Introduction
Approximately 322 species of marine fishes are present in the Southern Ocean, consisting mainly of Nototheniidae, Zoarcidae and Liparidae families (Barrera-Oro Reference Barrera-Oro2002, Eastman Reference Eastman2005). The nototheniid Lepidonotothen larseni (Lönnberg) is an endemic species which inhabits inshore and offshore waters between 30 and 550 m depth (Gon & Heemstra Reference Gon and Heemstra1990, Barrera-Oro Reference Barrera-Oro2002). It has a wide geographical distribution, including the Balleny Islands, west Antarctic Peninsula, South Shetland and South Orkney islands, Shag Rocks, South Georgia, South Sandwich, Bouvet, and Indian Ocean sector islands, such as the Prince Edward Islands and Iles Crozet (Duhamel & Pletikosic Reference Duhamel and Pletikosic1983, Gon & Heemstra Reference Gon and Heemstra1990, Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005). From an ecological point of view, L. larseni is an opportunistic plankton feeder able to migrate from the bottom to the surface of the water column to feed (Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005). It feeds mainly on zooplankton such as krill, hyperid amphipods, mysids, copepods and salps, and to a lesser extent on gammarid amphipods, isopods and polychaetes (Barrera-Oro Reference Barrera-Oro2002).
Nototheniidae have developed a wide range of feeding strategies, which allow them to utilize food resources in a variety of habitats. The fact that species have evolved niche difference allows the exploitation of prey in different habitats and may be a contributing factor to their dominance in fish communities (Targett Reference Targett1981). The mode of feeding is a major determinant of the ecological niche of fish species, and in Nototheniidae there is a trend towards pelagization of demersal species (Klingenberg & Ekau Reference Klingenberg and Ekau1996) which might be related to the food availability in the water column. Specifically, L. larseni undertakes vertical migrations with juveniles and adults having different distributions at horizontal and vertical scales (Frolkina et al. Reference Frolkina, Konstantinova and Trunov1998). Although there are some studies on the feeding habits of L. larseni (Targett Reference Targett1981, Barrera-Oro Reference Barrera-Oro2002, Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005) and on their nutritional condition (Eastman & Sidell Reference Eastman and Sidell2002), no comparative studies on juvenile and adult feeding ecology have been carried out so far.
In the inner ear of fishes there are complex polycrystalline structures of calcium carbonate, the otoliths (sagitta, asteriscus and lapillus), which are different in size, shape and position, conserved through the time. They are located inside sac-like structures (called respectively saccule, lagena and utricle), and connected by a thin otolithic membrane lying close to the macula, a sensory epithelium used for fish orientation (Popper & Zhongmin Reference Popper and Zhongmin2000). Because of its high specificity (Martínez Pérez et al. Reference Martínez Pérez, Chavez Arteaga, Tello and Morales2007), otoliths are used to determine age and growth of fishes (Campana Reference Campana2001), as well as in palaeoecology, palaeobiogeography, phylogeny and in trophic ecological studies (Koen Alonso et al. Reference Koen Alonso, Crespo, Pedraza and Coscarella2000, Assis Reference Assis2003, Reference Assis2005). Moreover, there is a relationship between fish ecotypes and the otolith shape because this structure shows adaptations to different environmental factors, such as depth, water temperature and substrate type (Volpedo & Echeverría Reference Volpedo and Echeverría2003, Colmenero et al. Reference Colmenero, Aguzzi, Lombarte and Bozzano2010, Lombarte et al. Reference Lombarte, Palmer, Matallanas, Gómez-Zurita and Morales-Nin2010). For example, Volpedo & Echeverría (Reference Volpedo and Echeverría2003) observed an elongated otolith with a well developed rostrum in pelagic species, whereas they are rounded with a not developed rostrum in demersal species. Based on diet, otolith shape and phylogeny of Nototheniidae, Lombarte et al. (Reference Lombarte, Palmer, Matallanas, Gómez-Zurita and Morales-Nin2010) stated that the relative size of sagitta has a great functional significance to determine the trophic niche, concluding that benthic fishes of this family have the largest sagittae in relation to body size.
