.a. Reactions in sapphirine–spinel granulite
Spinel, partially or wholly rimmed by sapphirine, occurs along the interstices of coarse prismatic aggregates of orthopyroxene. This textural relationship may be attributed to the reaction
 \begin{eqnarray}
&&\textrm{ 5 Mg - tschermakite + 4 spinel}\nonumber\\
&&\quad \textrm{ = 2 sapphirine}_{{\rm (7:9:3)}} \textrm{ + enstatite}
\end{eqnarray}
\begin{eqnarray}
&&\textrm{ 5 Mg - tschermakite + 4 spinel}\nonumber\\
&&\quad \textrm{ = 2 sapphirine}_{{\rm (7:9:3)}} \textrm{ + enstatite}
\end{eqnarray}
(in orthopyroxene).
The composition of sapphirine lies in the three-phase field of the spinel–sillimanite–quartz reaction:
Co-linear positions of the three phases point to degenerated systems with the three-phase reactions
and
The phase relations can be displayed in the model system K2O–FeO–MgO–A12O3–SiO2–H2O (KFMASH) in which FeO and MgO are treated as separate components. This can be simplified by excluding the following minerals:
(1) Osumilite, though present at the thermal peak of metamorphism, has been completely replaced by symplectite of orthopyroxene–quartz–K-feldspar–cordierite;
(2) K-feldspar is ubiquitous and thus can be treated as an excess phase; and
(3) biotite, because it was involved predominantly in retrograde reactions.
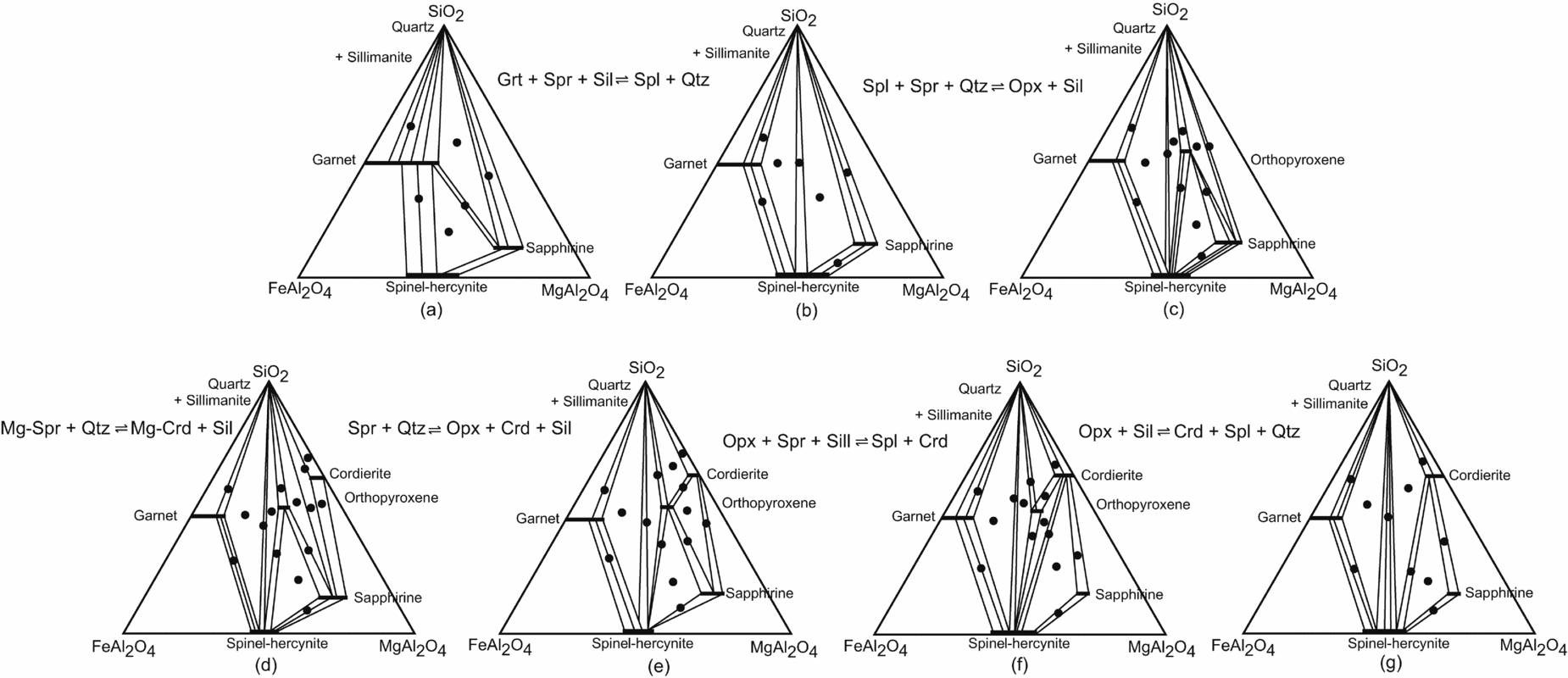
As most of the sapphirine and spinel-bearing assemblages of the study area contain sillimanite, the phase relations can be shown in plane projection from sillimanite onto the SiO2–FeAl2O4–MgAl2O4 plane of the FeO–MgO–A2O3–SiO2 tetrahedron (Fig. 10). A plausible sequence of metamorphic reactions, based upon the textural relationships, is shown in a series of sillimanite projections (Fig. 10).
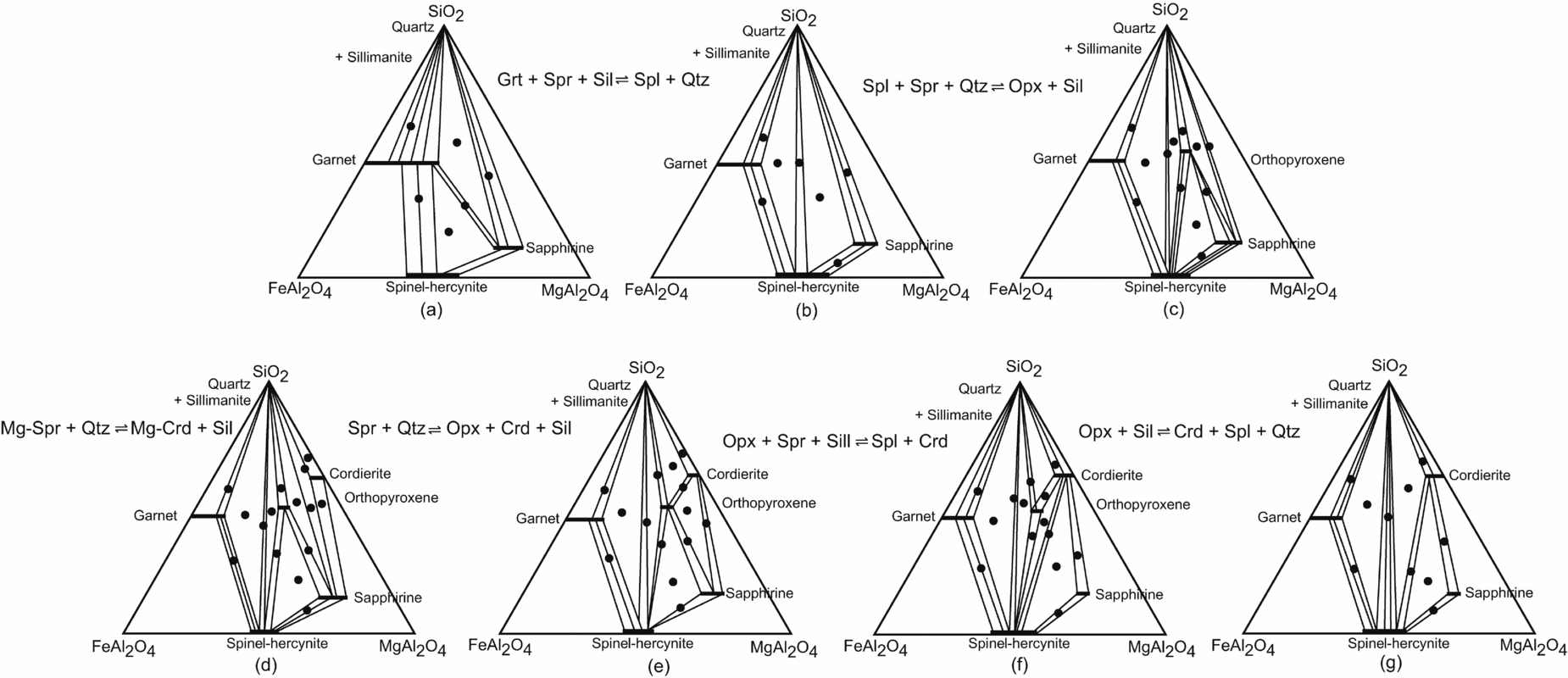
Figure 10. Phase compatibility relationships from the sapphirine–spinel granulites shown in the projection from sillimanite on to the SiO2–FeAl2O4–MgAl2O4 plane of the FeO–MgO–Al2O3–SiO2 tetrahedron; sequence of reactions is deduced from the coronas and other reaction textures and phase compatibility relationships of the mineral phases (a–g). Filled circles show the two- and three-phase assemblages observed.