The sagitta of L. larseni has been already morphologically described (Hecht Reference Hecht1987), but there are no data on morphology and morphometry of asteriscus and lapillus. The sagitta is characterized by an ovate to fusiform shape and a smooth and gently rounded external surface. The sulcus is ostial and a constricted collum separates ostium and cauda. The prominent ventral area and the rostrum are well developed in larger fishes (Radtke & Targett Reference Radtke and Targett1984, Volpedo et al. Reference Volpedo, Tombari and Echeverría2008).
The aims of this study are: a) to describe in more detail the morphology of asteriscus, lapillus and sagitta otoliths of L. larseni, obtaining useful relationships between fish size and several morphometric measurements of otoliths; b) to compare the feeding habits of juveniles and adults by their stomach contents analysis, nutritional condition and stomach repletion; and c) to analyse the relationship between ontogenetic change of sagittal otolith shape and feeding ecology of juveniles and adults.
Materials and methods
Fifty individuals of L. larseni were collected at the South Shetland Islands (stations 11, 13, 14) and the Antarctic Peninsula (station 22) during the summer campaign of 2011 carried out by the ARA Puerto Deseado vessel (Fig. 1). Fishes were collected at noon (13h00), evening (15h30) and late evening (18h00) with demersal (25 mm mesh size) and pilot (50 mm mesh size) nets. Sampling gears were deployed at a speed of 2–3 knots and towed on the bottom (mean depth 222 m) for 15–20 minutes. In each station surface temperature and salinity data were obtained using a Seabird SBE 21 thermosalinograph. Fishes were frozen at -10°C and total length (TL, ±1 mm), standard length (SL, ±1 mm) and weight (W, ±1 g) were measured. To compare mouth sizes, the upper maxilla length (A, ±1 mm) was measured (Klingenberg & Ekau Reference Klingenberg and Ekau1996). As the gonads were frequently destroyed in most samples, possibly after freezing, juveniles and adults were classified by their size, as they attain sexual maturity at 110 mm TL (Duhamel & Pletikosic Reference Duhamel and Pletikosic1983). Fish length-weight relationships were estimated by linear regression applied to log-transformed data.

Fig. 1 Sampling stations at the South Shetland Islands and Antarctic Peninsula during the 2011 summer campaign. Surface temperature: 0.6–2.4°C. Surface salinity: 34–34.1‰. Sampled depth: 210–222 m. Scale bar: 20 km.
Nutritional condition of L. larseni was evaluated by Le Cren condition index (LCCI) with the following equation LCCI = W/Wexp, where W is the observed weight of fish and Wexp is the expected weight calculated using the growth curve obtained from the regression between W and TL of the entire dataset. This index allows us to find out weight deviations of a certain length from the expected weight. Values higher than one indicates good nutritional condition of fishes, and lower values reflect a poor condition (Le Cren Reference Le Cren1951). A t-test was applied to analyse differences between LCCI of juveniles and adults. Each stomach was excised and preserved in 70% alcohol solution. Based on the stomach fullness, three different stages were arbitrarily defined: I (empty < 25% of gut content), II (partially full 25–75%) and III (full > 75%). For each stage, the stomach repletion index was calculated by the equation IR = (Wf / W)*100, where W is the fish weight and Wf is the ingested food weight (±0.001 g), obtained as difference between the weights of the stomach (with all the food) and the empty stomach.
The stomach contents were preserved in formaldehyde 5% for further analysis. Each zooplanktonic prey item was photographed and identified to the lowest possible taxonomic level according to Boltovskoy (Reference Boltovskoy1999). The frequency of occurrence (F%) was obtained by counting in which percentage of stomachs the different prey items were present, whereas the numerical abundance (N%) was obtained by the equation (np*100) / Np, where np is the number of each prey and Np is the total number of prey in the stomachs. Prey size (length and width) was recorded, evaluating differences between juvenile and adult fishes, and also relating these measurements to fish mouth size.
Sagitta, asteriscus and lapillus otoliths were extracted from the othic capsule, making a transversal cut on the dorsal and posterior part of the cranium under a stereomicroscope to help with their removal. They were cleaned with a sodium hypochlorite solution for 5–8 min and stored dry to further analysis. The inner and outer faces of sagitta and asteriscus and dorsal and ventral faces of lapillus were photographed and described according to Volpedo & Echeverría (Reference Volpedo and Echeverría2000), Assis (Reference Assis2003, Reference Assis2005) and Volpedo & Fuchs (Reference Volpedo and Fuchs2010). Maximum otolith length (OL) and maximum otolith width (OW) were measured from all otolith types, whereas rostrum length (RL), cauda length (CL) and “from cisure length” (FCL; considering from the cisure to the cauda base), were taken only on sagitta (Fig. 2). E and R indexes were calculated only for sagitta by the equations E % = OW / OL and R % = RL / OL, respectively. E determines the otolith shape, while R is the percentage of the rostrum respect to otolith length (Volpedo & Echeverría Reference Volpedo and Echeverría2003). Using all these variables, a multivariate discriminant analysis was carried out using the linear canonical function of Fisher (Zar Reference Zar1999). For sagitta and lapillus, morphometric relationships between these variables and fish lengths were assessed by linear regression models (Zar Reference Zar1999). InfoStat 2011 (Di Rienzo et al. Reference Di Rienzo, Casanoves, Balzarini, Gonzalez, Tablada and Robledo2011) was used for statistical analysis.

Fig. 2 Morphometric and morphological characteristics of Lepidonotothen larseni otoliths. a. Sagitta otolith (scale: 1 mm); outer face: (a) maximum length, OL, (b) maximum width, OW; inner face: (c) from cisure length, FCL (cauda base to cisure), (d) cauda length, CL, (e) rostrum length, RL. b. Asteriscus otolith (scale: 0.5 mm); outer face: (f) maximum length, OL, (g) maximum width, OW. c. Lapillus otolith (scale: 0.5 mm); dorsal face: (h) maximum length, OL, (i) maximum width, OW. Abbreviations: A = anterior, P = posterior, D = dorsal, V = ventral, I = inner, O = outer.
Results
Analysis of the nutritional condition, stomach repletion index and stomach content
Total length of fishes varied between 70 and 185 mm and total weight (W) varied between 2 and 60 g, with 46% of juveniles and 54% adults. The length-weight relationship obtained for all the specimens was W = 6.28 x 10-7 TL3.49 (r 2 = 0.96), which indicated a positive allometric growth (b coefficient > 3). The nutritional condition of fishes estimated by LCCI ranged between 0.65 and 1.52, with a high percentage of fishes in good nutritional condition (LCCI > 1). No significant differences between juveniles (LCCI mean = 1.007) and adults (LCCI mean = 1.024) were found (Fig. 3a; t = 0.33, P-value = 0.745). Stomach repletion index (IR) varied between 0.01 and 5.7, with a general decreasing trend from noon to late evening (Fig. 3b). Overall, 53% of fishes presented full stomach, 36% partially full stomach and 11% empty stomach. Stomach fullness significantly changed with fish size, as 20%, 45% and 35% of juveniles and 4%, 28% and 68% of adults were at stage I, II and III, respectively. Full stomachs (stage III) were observed mainly during late evening for adults and during noon hours for juveniles.

Fig. 3 a. Le Cren condition index (LCCI) of Lepidonotothen larseni, and b. stomach repletion index (IR) in different periods of the day: noon (13h00), evening (15h30) and late evening (18h00). Dark hours: black horizontal bars. Juveniles (filled circle) and adults (open circle).
In stomach content analysis, copepods and amphipods were more frequent and abundant in juveniles, whereas euphausiids were in adults. Polychaetes, cladocerans and isopods were only present in juveniles, while ostracods and appendicularians were only found in adults. Pteropods and decapod larvae were less frequent and abundant, but they were present in stomach contents of both stages (Fig. 4). In a great percentage of stomachs (33.3%) inorganic material was found, mainly stones.

Fig. 4 a. Frequency of occurrence, and b. numerical abundance of each prey consumed by Lepidonotothen larseni. Juveniles (black bars) and adults (white bars).