The sequence of reaction coronas in these rocks indicates earlier stability of sapphirine+quartz and spinel+quartz during the thermal peak of metamorphism. Experimental work in the MgO–Al2O3–SiO2–H2O (MASH) and FeO–MgO–Al2O3–SiO2–H2O (FMASH) systems has demonstrated the stability of the association sapphirine+spinel with quartz at high-pressure – high-temperature conditions (Schreyer & Seifert, Reference Schreyer and Seifert1969; Hensen & Green, Reference Hensen and Green1971; Chatterjee & Schreyer, Reference Chatterjee and Schreyer1972). The spinel–sapphirine–(garnet+sillimanite) corona in sample no. 1110 may be attributed to a discontinuous Fe2+–Mg reaction (Fig. 10a, b):
 \begin{eqnarray}
&&\textrm{ 13 spinel + 11 quartz = 2 garnet }\nonumber\\
&&\quad\textrm{ +\, 2 sapphirine}_{_{{\rm (7:9:3)}} } \textrm{ + 2 sillimanite}
\end{eqnarray}
\begin{eqnarray}
&&\textrm{ 13 spinel + 11 quartz = 2 garnet }\nonumber\\
&&\quad\textrm{ +\, 2 sapphirine}_{_{{\rm (7:9:3)}} } \textrm{ + 2 sillimanite}
\end{eqnarray}
In Figure 10a two continuous Fe2+–Mg reactions would occur in the three-phase fields garnet–sapphirine–spinel and garnet–sapphirine–quartz, which are, respectively:
 \begin{equation}
\eqcellsep\eqcellsep\textrm{ garnet + 32 spinel + 12 sillimanite}\nonumber\\
\eqcellsep\eqcellsep\quad\textrm{ = 10 sapphirine}_{{\rm (7:9:3)}}\end{equation}\\
\begin{equation}
\eqcellsep\eqcellsep\textrm{ garnet + 32 spinel + 12 sillimanite}\nonumber\\
\eqcellsep\eqcellsep\quad\textrm{ = 10 sapphirine}_{{\rm (7:9:3)}}\end{equation}\\
Sapphirine rims around spinel, separating spinel from two other reactants garnet+sillimanite and sapphirine rimmed by garnet+sillimanite in a matrix of quartz as observed in sample no. 1110 are textural evidence of reactions (16) and (17), respectively.
6.b. Reactions involving orthopyroxene
The spinel–sapphirine–sillimanite–orthopyroxene cor-ona (Fig. 7b) in the matrix of quartz may be attributed to the discontinuous Fe2+–Mg reaction:
 \begin{eqnarray}
&&\textrm{ sapphirine}_{{\rm (2:2:1)}} \textrm{ + 2 spinel + 10 quartz}\nonumber\\
&&\quad\textrm{ = 3 orthopyroxene + 6 sillimanite}\end{eqnarray}
\begin{eqnarray}
&&\textrm{ sapphirine}_{{\rm (2:2:1)}} \textrm{ + 2 spinel + 10 quartz}\nonumber\\
&&\quad\textrm{ = 3 orthopyroxene + 6 sillimanite}\end{eqnarray}
The reaction is evident from the formation of orthopyroxene–spinel, orthopyroxene–sapphirine and orthopyroxene–quartz joins within the three-phase field of spinel–sapphirine–quartz in the sillimanite projection (Fig. 10b, c).
In the sillimanite projection shown in Figure 10c, three continuous Fe+2–Mg reactions, which may occur in the three-phase fields of sapphirine–orthopyroxene–quartz, spinel–orthopyroxene–quartz and sapphirine–cordierite–quartz, respectively, are as follows:
 \begin{equation}
\eqcellsep\eqcellsep \textrm{ 4 sapphirine}_{(7:9:3)} + 26\,\textrm{ quartz} = {\rm 7}\,\textrm{ orthopyroxene} \nonumber\\
\eqcellsep\eqcellsep\quad +\, 18\,\textrm{ sillimanite}\end{equation}\\
\begin{equation}
\eqcellsep\eqcellsep \textrm{ 4 sapphirine}_{(7:9:3)} + 26\,\textrm{ quartz} = {\rm 7}\,\textrm{ orthopyroxene} \nonumber\\
\eqcellsep\eqcellsep\quad +\, 18\,\textrm{ sillimanite}\end{equation}\\
Coronas of sapphirine–sillimanite–orthopyroxene, spinel sillimanite–orthopyroxene and sapphirine–sillimanite–cordierite can be explained by the reactions (19), (20) and (21), respectively.
Inclusions of spinel within garnet and sillimanite indicate the reaction:
within the three-phase field of garnet–spinel–quartz (Fig. 10b, c). At a later stage sapphirine became incompatible with quartz, presumably due to the discontinuous Fe2+–Mg reaction
which may explain the corona of sapphirine–sillimanite–cordierite–orthopyroxene. The corona spinel–sillimanite–cordierite–orthopyroxene may be attributed to two continuous Fe2+–Mg reactions:
and
In the sillimanite projections (Fig. 10e), the sapphirine–quartz tie line is intersected by the cordierite–orthopyroxene tie line. This reaction is evident from the diagram (MgO+FeO)–(Al2O3+Fe2O3+Cr2O3)–SiO2 (Fig. 9), in which the composition of cordierite plots on the spinel–quartz tie line.
In the sillimanite projection the Reaction (25) suggests that the P–T trajectory crossed over the two reactions:
 \begin{equation}
\hspace*{-15pt}\eqcellsep\eqcellsep\textrm{ 16 sapphirine}_{{\rm (7:9:3)}} \textrm{ + 33 orthopyroxene }\nonumber\\
\hspace*{-15pt}\eqcellsep\eqcellsep\quad\textrm{ + 50 sillimanite = 66 spinel + 28 cordierite}\end{equation}\\
\begin{equation}
\hspace*{-15pt}\eqcellsep\eqcellsep\textrm{ 16 sapphirine}_{{\rm (7:9:3)}} \textrm{ + 33 orthopyroxene }\nonumber\\
\hspace*{-15pt}\eqcellsep\eqcellsep\quad\textrm{ + 50 sillimanite = 66 spinel + 28 cordierite}\end{equation}\\
 \begin{equation}
\hspace*{-15pt}\eqcellsep\eqcellsep\textrm{ 3 orthopyroxene + 6 sillimanite = 2 spinel }\nonumber\\
\hspace*{-15pt}\eqcellsep\eqcellsep\quad\textrm{ +\, 2 cordierite + 2 quartz}\end{equation}
\begin{equation}
\hspace*{-15pt}\eqcellsep\eqcellsep\textrm{ 3 orthopyroxene + 6 sillimanite = 2 spinel }\nonumber\\
\hspace*{-15pt}\eqcellsep\eqcellsep\quad\textrm{ +\, 2 cordierite + 2 quartz}\end{equation}
The presence of coarse-grained porphyroblasts of cordierite in contact with orthopyroxene, in a zone depleted in quartz or sillimanite, can be explained by the continuous Fe2+–Mg Reaction (25).
6.c. Possible reactions involving osumilite
Osumilite was most likely stable during the early stage of metamorphism of sapphirine–spinel-bearing granulite but has been replaced by symplectite containing cordierite–orthopyroxene–K-feldspar–quartz after the thermal peak of metamorphism. The presence of inclusions of coarse prisms of orthopyroxene and sapphirine within cordierite–orthopyroxene–K-feldspar–quartz osumilite symplectites suggests osumilite formation by the prograde continuous Fe2+–Mg reaction
 \begin{eqnarray}
\hspace*{-10pt}&&\textrm{ 4 sapphirine}_{{\rm (7:9:3)}} \textrm{ + 2 orthopyroxene }\nonumber\\
\hspace*{-10pt}&&\quad\textrm{+ 9 K - feldspar + 53 quartz = 9 osumilite}\end{eqnarray}
\begin{eqnarray}
\hspace*{-10pt}&&\textrm{ 4 sapphirine}_{{\rm (7:9:3)}} \textrm{ + 2 orthopyroxene }\nonumber\\
\hspace*{-10pt}&&\quad\textrm{+ 9 K - feldspar + 53 quartz = 9 osumilite}\end{eqnarray}
Sapphirine rimmed by sillimanite and/or cordierite within the symplectite may be attributed to the reactions:
 \begin{equation}
\eqcellsep\eqcellsep\textrm{ sapphirine + sillimanite + osumilite}\nonumber\\
\eqcellsep\eqcellsep\quad\textrm{ = cordierite +\, K - feldspar + quartz}\end{equation}\\
\begin{equation}
\eqcellsep\eqcellsep\textrm{ sapphirine + sillimanite + osumilite}\nonumber\\
\eqcellsep\eqcellsep\quad\textrm{ = cordierite +\, K - feldspar + quartz}\end{equation}\\
The common presence of cordierite + orthopyroxene + K-feldspar + quartz and sillimanite + orthopyroxene + K-feldspar + quartz symplectites in the sapphirine–spinel-bearing granulite are, respectively, related to the breakdown of osumilite due to the continuous Fe+2–Mg reactions (29) and (30).
Retrograde phlogopite replacing the symplectite of cordierite–orthopyroxene–K-feldspar–quartz formed from the breakdown of osumilite may be related to the reaction
 \begin{eqnarray}
&&\textrm{ orthopyroxene + cordierite + K - feldspar + H}_{\rm 2} {\rm O}\nonumber\\
&&\quad\textrm{ = phlogopite + quartz}\end{eqnarray}
\begin{eqnarray}
&&\textrm{ orthopyroxene + cordierite + K - feldspar + H}_{\rm 2} {\rm O}\nonumber\\
&&\quad\textrm{ = phlogopite + quartz}\end{eqnarray}
6.d. Oxidation reactions
The presence of ilmenite–haematite intergrowth within spinel in the spinel–sillimanite–orthopyroxene coronas and elevated Fe3+ contents of sapphirine, spinel and sillimanite suggest oxidizing conditions during the formation of these coronas.