Prey of different sizes (in length and width) were found in stomachs of juveniles and adults, both sizes being larger in adult fishes with a bigger mouth size (Table I). Diet consisted mainly of several copepods, such as ciclopoid, calanoid (Aetideus armatus Boeck, Calanus simillimus Giesbrecht, Metridia gerlachei Giesbrecht, Onchocalanus sp. and Rhincalanus sp.), harpaticoid (Microsetella norvegica Boeck) and poecilostomatoid, both gammarid and hyperiid (Vibilia antarctica Stebbing) amphipods and euphausiids (Euphausia superba Dana). Prey consumed to a lesser extent were pteropods (Limacinidae, Pleumodermatidae and Cavoliniidae, mainly Clio pyramidata sulcata Pfeffer), ostracods, polychaetes (Vanadis and Travisiopsis spp.), decapods (zoea and megalopa larvae), cladocerans, isopods and appendicularians (Oikopleuridae).
Table I Mean values of fish mouth size of juveniles and adults of Lepidonotothen larseni and mean lengths and widths of their ingested prey. Minimum and maximum values are indicated between parentheses.

Otolith morphology and morphometry
A detailed description of the sagitta, considering both new characteristics and characteristics already mentioned in previous studies, is reported below, as well as, for the first time, those referred to the asteriscus and lapillus.
Sagitta (Fig. 5a, see also Fig. 2a)
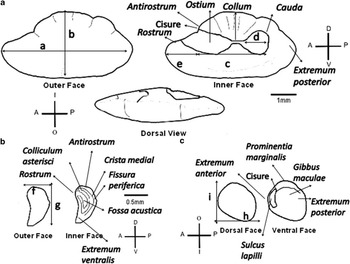
Oval shape with a developed projection in the extremum posterior. The dorsal border is slightly scalloped and the ventral one is regular. The otolith has a slightly pronounced cisure, and well marked rostrum and antirostrum. A medium sulcus with a constricted collum which delimits ostium from cauda. The ostium is discoid and opened in the anterior margin of the otolith. The cauda is elliptic and closed far from the posterior edge. The inner and outer faces are smooth and slightly convex. In juveniles, the sagitta is more rounded with smooth borders not scalloped and the rostrum is less pronounced. Moreover, the inner face is flat and the outer one is convex.

Fig. 5 a. Sagitta, b. asteriscus, and c. lapillus otoliths of juvenile and adult Lepidonotothen larseni. Abbreviations: A = anterior, P = posterior, D = dorsal, V = ventral, I = inner, O = outer.
Asteriscus (Fig. 5b, see also Fig. 2b)
Vertical otolith with a pronounced shape, and a sharp extremum ventralis. Smooth edges and inner and outer faces are smooth and convex. Rostrum, antirostrum and major and minor cisures are not differentiated. Fossa acustica is scarcely developed and ventrally curved. Crista medial is curved to the ventral area following the fossa acustica shape, occupying almost all the otolith length. Fissura periferica is larger than wider accompanying the crista medial length.
Lapillus (Fig. 5c, see also Fig. 2c)
Quadrangular otolith with a scarcely differentiated extremum anterior and a not distinguishable extremum posterior. Smooth edges, the ventral face is convex and the dorsal one is slightly concave. Gibbus maculae are scarcely differentiated. Sulcus lapilli is not delimited by a presulcal area (extended sulcus). Cisure is not differentiated. Prominentia marginalis conspicuous and rounded, not delimited in the profile. In juveniles the sulcus is delimited by a presulcal area (opened sulcus).
Morphometric analysis was made for the three otoliths of juvenile and adult fishes. Table II shows the mean values of maximum OL and maximum OW of sagitta, asteriscus and lapillus, and FCL, CL, RL, E and R indexes of sagitta. Cauda and rostrum lengths were the most variable measurements, while E and R were the least ones for juveniles as for adults (see coefficient of variation values in the table). Significant differences in all the morphometric variables were observed between juveniles and adults, which were reflected in a change in their otolith shape. In detail, OL (t = -9.98, P-value < 0.0001) and R (t = -2.40, P-value = 0.0202) were higher in adults, whereas E was higher in juveniles (t = 2.84, P-value = 0.0065). Asteriscus measurements (OL and OW) were more variable in adults than in juveniles, while in lapillus an inverse pattern was detected. All the relationships estimated between otolith measurements (sagitta and lapillus) and fish length (TL and SL) and between OW and OL were statistically significant (Table III, P < 0.0001 in all cases). In the case of asteriscus these relationships were not investigated owing to the small number of samples.