In the sapphirine–spinel-bearing rocks spinel is separated from quartz presumably by a discontinuous Fe–Mg–Ti reaction:
 \begin{eqnarray}
&&\textrm{ spinel + rutile + quartz = ilmo - hematite}\nonumber\\
&&\quad\textrm{ + sillimanite + orthopyroxene + O}_{\rm 2}\end{eqnarray}
\begin{eqnarray}
&&\textrm{ spinel + rutile + quartz = ilmo - hematite}\nonumber\\
&&\quad\textrm{ + sillimanite + orthopyroxene + O}_{\rm 2}\end{eqnarray}
In some reaction coronas spinel–haemo-ilmenite±corundum is rimmed by sillimanite. This may be attributed to the reactions
and
Spinelss(1) has a higher Fe+2/Mg ratio than spinelss(2).
Rutile is acting as a reactant in these reactions, which is evident from its occurrence along with spinel in the core of the coronas in relatively less oxidized rocks. The corona types (Fig. 7a) related to reactions (33) and (34) are: (spinel–haemo-ilmenite±corundum)–sillimanite–orthopyroxene, (spinel–haemo-ilmenite)–sillimanite–orthopyroxene and (spinel–haemo-ilmenite)–sapphirine–sillimanite–orthopyroxene.
7. Petrogenetic grids
At low fO2, the [Spl]-, [Qtz]-, [Opx]- and [Sil]-absent invariant points are stable, whereas the [Crd]-, [Grt]- and [Spr]-absent invariant points are metastable. A particular invariant point stable at low fO2 becomes metastable at high fO2 as the topology of the diagram is inverted and a number of univariant and divariant mineral equilibria are restricted in their occurrence to either low or high fO2, respectively. Analytical data on sapphirine, spinel, garnet, orthopyroxene and sillimanite indicate that these phases may contain significant amounts of Fe3+ and, therefore, crystallized at relatively high fO2.
The association of spinel–sapphirine–quartz with garnet and sillimanite and the corona type spinel–sapphirine–(garnet–sillimanite) in a matrix of quartz are related to the (Opx)-absent univariant Fe–Mg reaction (Fig. 11) emerging from the [Crd] invariant point, which is compatible in the FMAS grids for high fO2 proposed by Hensen (Reference Hensen1986). Similarly, the association of spinel–sapphirine–quartz with orthopyroxene–sillimanite and the corona type spinel–sapphirine–sillimanite, spinel–sillimanite–orthopyroxene and sapphirine–sillimanite–orthopyroxene are related to the univariant reaction (Fig. 11) emerging from the [Crd]- and [Grt]-absent invariant points. The coronas sapphirine–sillimanite–cordierite–orthopyroxene and sapphirine–sillimanite–cordierite in a matrix of quartz are related to the (Spl)-absent reaction (Fig. 11) from the [Grt]-absent invariant point.

Figure 11. P–T petrogenetic grid in the FeO–MgO–Al2O3–SiO2 (FMAS) system at high fO2 after Hensen (Reference Hensen1986). The mineral assemblages in the divarient fields are shown by AFM projection. Minerals in parentheses represent univariant reactions diverging from the invariant points, indicated by square brackets. The sequence of development of coronas and other reaction textures suggest a retrograde path passing through the [Crd]-absent invariant point and along the (Spr)-absent univariant reaction towards the [Spr]-absent invariant point. The presence of orthopyroxene coexisting with sillimanite in many samples of sapphirine–spinel-bearing rocks in the area suggests a prograde path passing from the low- to the high-temperature side of the (Spl)-absent univariant curve. Thus the P–T trajectory appears to have been clockwise with nearly isothermal, presumably rapid decompression during the metamorphic evolution.
Furthermore, the corona of spinel–sillimanite–cordierite–orthopyroxene can be explained by combination of the reactions (23) and (24). Similarly the corona rim of cordierite between spinel and quartz is due to Reaction (13). These reactions are related to the univariant Reaction (26) from (Grt)- and (Spr)-absent reactions.
Thus, it is evident that the [Crd]-, [Grt]- and [Spr]-absent invariant points are stable in the sapphirine–spinel-bearing rocks of the study area. Therefore, the petrogenetic grid in the FMAS system for high fO2 (Fig. 11), as proposed by Hensen (Reference Hensen1986), is applicable to these rocks.
The sequence of development of coronas and other reaction textures is shown in the petrogenetic grid in the FMASH system after Hensen (Reference Hensen1986) at high fO2 (Fig. 11). The earlier recognizable stage in the mineral history using the above petrogenetic grid is represented on the high-pressure side by orthopyroxene or garnet with Mg-rich cores coexisting with sillimanite. The spinel–quartz and sapphirine–quartz grain contacts indicate that these minerals coexisted during the thermal peak of metamorphism. The development of reaction textures, especially polymineralic coronas including coronas of spinel–sapphirine–(garnet+sillimanite), spinel–sapphirine–sillimanite–orthopyroxene and spinel–sillimanite–cordierite–orthopyroxene in a matrix of quartz, as well as rims of cordierite between spinel and quartz, demonstrate decreasing P–T conditions during the dynamic uplift history. The sequence of the development of reaction coronas also confirms a retrograde path passing through the [Crd]-absent invariant point to (Spl)-absent univariant reaction from [Grt], to the univariant reaction from the [Spr]- and [Grt]-absent invariant points (Fig. 11). Despite errors inherent in the calculations, the texturally defined sub-assemblages in the sapphirine–spinel-bearing granulite yield distinct P–T conditions for the various evolutionary stages. The P–T estimates calculated through the application of THERMOCALC (version 3.21; Holland & Powell, Reference Holland and Powell1998) indicate decreasing P–T combinations of about 10.5 kbar/1000°C, 9 kbar/900°C and 5 kbar/800°C, the latter two conforming to the [Cdr]- and [Spr]-absent invariant points, respectively.
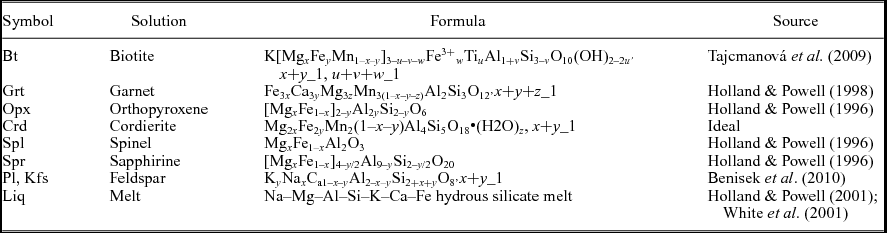
8. P–T pseudosection modelling for sapphirine-bearing granulite
Based on the dominant minerals and their chemical compositions, the phase equilibria of the Diguva Sonaba granulites from the EGMB can be modelled in the system Na2O–CaO–K2O–FeO–MgO–Al2O3–SiO2–TiO2–H2O (NCKFMASTH). A P–T pseudosection for the sapphirine-bearing granulite (sample no. 1111) was calculated using the software Perple_X 6.66 (Connolly, Reference Connolly2005) (Fig. 12). Solution models used in the calculations are given in Table 1. Comparing the textural observations with the calculated pseudosection, the P–T path associated with the series of changes that occurred in the rock is inferred. In sample no. 1111, the prograde assemblage Bt–Opx–Grt–Pl–Ky is quadravariant (v = 4) in NCKFMASTH and its conversion to the assemblage Opx–Grt–Pl–Sil suggests biotite dehydration. The peak assemblage Grt–Opx–Sil–Pl is stable between 11 and 12 kbar at temperatures above 1000°C. High Al contents (0.19) in coarse-grained orthopyroxene indicate temperatures of c. 1000°C. A P–T path may be drawn on the basis of calculated isopleths and measured mineral chemistry obtained by EPMA. As seen in Figure 12, garnet is stable above 10 kbar and contoured isopleths of XMg for the garnet have flat slopes. Symplectitic intergrowths provide evidence for decompression. For instance, the formation of different coronas indicates entry into the Opx–Pl–Sil/Spr fields with decreasing pressure, whereas the subsequent formation of cordierite at lower pressure led to Crd–Spr/Opx symplectites. Decrease in Al content in orthopyroxene (0.15–0.07) and formation of retrograde biotite reflect continued cooling below the solidus at temperature less than 800°C (hydration stage).

Figure 12. Calculated P–T pseudosections for sapphirine-bearing granulite without spinel (sample no. 1111) in the system NCKFMASHT (+Qtz+Rt+Liq (above solidus)). Bulk composition in mole% (Na2O 2.50, CaO 1.39, K2O 1.23, FeO 3.69, MgO 19.24, Al2O3 10.83, SiO2 59.03, H2O 1.76, TiO2 0.31). The solidus (for aH2O = 0.02) is marked by the dashed line. The pseudosection is contoured for the isopleths of (a) XMg(Grt) and (b) XAl(Opx). The stippled areas mark peak metamorphic P–T ranges inferred from mineral compositional data. Prograde and retrograde paths are marked by dashed and continuous lines, respectively.