Table II Mean measurements of sagitta, asteriscus and lapillus otoliths of Lepidonotothen larseni (maximum otolith length (OL), maximum otolith width (OW), from cisure length (FCL), cauda length (CL), rostrum length (RL), E and R indexes. Standard deviation (SD) and coefficient of variation (CV) are indicated. Juveniles: 69.63–109.72 mm TL (total length), 62.52–97.96 mm SL (standard length). Adults: 110.67–183.00 mm TL, 97.50–164.79 mm SL. n = number of fishes analysed.

Table III Relationships (Y = a + bX) obtained between different measurements of sagitta and lapillus otoliths of L. larseni and fish size (TL: total length, SL: standard length). Standard errors of a and b coefficients between parentheses. See Table II for abbreviations.

The discriminant analysis of sagitta allowed us to identify the best linear function of some selected otolith measurements discriminating juveniles from adults, previous assumptions verified as the homogeneity matrix of covariance was not significant (Bartlett's test, P-value = 0.9992). Starting from two groups of fishes in relation to fish size (i.e. assuming 110 mm TL as limit between juveniles and adults), one eigenvalue was obtained (3.22), being the maximum OL (-4.02), the RL (2.85) and the R index (-1.29), the best variables which separated juveniles and adults. Conversely, maximum OW (0.11), FCL (0.16), CL (0.52) and E index (-0.79) were not significant for the stages discrimination. The fifty scores obtained applying this linear canonical function was plotted in Fig. 6.

Fig. 6 Plot of scores of juveniles (total length < 110 mm) and adults (total length > 110 mm) applying the linear canonical function of the discriminant analysis (y = 2.84615898*RL + 0.52169245*CL + 0.16328178*FCL + 0.11448414*OW - 4.017396*OL - 1.2973963*R - 0.7934913*E). For morphometric abbreviations see Fig. 2 caption. Juveniles (filled circle) and adults (open circle).
Discussion
The stomach contents analysis and the study of morphology and morphometry of sagitta, asteriscus and lapillus otoliths provided new insights on feeding behaviour and preferred habitat of the L. larseni population inhabiting the South Shetland Islands and the Antarctic Peninsula. Asteriscus and lapillus were described for the first time, while previous description of sagitta was improved by adding inner and outer faces descriptions including the ostium and cauda. The morphometric analysis of the sagitta and lapillus enabled us to relate different variables (OL, OW, RL, FCL and CL) in relation to fish size. Otolith morphology and morphometry are potential tools in identifying juveniles or adults of L. larseni in predator guts (Koen Alonso et al. Reference Koen Alonso, Crespo, Pedraza and Coscarella2000, Martínez Pérez et al. Reference Martínez Pérez, Chavez Arteaga, Tello and Morales2007). As otoliths can be partially deteriorated by chemical or mechanical abrasion in the stomach of predators (Granadeiro & Silva Reference Granadeiro and Silva2000), rostrum or sulcus often being eroded, we provided the FCL as well.
The sagitta showed morphological and morphometric variations during their ontogenetic development. The discriminant analysis of this otolith showed that the variables which mostly contribute to discriminate juveniles from adults were maximum OL, RL and R index. Although R index had the lowest score of these variables, it is of particular interest, as it provides evidence of an ontogenetic change of development rate of the rostrum with respect to the maximum OL (i.e. allometry). As it was expected, all other variables (RL and OL), contribute to discriminate juveniles and adults, as all increase with fish size.
The differentiation of sagittae shape could be linked to a change of fish habitat. Lombarte et al. (Reference Lombarte, Palmer, Matallanas, Gómez-Zurita and Morales-Nin2010) observed that benthic dwellers of Nototheniidae had the largest sagittae in relation to body size, pelagic species had smaller and rounder sagittae than benthic species, and epibenthic species showed intermediate relative sizes. Particularly, they corroborated the epibenthic habitat of adults of L. larseni by using geometric techniques relating diet, phylogeny and otolith shape. In the present study, juveniles of L. larseni presented a shorter and wider sagitta than adults, resulting in a major E index because of a rounded shape and a minor R index because of a less developed rostrum. This could indicate that juveniles live in the water column for a long time before settling, while adults could have an epibenthic habitat leaving the bottom during the night to feed in the water column (Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005). This different distribution pattern is reflected in the type of prey consumed (indeed, more pelagic copepods were found in juvenile stomachs). In addition, the values obtained of E (56.34 ± 3.57) and R (22.75 ± 2.34) indexes for adults are similar to those reported in previous studies (Hecht Reference Hecht1987, Volpedo et al. Reference Volpedo, Tombari and Echeverría2008).