Table 1. Solution notation, formulae and model sources for phase diagram calculation
9. Discussion
The FMAS grid for high fO2 (after Hensen, Reference Hensen1986) provides a useful framework to constrain semiquantitatively the P–T history of sapphirine-bearing granulites (Fig. 11):
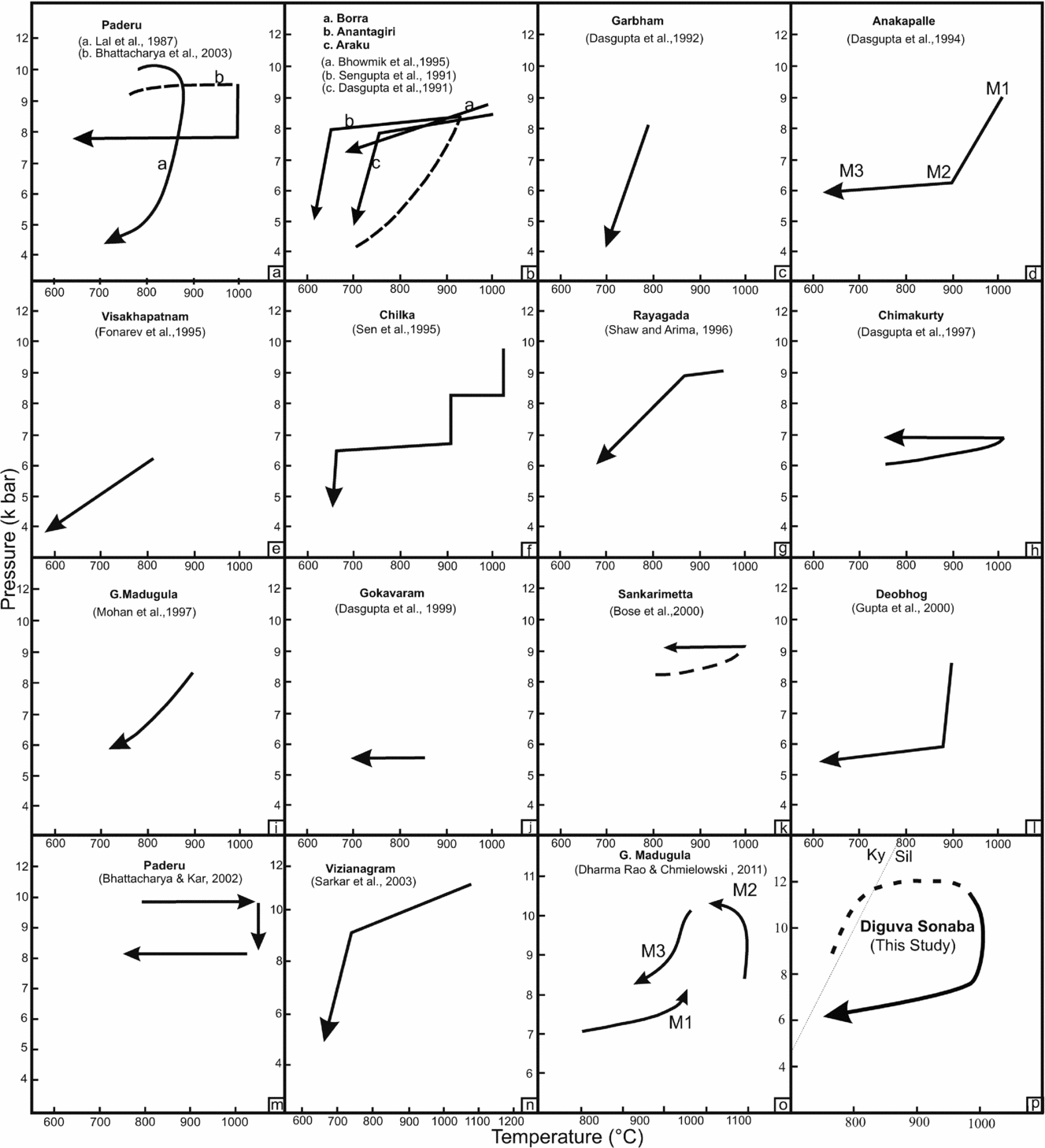
(i) A clockwise P–T path is indicated by the presence of prograde mineral relics and textural evidence for the presence of sillimanite pseudomorphs after kyanite blades (Fig. 13p).

Figure 13. (a–p) P–T paths suggested by various workers for the granulites in different areas of the EGMB.
(ii) During the thermal peak of metamorphism, sapphirine+quartz and spinel+quartz were stable at P–T conditions of 10–12 kbar and > 950°C. Garnet porphyroblasts, orthopyroxene prisms, blocky sillimanite and corroded biotite are interpreted as part of the primary parageneses. Moreover, abundant symplectitic intergrowths of cordierite+quartz+K-feldspar+orthopyroxene are interpreted as breakdown products of peak-metamorphic osumilite.
(iii) During the subsequent corona formation stage, sapphirine and spinel were isolated from quartz by several spectacular corona-forming reactions that indicate isobaric cooling. The retrograde P–T path passes through the (Opx)- and (Grt)-absent reactions around the [Crd]-absent invariant point.
(iv) Subsequent development of cordierite is due to decompression and indicates a P–T path passing through (Grt, Spl) from the [Spr]-absent invariant point of the P–T grid for the FMAS system at high fO2.
(v) During a late stage and decreasing P–T conditions, biotite, biotite–quartz intergrowths, biotite symplectites and fibrolite matted with biotite replaced earlier grown garnet porphyroblasts, orthopyroxene prisms, cordierite and K-feldspar.
The decompressional P–T trajectory may be ascribed to a geotectonic setting dominated by exhumation after crustal thickening. The exhumation mechanism, however, remains uncertain. Tectonic models for granulite formation, which take into account a decompressive P–T path, include (i) crustal thickening due to collisional models (Thompson & England, Reference Thompson and England1984) and (ii) an extensional regime involving a crust of near-normal thickness (Sandiford & Powell, Reference Sandiford and Powell1986). Calculated peak P–T conditions suggest that the Diguva Sonaba sapphirine-spinel-bearing granulites were buried to depths of c. 42 km. The crustal thickness beneath the EGMB is now c. 35 km (Kumar et al. Reference Kumar, Singh, Gupta and Mishra2004), indicating that the total crustal thickness may have been as much as c. 77 km. Such a crustal thickness could be possibly generated by collision tectonics. Clockwise and anticlockwise paths may be distinguished on the basis of the geometry of the prograde path and not on that of the retrograde path. However, for most of the EGMB, the prograde P–T trajectory is not well documented (Fig. 13). The EGMB has been the subject of numerous petrological studies involving sapphirine-bearing granulite, all of which describe a great variety of different, in places contradictory, P–T paths (Fig. 13). A P–T path involving initial isobaric cooling followed by isothermal decompression has been advocated by several workers in the EGMB (Kamineni & Rao, Reference Kamineni and Rao1988; Sengupta et al. Reference Sengupta, Dasgupta, Bhattacharya, Fukoka, Chakraborti and Bhowmik1990; Dasgupta et al. Reference Dasgupta, Sengupta, Ehl, Raith and Bardhan1995; Rao et al. Reference Rao, Kamineni, Arima, Yoshida, Yoshida, Santosh and Rao1995; Shaw & Arima, Reference Shaw and Arima1998; Dharma Rao, Santosh & Chmielowski, Reference Dharma Rao, Santosh and Chmielowski2012) but in the absence of well-constrained P–T histories ambiguity remains concerning clockwise and anticlockwise P–T paths. Recently, Korhonen et al. (Reference Korhonen, Saw, Clark, Brown and Bhattacharya2011, Reference Korhonen, Clark, Brown, Bhattacharya and Taylor2013) suggested that the EGMB records a single long-lived high-grade metamorphic evolution and the U–Pb zircon age populations between c. 1130 Ma and c. 930 Ma correspond to a single prograde and retrograde metamorphic evolution. Discrimination between these tectonic models is also hindered mainly by a lack of data on the cooling history and inadequate systematic isotopic age data in the EGMB. In the present study, the overall textural evidence, as well as semiquantitative petrogenetic grid and pseudosection approach point to a clockwise UHT P–T path in the Diguva Sonaba granulites, which is marked by post-peak isothermal decompression (Fig. 13p).
10. Conclusions
The Diguva Sonaba area is part of the multiply deformed granulite-facies terrain of the EGMB, India. A prograde P–T path has been inferred for the mineral assemblages in the Diguva Sonaba granulites. An early stage during the thermal peak is recognized by the coexistence of sapphirine+quartz, and spinel+quartz in textural equilibrium. Garnet, orthopyroxene, sillimanite, biotite and possibly osumilite are interpreted as part of the peak metamorphic parageneses. This was followed by several cordierite-forming reactions related to decompression. During the subsequent corona formation stage, sapphirine and spinel became isolated from quartz by several spectacular coronas that can be explained by nearly isobaric cooling. During a late stage, biotite, garnet–quartz intergrowths, biotite–quartz symplectite and fibrolite matted with biotite replaced earlier garnet porphyroblasts, orthopyroxene, cordierite and K-feldspar during retrograde overprint. Reaction textures, petrogenetic grid and P–T pseudosection constraints suggest extreme P–T conditions of 10–12 kbar and > 950°C and a high-temperature decompression path for the sapphirine–spinel-bearing granulites. Based on prograde and retrograde mineral assemblages as well as a sequence of isopleths, a clockwise metamorphic P–T trajectory could be defined.
Acknowledgements
Discussions with, and numerous helpful suggestions by, Professor R. K. Lal are deeply appreciated. The authors acknowledge the detailed technical advice given by J. A. D. Connolly on the use of the Perple_X software. The manuscript benefitted from constructive reviews by M. Okrusch and the editor. This work was financially supported by the Ministry of Earth Sciences, (MoES) New Delhi, under the research project (P-07-578), and by the DFG (Deutsche Forschungsgemeinschaft; grant No. Fr 2183/7–1).
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0016756814000399.