Lepidonotothen larseni presented a positive allometric growth (b > 3), as they increased in weight faster than in length. The same pattern of allometric growth was observed in L. larseni of 140–203 mm TL collected at the South Shetland Islands by Eastman & Sidell (Reference Eastman and Sidell2002). Interestingly, both juveniles and adults showed a good nutritional condition (LCCI > 1), possibly owing to the favourable environmental conditions (primarily food availability) experienced during summer season. In the sub-Antarctic Prince Edward Islands, L. larseni fed during all of the 24 hour cycle, but the peak of feeding activity occurred at evening when they left the bottom to feed in the water column (Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005). An increase of feeding activity during early night and morning was confirmed elsewhere (Shandikov Reference Shandikov1986). In our study, even though the stomach repletion presented a general decreasing trend from noon to late evening, adults exhibited a full stomach degree of fullness (stage III) during late evening, while in juveniles this peak was at noon hours. Spatial and temporal distribution varied with ontogeny, as juveniles were caught exclusively at noon and evening whereas adults were caught exclusively at evening and late evening. Probably, this pattern is related to asynchronous vertical migrations of juveniles and adults which contribute to smooth spatial and food overlap between them.
The composition and sizes of prey consumed by L. larseni differed between juveniles and adults. While adults fed on small and large prey (in length and width), juveniles were limited to feed on small prey because of their comparatively smaller mouth size (Duarte et al. Reference Duarte, Ibáñez and Chong2007). The diet composition of L. larseni was similar to those found in previous studies (Targett Reference Targett1981, Barrera-Oro & Tomo Reference Barrera-Oro and Tomo1987, Pakhomov & Pankratov Reference Pakhomov and Pankratov1992, Takahashi & Iwami Reference Takahashi and Iwami1997, Frolkina et al. Reference Frolkina, Konstantinova and Trunov1998, Barrera-Oro Reference Barrera-Oro2002, Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005), consisting mainly of copepods, amphipods and euphausiids both in frequency of occurrence and abundance. Unlike the findings of Targett (Reference Targett1981), we did not find fishes or mysids in their stomach contents. In agreement with Barrera-Oro & Tomo (Reference Barrera-Oro and Tomo1987), we observed that diet changes with ontogeny, and particularly, the increase in size of L. larseni is related to a decrease in the consumption of copepods and an increase in that of krill. On the other hand, the presence of stones and benthic gammarid amphipods in the stomachs analysed confirmed the epibenthic habitat of adult fishes. Previous studies on other nototheniid species, such as Lepidonotothen nudifrons Lönnberg, Gobionotothen gibberifrons Lönnberg and Gobionotothen marionensis (Günther), showed that they all present morphological and behavioural adaptations to different habitats to avoid food overlapping (Targett Reference Targett1981, Takahashi Reference Takahashi1983, Bushula et al. Reference Bushula, Pakhomov, Kaehler, Davis and Kalin2005).
Acknowledgements
The authors wish to thank Juliana Giménez from University of Buenos Aires, Juan Martín Díaz de Astarloa from University of Mar del Plata and Daniel Fernández from CADIC, Tierra del Fuego, for providing the material collected in the ARA Puerto Deseado campaign funded by CONICET. We are also grateful to Fernando Ramírez and Carlos Assis for the collaboration and suggestions for the copepod and otolith studies respectively. Thanks to Mariela Spinelli for help with editing the figures. This research was support by a UBACYT grant (no. 20020100200048) to F. Capitanio. We thank two referees, Dr La Mesa and an anonymous reviewer, for their valuable comments and constructive inputs that greatly improved the manuscript.